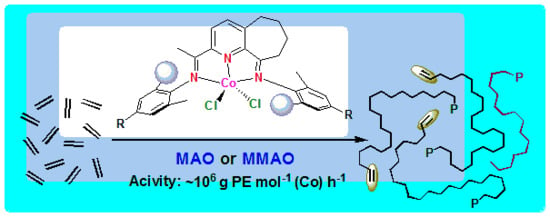
Highly Linear Polyethylenes Achieved Using Thermo-Stable and Efficient Cobalt Precatalysts Bearing Carbocyclic-Fused NNN-Pincer Ligand
Abstract
:1. Introduction
2. Results
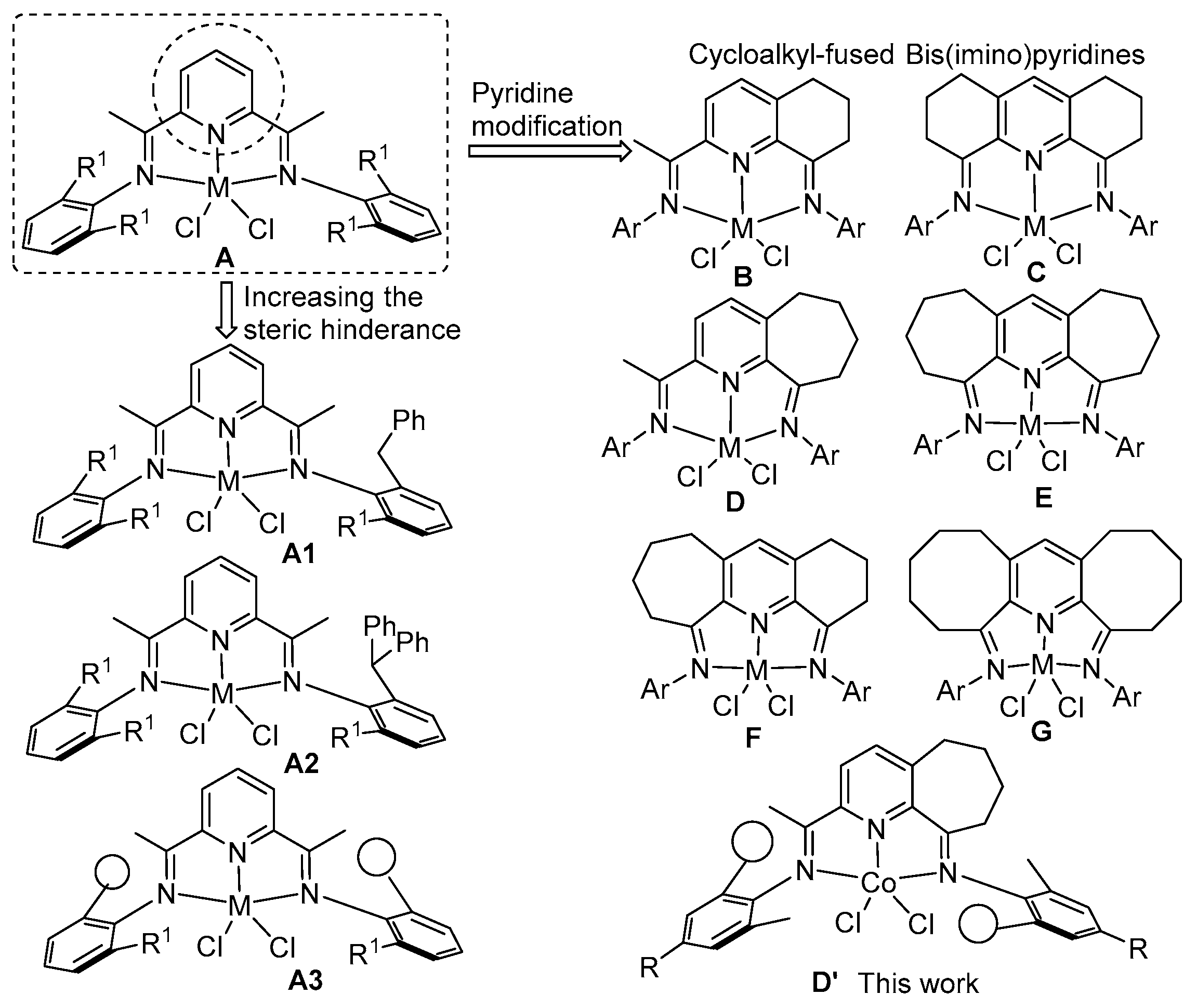
2.1. Synthesis and Characterization of Ligands and Cobalt Complexes Co1–Co6
2.2. Single-Crystal X-ray Diffraction Study
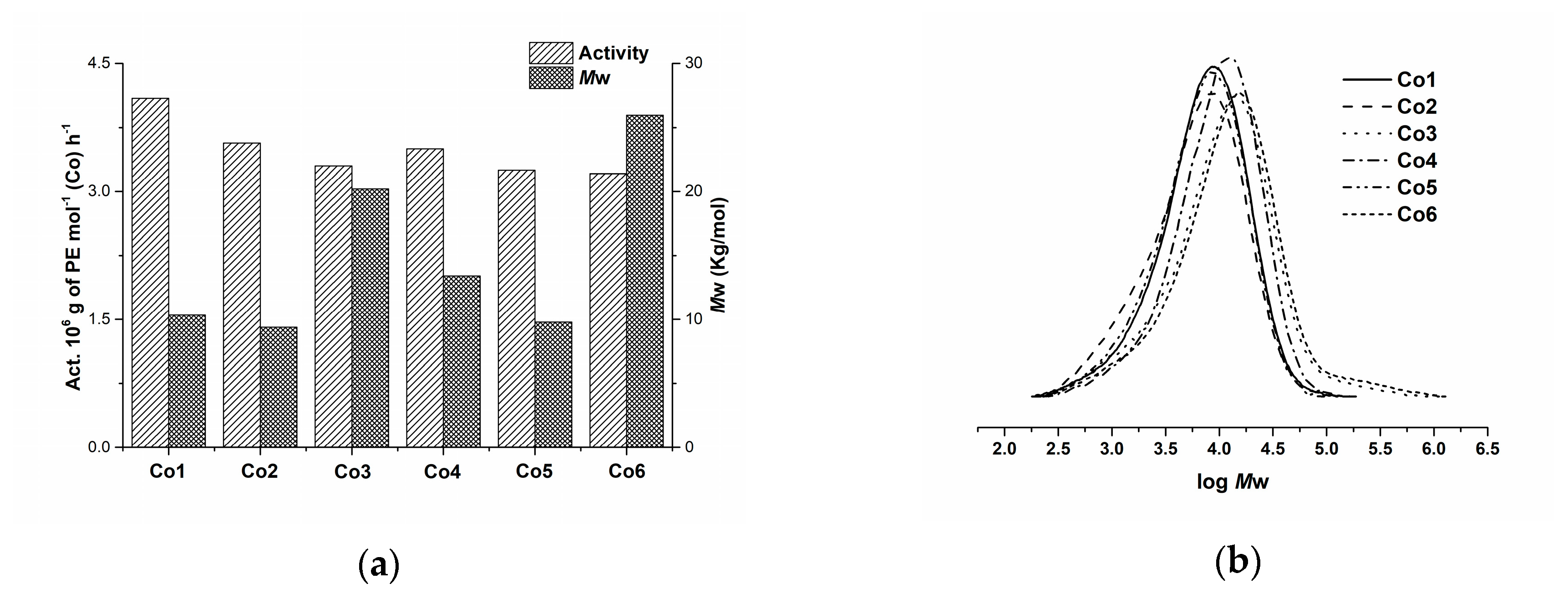
2.3. Ethylene Polymerization
2.3.1. Ethylene Polymerization using Co2/MAO
2.3.2. Ethylene Polymerization Using Co2/MMAO
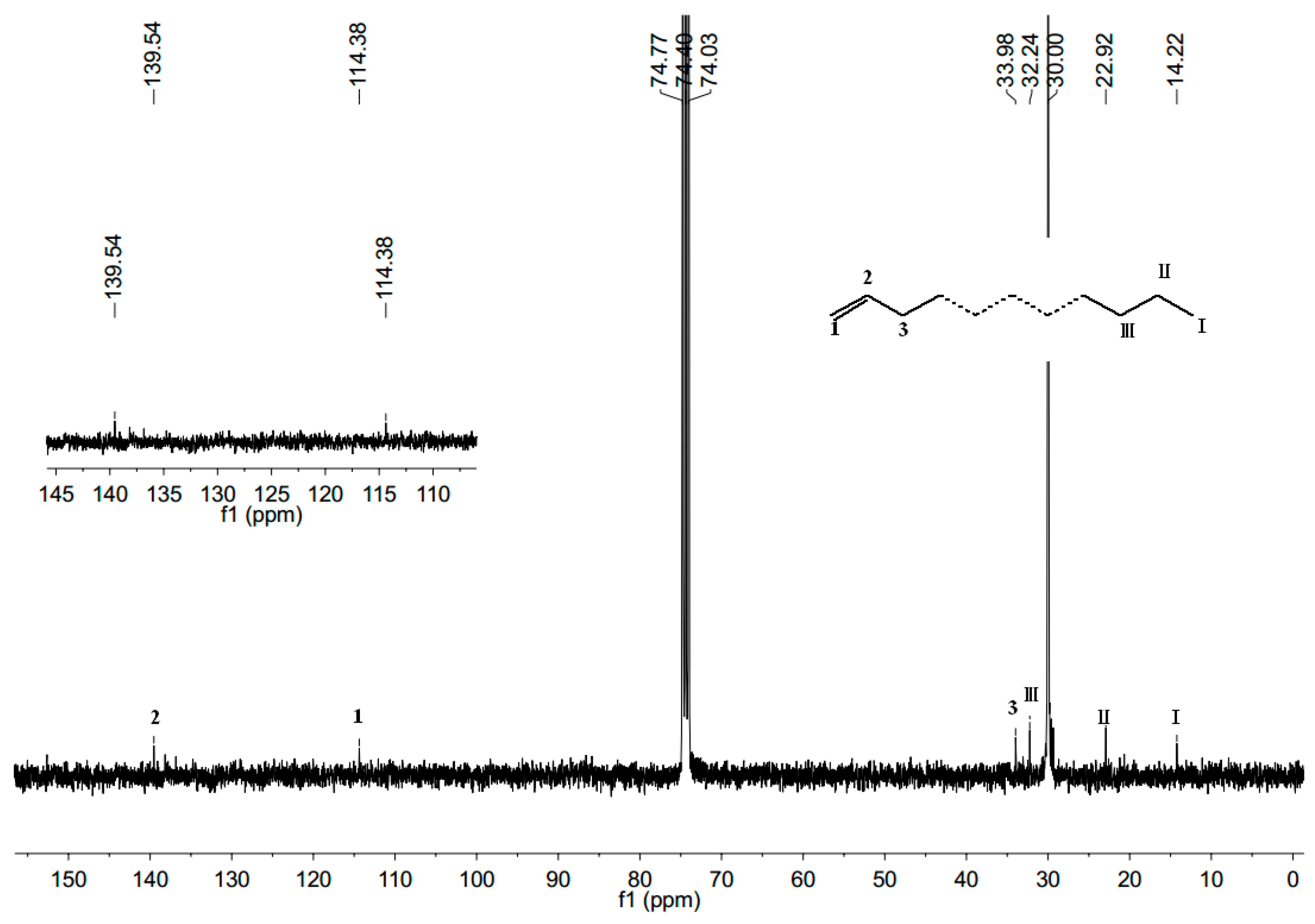
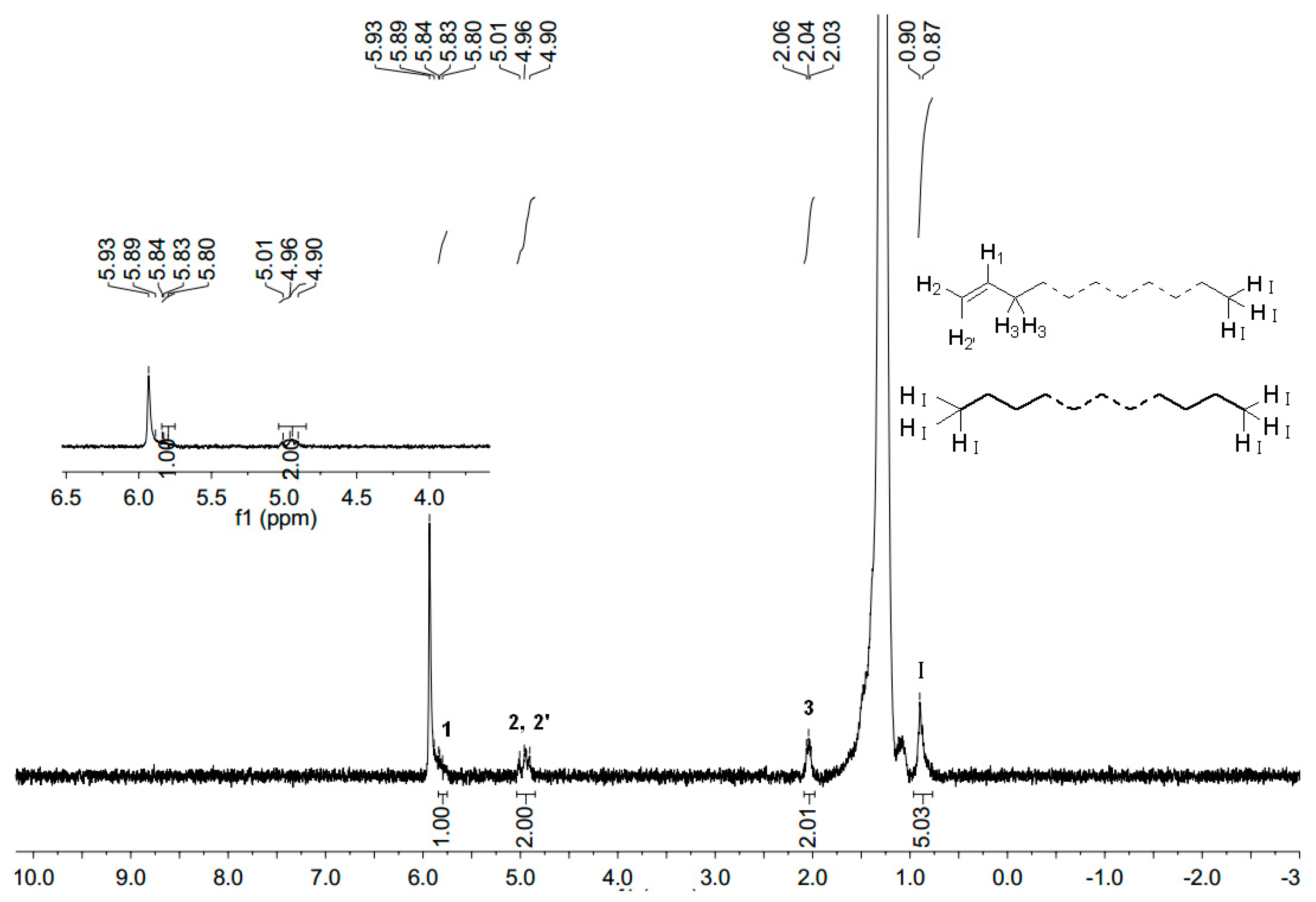
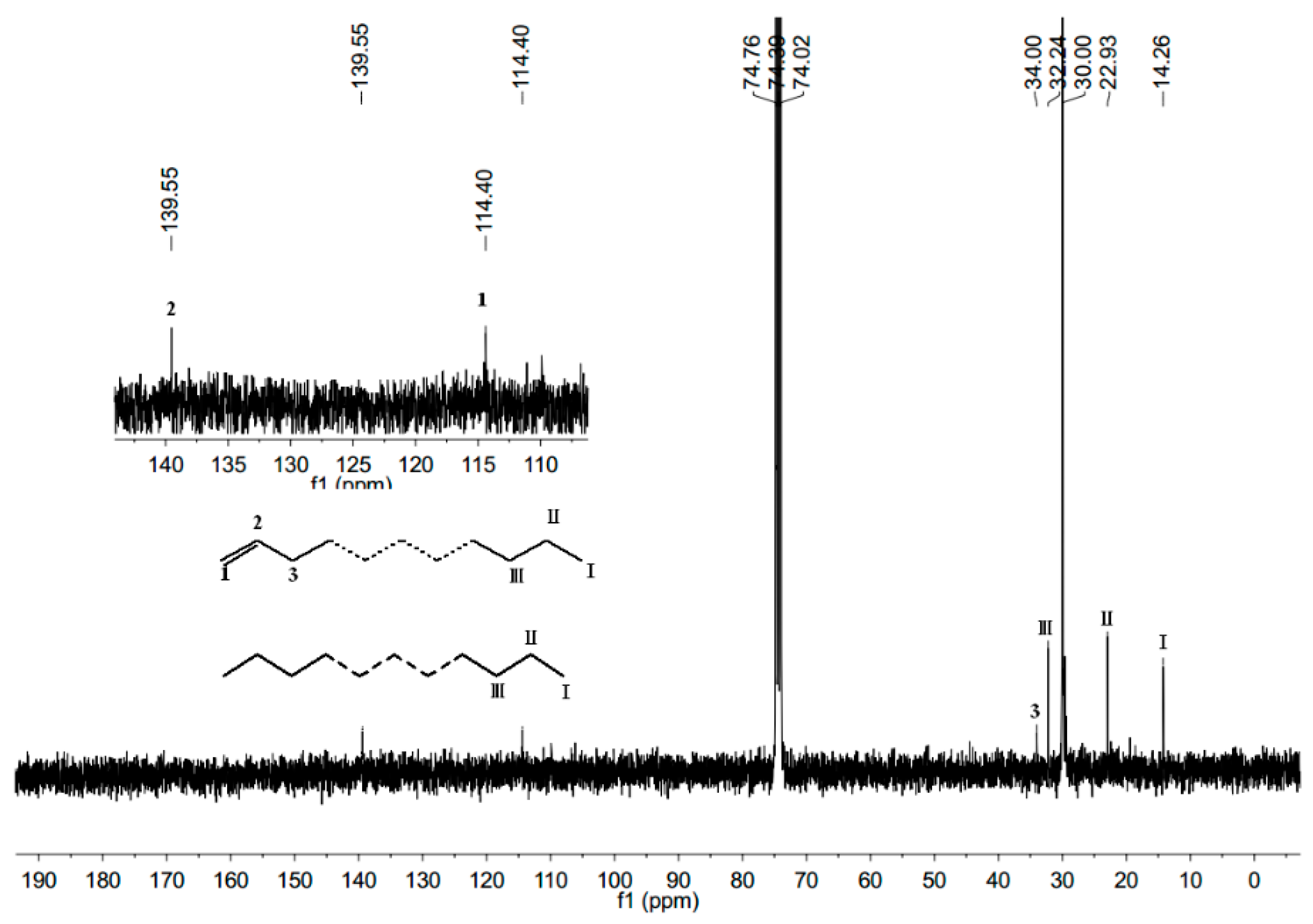
2.4. Characterization of Polyethylene
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis of 2-(1-ArN) C2H3-9-ArN-5,6,7,8-C5H8C5H3N (L1–L6)
3.3. Synthesis of [2-(1-ArN) C2H3-9-ArN-5,6,7,8-C5H8C5H3N] CoCl2 (Co1–Co6)
3.4. X-ray Crystallographic Studies
3.5. General Procedure for Ethylene Polymerization under 5/10 Atm Pressure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PE | Polyethylene |
| ORTEP | Oak Ridge Thermal Ellipsoid Plot |
| CIF | Calibration Index File |
| GPC | Gel Permeation Chromatography |
| MAO | methylaluminoxane |
| MMAO | Modified methylaluminoxane |
| PDI | Polydispersity index |
| Tm | Melting temperature |
References
- Small, B.L.; Brookhart, M.; Bennett, A.M.A. Highly active iron and cobalt catalysts for the polymerization of ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M. Iron-based catalysts with exceptionally high activities and selectivities for oligomerization of ethylene to linear α-olefins. J. Am. Chem. Soc. 1998, 120, 7143–7144. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; McTavish, S.J.; Solan, G.A.; White, A.J.P.; Williams, D.J. Novel olefin polymerization catalysts based on iron and cobalt. Chem. Commun. 1998, 7, 849–850. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Bruce, M.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; Mastroianni, S.; McTavish, S.J.; Redshaw, C.; Solan, G.A.; Stromberg, S.; et al. Iron and cobalt ethylene polymerization catalysts bearing 2,6-bis(Imino)pyridyl ligands: Synthesis, structures, and polymerization studies. J. Am. Chem. Soc. 1999, 121, 8728–8740. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Mastroianni, S.; Solan, G.A.; Baugh, S.P.D.; Redshaw, C.; Gibson, V.C.; White, A.J.P.; Williams, D.J.; Elsegood, M.R. Oligomerisation of ethylene by bis(imino)pyridyliron and -cobalt complexes. Chem. Eur. J. 2000, 6, 2221–2231. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Zhang, W.; Sun, W.-H. Carbocyclic-fused N,N,N-pincer ligands as ring-strain adjustable supports for iron and cobalt catalysts in ethylene oligo-/polymerization. Coord. Chem. Rev. 2018, 363, 92–108. [Google Scholar] [CrossRef]
- Small, B.L. Discovery and development of pyridine-bis(imine) and related catalysts for olefin polymerization and oligomerization. Acc. Chem. Res. 2015, 48, 2599–2611. [Google Scholar] [CrossRef]
- Burcher, B.; Breuil, P.-A.R.; Magna, L.; Olivier-Bourbigou, H. Iron-catalyzed oligomerization and polymerization Reactions. Top. Organomet. Chem. 2015, 50, 217–258. [Google Scholar]
- Flisak, Z.; Sun, W.-H. Progression of diiminopyridines: From single application to catalytic versatility. ACS Catal. 2015, 5, 4713–4724. [Google Scholar] [CrossRef]
- Ma, J.; Feng, C.; Wang, S.; Zhao, K.-Q.; Sun, W.-H.; Redshaw, C.; Solan, G.A. Bi- and tri-dentate imino-based iron and cobalt pre-catalysts for ethylene oligo-/polymerization. Inorg. Chem. Front. 2014, 1, 14–34. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Sun, W.-H.; Redshaw, C. Tailoring iron complexes for ethylene oligomerization and/or polymerization. Dalton Trans. 2013, 42, 8988–8997. [Google Scholar] [CrossRef]
- Gibson, V.C.; Solan, G.A. Olefin Oligomerizations and Polymerizations Catalyzed by Iron and Cobalt Complexes Bearing Bis(imino)pyridine Ligands. In Catalysis without Precious Metals; Bullock, M., Ed.; Wiley-VCH: Weinheim, Germany, 2010; pp. 111–141. [Google Scholar]
- Gibson, V.C.; Solan, G.A. Iron-based and cobalt-based olefin polymerisation catalysts. Top. Organomet. Chem. 2009, 26, 107–158. [Google Scholar]
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: Surprisingly reactive ligands and a gateway to new families of catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, C.; Giambastiani, G.; Rios, I.G.; Mantovani, G.; Meli, A.; Segarra, A.M. Ethylene oligomerization, homopolymerization and copolymerization by iron and cobalt catalysts with 2,6-(bis-organylimino)pyridyl ligands. Coord. Chem. Rev. 2006, 250, 1391–1418. [Google Scholar] [CrossRef]
- Mahmood, Q.; Guo, J.; Zhang, W.; Ma, Y.; Liang, T.; Sun, W.-H. Concurrently improving the thermal stability and activity of ferrous precatalysts for the production of saturated/unsaturated polyethylene. Organometallics 2018, 37, 957–970. [Google Scholar] [CrossRef]
- Mahmood, Q.; Yue, E.; Guo, J.; Zhang, W.; Ma, Y.; Hao, X.; Sun, W.-H. Nitro-functionalized bis(imino)pyridylferrous chlorides as thermo-stable precatalysts for linear polyethylenes with high molecular weights. Polymer 2018, 159, 124–137. [Google Scholar] [CrossRef]
- Semikolenova, N.V.; Sun, W.-H.; Soshnikov, I.E.; Matsko, M.A.; Kolesova, O.V.; Zakharov, V.A.; Bryliakov, K.P. Origin of “multisite-like” ethylene polymerization behavior of the single-site nonsymmetrical bis(imino)pyridine iron(II) complex in the presence of modified methylaluminoxane. ACS Catal. 2017, 7, 2868–2877. [Google Scholar] [CrossRef]
- Zhao, W.; Yue, E.; Wang, X.; Yang, W.; Chen, Y.; Hao, X.; Cao, X.; Sun, W.-H. Activity and stability spontaneously enhanced toward ethylene polymerization by employing 2-(1-(2,4-Dibenzhydrylnaphthyl- imino)ethyl)-6-(1-(arylimino)ethyl)pyridyliron(II) dichlorides. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 988–996. [Google Scholar] [CrossRef]
- Mitchell, N.E.; Anderson, W.C.; Long, B.K. Mitigating chain-transfer and enhancing the thermal stability of co-based olefin polymerization catalysts through sterically demanding ligands. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3990–3996. [Google Scholar] [CrossRef]
- Yue, E.; Zeng, Y.; Zhang, W.; Sun, Y.; Cao, X.-P.; Sun, W.-H. Highly linear polyethylenes using the 2-(1-(2,4-dibenzhydrylnaphthylimino)ethyl)-6-(1-(arylimino)ethyl)pyridylcobalt chlorides: Synthesis, characterization and ethylene polymerization. Sci. China Chem. 2016, 59, 1291–1300. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Du, S.; Guo, C.-Y.; Hao, X.; Sun, W.-H. 2-(1-(2,4-Bis((di(4-fluorophenyl)ethyl)- methylphenylimino)ethyl)-6-(1-(arylimino)ethyl)pyridylmetal (iron or cobalt) complexes: Synthesis, characterization, and ethylene polymerization behavior. Macromol. Chem. Phys. 2014, 215, 1797–1809. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Liang, T.; Redshaw, C.; Li, Y.; Sun, W.-H. Synthesis, characterization and catalytic behavior toward ethylene of 2-[1-(4,6-dimethyl-2-benzhydrylphenylimino)ethyl]-6-[1-(arylimino)ethyl] pyridylmetal (iron or cobalt) chlorides. Dalton Trans. 2013, 42, 9188–9197. [Google Scholar] [CrossRef]
- Smit, T.M.; Tomov, A.K.; Britovsek, G.J.P.; Gibson, V.C.; White, A.J.P.; Williams, D.J. The effect of imine-carbon substituents in bis(imino)pyridine-based ethylene polymerisation catalysts across the transition series. Catal. Sci. Technol. 2012, 2, 643–655. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, J.; Song, S.; Yang, W.; Liu, H.; Hao, X.; Redshaw, C.; Sun, W.-H. Controlling the ethylene polymerization parameters in iron pre-catalysts of thetype 2-[1-(2,4-dibenzhydryl-6-methylphenylimino) ethyl]-6-[1-(arylimino)ethyl] pyridyliron dichloride. Polymer 2012, 53, 130–137. [Google Scholar] [CrossRef]
- Cao, X.; He, F.; Zhao, W.; Cai, Z.; Hao, X.; Shiono, T.; Redshaw, C.; Sun, W.-H. 2-[1-(2,6-Dibenzhydryl-4-chlorophenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridyliron(II) dichlorides: Synthesis, characterization and ethylene polymerization behavior. Polymer 2012, 53, 1870–1880. [Google Scholar] [CrossRef]
- Lai, J.; Zhao, W.; Yang, W.; Redshaw, C.; Liang, T.; Liu, Y.; Sun, W.-H. 2-[1-(2,4-Dibenzhydryl-6-methylphenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridylcobalt(II) dichlorides: Synthesis, characterization and ethylene polymerization behavior. Polym. Chem. 2012, 3, 787–793. [Google Scholar] [CrossRef]
- He, F.; Zhao, W.; Cao, X.-P.; Liang, T.; Redshaw, C.; Sun, W.-H. 2-[1-(2,6-Dibenzhydryl-4-chloro phenylimino)ethyl]-6-[1-aryliminoethyl]pyridylcobalt dichlorides: Synthesis, characterization and ethylene polymerization behavior. J. Organomet. Chem. 2012, 713, 209–216. [Google Scholar] [CrossRef]
- Yu, J.; Huang, W.; Wang, L.; Redshaw, C.; Sun, W.-H. 2-[1-(2,6-Dibenzhydryl-4-methylphenylimino) ethyl]-6-[1-(arylimino)ethyl]-pyridylcobalt(II) dichlorides: Synthesis, characterization and ethylene polymerization behavior. Dalton Trans. 2011, 40, 10209–10214. [Google Scholar] [CrossRef]
- Yu, J.; Liu, H.; Zhang, W.; Hao, X.; Sun, W.-H. Access to highly active and thermally stable iron procatalysts using bulky 2-[1-(2,6-dibenzhydryl-4-methylphenylimino)ethyl]-6-[1-(arylimino)ethyl] pyridine Ligands. Chem. Commun. 2011, 47, 3257–3259. [Google Scholar] [CrossRef]
- Guo, L.; Gao, H.; Zhang, L.; Zhu, F.; Wu, Q. An unsymmetrical iron(II) bis(imino)pyridyl catalyst for ethylene polymerization: Effect of a bulky ortho substituent on the thermostability and molecular weight of polyethylene. Organometallics 2010, 29, 2118–2125. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.-H.; Han, L.; Yang, H.; Hu, Y.; Jin, X. Late transition metal complexes bearing 2,9-bis(imino)-1,10-phenanthrolinyl ligands: Synthesis, characterization and their ethylene activity. J. Organomet. Chem. 2002, 658, 62–70. [Google Scholar] [CrossRef]
- Guo, L.; Zada, M.; Zhang, W.; Vignesh, A.; Zhu, D.; Ma, Y.; Liang, T.; Sun, W.-H. Highly linear polyethylenes tailored by 2,6-bis[1-(p-dibenzo-cycloheptylarylimino)ethyl]pyridylcobalt dichlorides. Dalton Trans. 2019. [Google Scholar] [CrossRef]
- Oleinik, I.I.; Oleinik, I.V.; Abdrakhmanov, I.B.; Ivanchev, S.S.; Tolstikov, G.A. Design of arylimine postmetallocene catalytic systems for olefin polymerization: I. Synthesis of substituted 2-cycloalkyl- and 2,6-dicycloalkylanilines. Russ. J. Gen. Chem. 2004, 74, 1423–1427. [Google Scholar] [CrossRef]
- Ivancheva, S.S.; Yakimansky, A.V.; Rogozin, G.D. Quantum-chemical calculations of the effect of cycloaliphatic groups in a-diimine and bis(imino)pyridine ethylene polymerization precatalysts on their stabilities with respect to deactivation reactions. Polymer 2004, 45, 6453–6459. [Google Scholar] [CrossRef]
- Ivanchev, S.S.; Tolstikov, G.A.; Badaev, V.K.; Oleinik, I.I.; Ivancheva, N.I.; Rogozin, D.G.; Oleinik, I.V.; Myakin, S.V. New bis(arylimino)pyridyl complexes as components of catalysts for ethylene polymerization. Kinet. Catal. 2004, 45, 176–182. [Google Scholar] [CrossRef]
- Tolstikov, G.A.; Ivanchev, S.S.; Oleinik, I.I.; Ivancheva, N.I.; Oleinik, I.V. New multifunctional bis(imino)pyridine–iron chloride complexes and ethylene polymerization catalysts on their basis. Dokl. Phys. Chem. 2005, 404, 208–211. [Google Scholar] [CrossRef]
- Ba, J.; Du, S.; Yue, E.; Hu, X.; Flisak, Z.; Sun, W.-H. Constrained formation of 2-(1-(arylimino)ethyl)-7-arylimino-6,6-dimethylcyclopentapyridines and their cobalt(II) chloride complexes: Synthesis, characterization and ethylene polymerization. RSC Adv. 2015, 5, 32720–32729. [Google Scholar] [CrossRef]
- Sun, W.-H.; Kong, S.; Chai, W.; Shiono, T.; Redshaw, C.; Hu, X.; Guo, C.; Hao, X. 2-(1-(Arylimino)ethyl)-8- arylimino-5,6,7-trihydroquinolylcobalt dichloride: Synthesis and polyethylene wax formation. Appl. Catal. A 2012, 447–448, 67–73. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, W.; Yue, E.; Liang, T.; Hu, X.; Sun, W.-H. Controlling the molecular weights of polyethylene waxes using the highly active precatalysts of 2-(1-aryliminoethyl)-9-arylimino- 5,6,7,8-tetrahydrocycloheptapyridylcobalt chlorides: Synthesis, characterization, and catalytic behavior. Dalton Trans. 2016, 45, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, W.; Sun, Y.; Hu, X.; Solan, G.A.; Sun, W.H. Thermally stable and highly active cobalt precatalysts for vinyl-polyethylenes with narrow polydispersities: Integrating fused-ring and imino-carbon protection into ligand design. New J. Chem. 2016, 40, 8012–8023. [Google Scholar] [CrossRef]
- Appukuttan, V.K.; Liu, Y.; Son, B.C.; Ha, C.-S.; Suh, H.; Kim, I. Iron and cobalt complexes of 2,3,7,8-tetrahydroacridine-4,5(1H,6H)-diimine sterically modulated by substituted aryl rings for the selective oligomerization to polymerization of ethylene. Organometallics 2011, 30, 2285–2294. [Google Scholar] [CrossRef]
- Du, S.; Zhang, W.; Yue, E.; Huang, F.; Liang, T.; Sun, W.-H. α,α′-Bis(arylimino)-2,3:5,6-bis(penta methylene)pyridylcobalt chlorides: Synthesis, characterization, and ethylene polymerization behavior. Eur. J. Inorg. Chem. 2016, 1748–1755. [Google Scholar] [CrossRef]
- Suo, H.; Oleynik, I.I.; Bariashir, C.; Oleynik, I.V.; Wang, Z.; Solan, G.A.; Ma, Y.; Liang, T.; Sun, W.-H. Strictly linear polyethylene using Co-catalysts chelated by fused bis(arylimino)pyridines: Probing ortho-cycloalkyl ring-size effects on molecular weight. Polymer 2018, 149, 45–54. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Guo, J.; Liu, Q.; Solan, G.A.; Liang, T.; Sun, W.-H. Bis(imino)pyridines fused with 6- and 7-membered carbocylic rings as N,N,N-scaffolds for cobalt ethylene polymerization catalysts. Dalton Trans. 2019, 48, 2582–2591. [Google Scholar] [CrossRef]
- Bariashir, C.; Wang, Z.; Suo, H.; Muhammad, Z.; Solan, G.A.; Ma, Y.; Liang, T.; Sun, W.-H. Narrow dispersed linear polyethylene using cobalt catalysts bearing cycloheptyl-fusedbis(imino)pyridines; probing the effects of ortho-benzhydryl substitution. Eur. Polym. J. 2019, 110, 240–251. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Mahmood, Q.; Liu, Q.; Ma, Y.; Hao, X.; Sun, W.-H. Bis(imino)pyridines incorporating doubly fused eight-membered rings as conformationally flexible supports for cobalt ethylene polymerization catalysts. Organometallics 2018, 37, 380–389. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.; Zhang, W.; Solan, G.A.; Liu, Q.; Liang, T.; Sun, W.-H. Enhancing thermostability of iron ethylene polymerization catalysts through N,N,N-chelation of doubly fused α,α′-bis(arylimino)-2,3:5,6-bis(hexamethylene)pyridines. Catal. Sci. Techn. 2019. [Google Scholar] [CrossRef]
- Huang, F.; Xing, Q.; Liang, T.; Flisak, Z.; Ye, B.; Hu, X.; Yang, W.; Sun, W.-H. 2-(1-Aryliminoethyl)-9-arylimino-5,6,7,8-tetrahydrocycloheptapyridyl iron(II) dichloride: Synthesis, characterization, and the highly active and tunable active species in ethylene polymerization. Dalton Trans. 2014, 43, 16818–16829. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yue, E.; Qu, M.; Oleynik, I.V.; Oleynik, I.I.; Li, K.; Liang, T.; Zhang, W.; Sun, W.-H. 8-(2-Cycloalkylphenylimino)-5,6,7-trihydro-quinolylnickel halides: Polymerizing ethylene to highly branched and lower molecular weight polyethylenes. Inorg. Chem. Front. 2015, 2, 223–227. [Google Scholar] [CrossRef]
- Tomov, A.K.; Gibson, V.C.; Britovsek, G.J.P.; Long, R.J.; van Meurs, M.; Jones, D.J.; Tellmann, K.P.; Chirinos, J.J. Distinguishing chain growth mechanisms in metal-catalyzed olefin oligomerization and polymerization systems: C2H4/C2D4 co-oligomerization/polymerization experiments using chromium, iron, and cobalt catalysts. Organometallics 2009, 28, 7033–7040. [Google Scholar] [CrossRef]
- Jones, D.J.; Gibson, V.C.; Green, S.M.; Maddox, P.J.; White, A.J.P.; Williams, D.J. Discovery and optimization of new chromium catalysts for ethylene oligomerization and polymerization aided by high-throughput screening. J. Am. Chem. Soc. 2005, 127, 11037–11046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hao, P.; Zuo, W.; Jie, S.; Sun, W.-H. 2-(Benzimidazol-2-yl)-1,10-phenanthrolyl metal (Fe and Co) complexes and their catalytic behaviors toward ethylene oligomerization. J. Organomet. Chem. 2008, 693, 483–491. [Google Scholar] [CrossRef]
- Xiao, T.; Hao, P.; Kehr, G.; Hao, X.; Erker, G.; Sun, W.-H. Dichlorocobalt(II) complexes ligated by bidentate 8-(benzoimidazol-2-yl)quinolines: Synthesis, characterization, and catalytic behavior toward ethylene. Organometallics 2011, 30, 4847–4853. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-integrated space-group and crystalstructure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
Sample Availability: Samples of the organic compounds and cobalt complexes are available from the authors. |

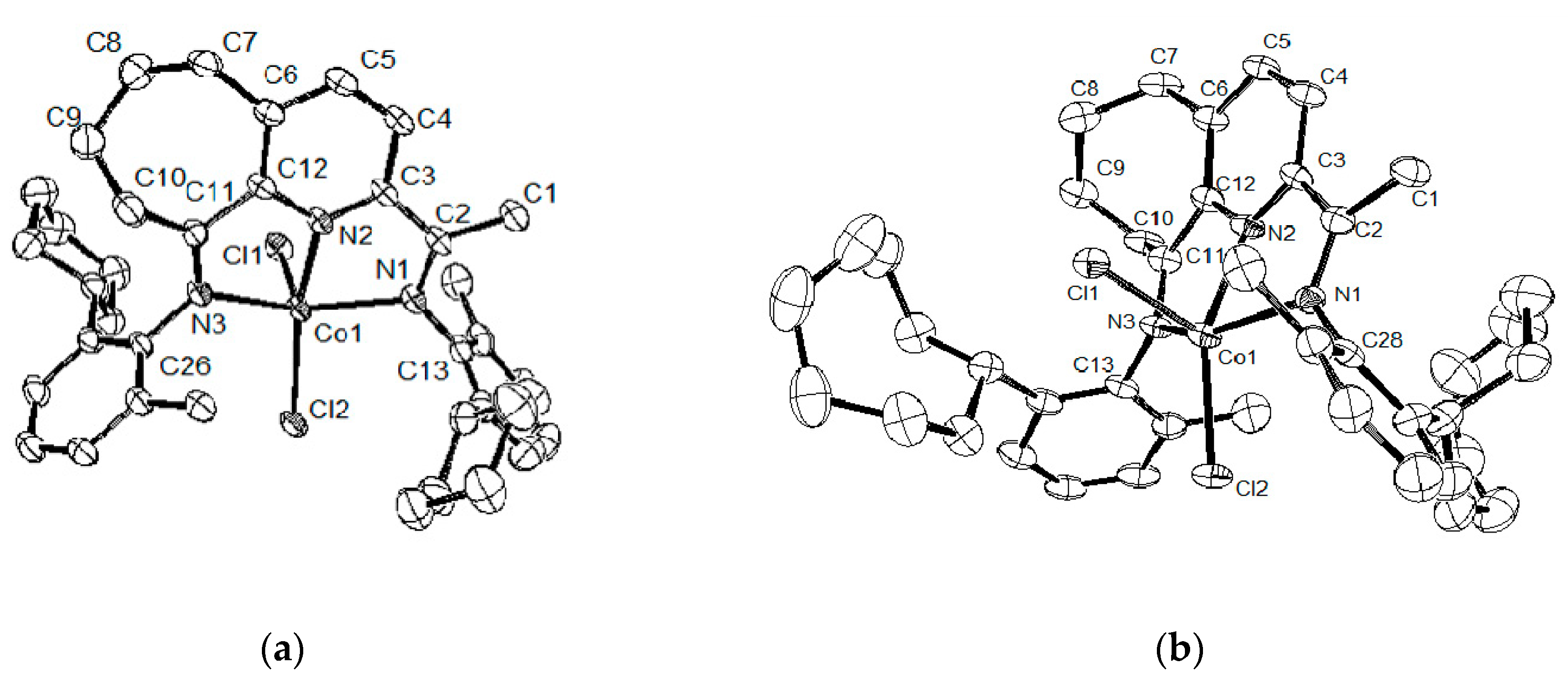




| Co2 | Co3 | Co2 | Co3 | ||
|---|---|---|---|---|---|
| Bond Lengths (Å) | |||||
| Co(1)–N(1) | 2.203(4) | 2.193(4) | Co(1)–N(2) | 2.063(4) | 2.043(4) |
| Co(1)–N(3) | 2.182(3) | 2.175(4) | Co(1)–Cl(1) | 2.2922(13) | 2.3062(15) |
| Co(1)–Cl(2) | 2.2646(12) | 2.2478(14) | N(1)–C(2) | 1.286(6) | 1.296(6) |
| N(3)–C(11) | 1.286(5) | 1.296(6) | |||
| Bond Angles (deg) | |||||
| N(1)–Co(1)–N(2) | 73.80(14) | 74.91(16) | N(1)–Co(1)–N(3) | 142.70(13) | 147.01(15) |
| N(2)–Co(1)–N(3) | 74.65(14) | 75.80(16) | N(1)–Co(1)–Cl(2) | 97.55(11) | 98.74(11) |
| N(2)–Co(1)–Cl(2) | 152.75(11) | 151.03(13) | N(3)–Co(1)–Cl(2) | 101.45(10) | 99.53(11) |
| N(1)–Co(1)–Cl(1) | 100.12(11) | 98.37(11) | N(2)–Co(1)–Cl(1) | 95.66(11) | 89.97(12) |
| N(3)–Co(1)–Cl(1) | 102.30(10) | 96.45(12) | Cl(2)–Co(1)–Cl(1) | 111.42(5) | 119.00(6) |
| C(11)–N(3)–Co(1) | 116.0(3) | 113.9(3) | C(2)–N(1)–Co(1) | 114.9(3) | 114.8(4) |
| Run | Precat. | Al/Co | T (°C) | t (min) | PE (g) | Act. b | Mwc | Mw/Mnc | Tm (oC) d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Co2 | 1000 | 30 | 30 | 3.66 | 2.44 | 22.97 | 3.7 | 133.7 |
| 2 | Co2 | 1000 | 40 | 30 | 3.74 | 2.49 | 17.94 | 3.8 | 131.4 |
| 3 | Co2 | 1000 | 50 | 30 | 4.33 | 2.89 | 14.21 | 3.0 | 131.1 |
| 4 | Co2 | 1000 | 60 | 30 | 2.70 | 1.80 | 9.37 | 2.0 | 130.2 |
| 5 | Co2 | 1000 | 70 | 30 | 1.45 | 0.96 | 6.56 | 2.5 | 130.1 |
| 6 | Co2 | 500 | 50 | 30 | Trace | - | - | - | - |
| 7 | Co2 | 1500 | 50 | 30 | 4.37 | 2.91 | 9.76 | 2.4 | 130.3 |
| 8 | Co2 | 2000 | 50 | 30 | 4.43 | 2.95 | 9.75 | 2.4 | 130.7 |
| 9 | Co2 | 2500 | 50 | 30 | 5.36 | 3.57 | 9.39 | 2.7 | 130.0 |
| 10 | Co2 | 3000 | 50 | 30 | 5.10 | 3.40 | 10.67 | 2.6 | 130.5 |
| 11 | Co2 | 3500 | 50 | 30 | 1.94 | 1.29 | 13.96 | 2.5 | 130.6 |
| 12 | Co2 | 2500 | 50 | 5 | 2.03 | 8.12 | 10.65 | 2.6 | 130.3 |
| 13 | Co2 | 2500 | 50 | 15 | 4.70 | 6.26 | 10.34 | 2.5 | 130.7 |
| 14 | Co2 | 2500 | 50 | 45 | 6.28 | 2.78 | 11.21 | 2.6 | 131.5 |
| 15 | Co2 | 2500 | 50 | 60 | 7.44 | 2.48 | 11.26 | 2.7 | 130.1 |
| 16 e | Co2 | 2500 | 50 | 30 | 4.28 | 2.85 | 13.04 | 2.7 | 130.8 |
| 17 | Co1 | 2500 | 50 | 30 | 6.13 | 4.09 | 10.34 | 2.4 | 130.4 |
| 18 | Co3 | 2500 | 50 | 30 | 4.95 | 3.30 | 20.20 | 3.9 | 132.4 |
| 19 | Co4 | 2500 | 50 | 30 | 5.25 | 3.50 | 13.40 | 2.4 | 131.6 |
| 20 | Co5 | 2500 | 50 | 30 | 4.87 | 3.25 | 9.78 | 2.4 | 130.2 |
| 21 | Co6 | 2500 | 50 | 30 | 4.82 | 3.21 | 25.96 | 4.4 | 132.8 |
| Run | Precat. | Al/Co | T (°C) | t (min) | PE (g) | Act. b | Mwc | Mw/Mnc | Tm (oC) d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Co2 | 2500 | 30 | 30 | 3.34 | 2.23 | 12.98 | 2.8 | 132.5 |
| 2 | Co2 | 2500 | 40 | 30 | 3.82 | 2.55 | 11.42 | 2.6 | 130.3 |
| 3 | Co2 | 2500 | 50 | 30 | 4.43 | 2.95 | 8.19 | 2.5 | 129.1 |
| 4 | Co2 | 2500 | 60 | 30 | 3.20 | 2.13 | 6.41 | 2.5 | 128.1 |
| 5 | Co2 | 1500 | 50 | 30 | 3.62 | 2.41 | 10.22 | 2.4 | 130.1 |
| 6 | Co2 | 2000 | 50 | 30 | 3.75 | 2.50 | 9.27 | 2.7 | 129.4 |
| 7 | Co2 | 3000 | 50 | 30 | 2.27 | 1.51 | 9.86 | 2.3 | 129.9 |
| 8 | Co2 | 2500 | 50 | 5 | 1.76 | 7.04 | 10.49 | 2.5 | 130.0 |
| 9 | Co2 | 2500 | 50 | 15 | 3.98 | 5.31 | 8.29 | 2.5 | 129.1 |
| 10 | Co2 | 2500 | 50 | 60 | 6.21 | 2.07 | 10.06 | 2.4 | 131.2 |
| 11 e | Co2 | 2500 | 50 | 30 | 2.85 | 1.90 | 8.90 | 2.5 | 129.9 |
| 12 | Co1 | 2500 | 50 | 30 | 5.01 | 3.34 | 10.01 | 2.4 | 130.0 |
| 13 | Co3 | 2500 | 50 | 30 | 3.74 | 2.49 | 14.72 | 2.2 | 130.9 |
| 14 | Co4 | 2500 | 50 | 30 | 4.87 | 3.25 | 10.79 | 2.4 | 129.8 |
| 15 | Co5 | 2500 | 50 | 30 | 3.98 | 2.65 | 9.22 | 2.6 | 129.6 |
| 16 | Co6 | 2500 | 50 | 30 | 3.36 | 2.24 | 15.94 | 4.5 | 133.3 |
| Co2 | Co3 | |
|---|---|---|
| CCDC No. | 1897124 | 1897125 |
| Crystal color | Brown | Yellow |
| Empirical formula | C38H47Cl2CoN3 | C42H55Cl2CoN3 |
| Formula weight | 675.61 | 731.72 |
| Temperature (K) | 173.15 | 173.15(2) |
| Wavelength (Å) | 0.71073 | 0.71073 |
| Crystal system | orthorhombic | tetragonal |
| Space group | Fdd2 | I41/a |
| a (Å) | 33.4157(6) | 34.3337(8) |
| b (Å) | 31.4531(6) | 34.3337(8) |
| c (Å) | 16.9829(4) | 14.5297(7) |
| α (°) | 90 | 90 |
| β (°) | 90 | 90 |
| γ (°) | 90 | 90 |
| Volume (Å3) | 17849.5(6) | 17127.7(11) |
| Z | 16 | 16 |
| Dcalcd (g cm−3) | 1.006 | 1.135 |
| μ (mm−1) | 0.528 | 0.555 |
| F(000) | 5712.0 | 6224.0 |
| Crystal size (mm3) | 0.288 × 0.193 × 0.102 | 0.274 × 0.144 × 0.118 |
| θ rang (°) | 4.56–63.054 | 3.044–66.158 |
| Limiting indices | −46 ≤ h ≤ 48, −43 ≤ k ≤ 46, −24 ≤ l ≤ 23 | −50 ≤ h ≤ 48, −47 ≤ k ≤ 51, −21 ≤ l ≤ 10 |
| No. of rflns collected | 70088 | 49080 |
| No. unique rflns [R(int)] | 0.0625(10266) | 0.1028(4627) |
| Completeness to θ (%) | 93.0 (θ = 25.00) | 90.6 (θ = 25.00) |
| Goodness of fit on F2 | 1.040 | 0.971 |
| Final R indices [I > 2σ(I)] | R1 = 0.0625, wR2 = 0.1560 | R1 = 0.1028, wR2 = 0.2154 |
| R indices (all data) | R1 = 0.0868, wR2 = 0.1705 | R1 = 0.2894, wR2 = 0.3034 |
| Largest diff. peak and hole (e Å−3) | 0.41 and −0.21 | 0.42 and −0.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Wang, Z.; Zhang, W.; Oleynik, I.I.; Vignesh, A.; Oleynik, I.V.; Hu, X.; Sun, Y.; Sun, W.-H. Highly Linear Polyethylenes Achieved Using Thermo-Stable and Efficient Cobalt Precatalysts Bearing Carbocyclic-Fused NNN-Pincer Ligand. Molecules 2019, 24, 1176. https://doi.org/10.3390/molecules24061176
Guo J, Wang Z, Zhang W, Oleynik II, Vignesh A, Oleynik IV, Hu X, Sun Y, Sun W-H. Highly Linear Polyethylenes Achieved Using Thermo-Stable and Efficient Cobalt Precatalysts Bearing Carbocyclic-Fused NNN-Pincer Ligand. Molecules. 2019; 24(6):1176. https://doi.org/10.3390/molecules24061176
Chicago/Turabian StyleGuo, Jingjing, Zheng Wang, Wenjuan Zhang, Ivan I. Oleynik, Arumugam Vignesh, Irina V. Oleynik, Xinquan Hu, Yang Sun, and Wen-Hua Sun. 2019. "Highly Linear Polyethylenes Achieved Using Thermo-Stable and Efficient Cobalt Precatalysts Bearing Carbocyclic-Fused NNN-Pincer Ligand" Molecules 24, no. 6: 1176. https://doi.org/10.3390/molecules24061176
APA StyleGuo, J., Wang, Z., Zhang, W., Oleynik, I. I., Vignesh, A., Oleynik, I. V., Hu, X., Sun, Y., & Sun, W.-H. (2019). Highly Linear Polyethylenes Achieved Using Thermo-Stable and Efficient Cobalt Precatalysts Bearing Carbocyclic-Fused NNN-Pincer Ligand. Molecules, 24(6), 1176. https://doi.org/10.3390/molecules24061176