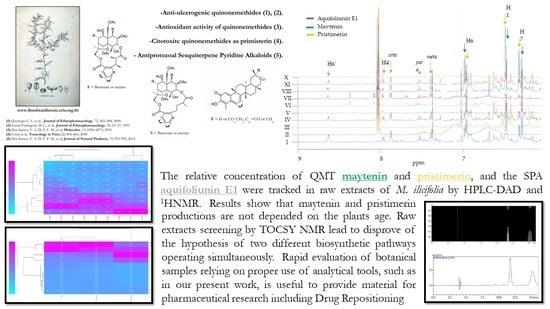
Ecological Insights to Track Cytotoxic Compounds among Maytenus ilicifolia Living Individuals and Clones of an Ex Situ Collection
Abstract
:1. Introduction
2. Results and Discussion
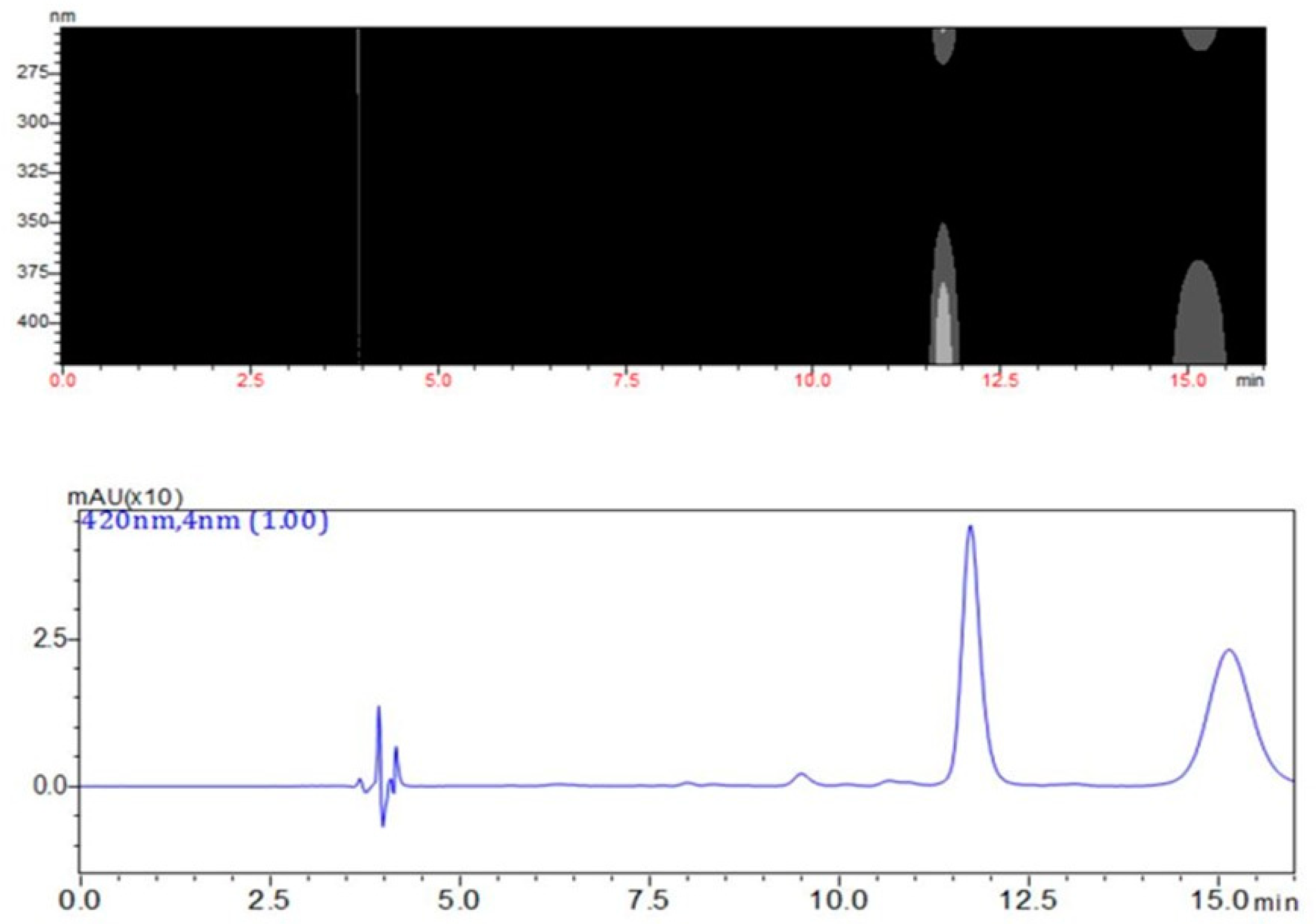
2.1. Measurement of QMTs and SPA in Root Barks from M. ilicifolia
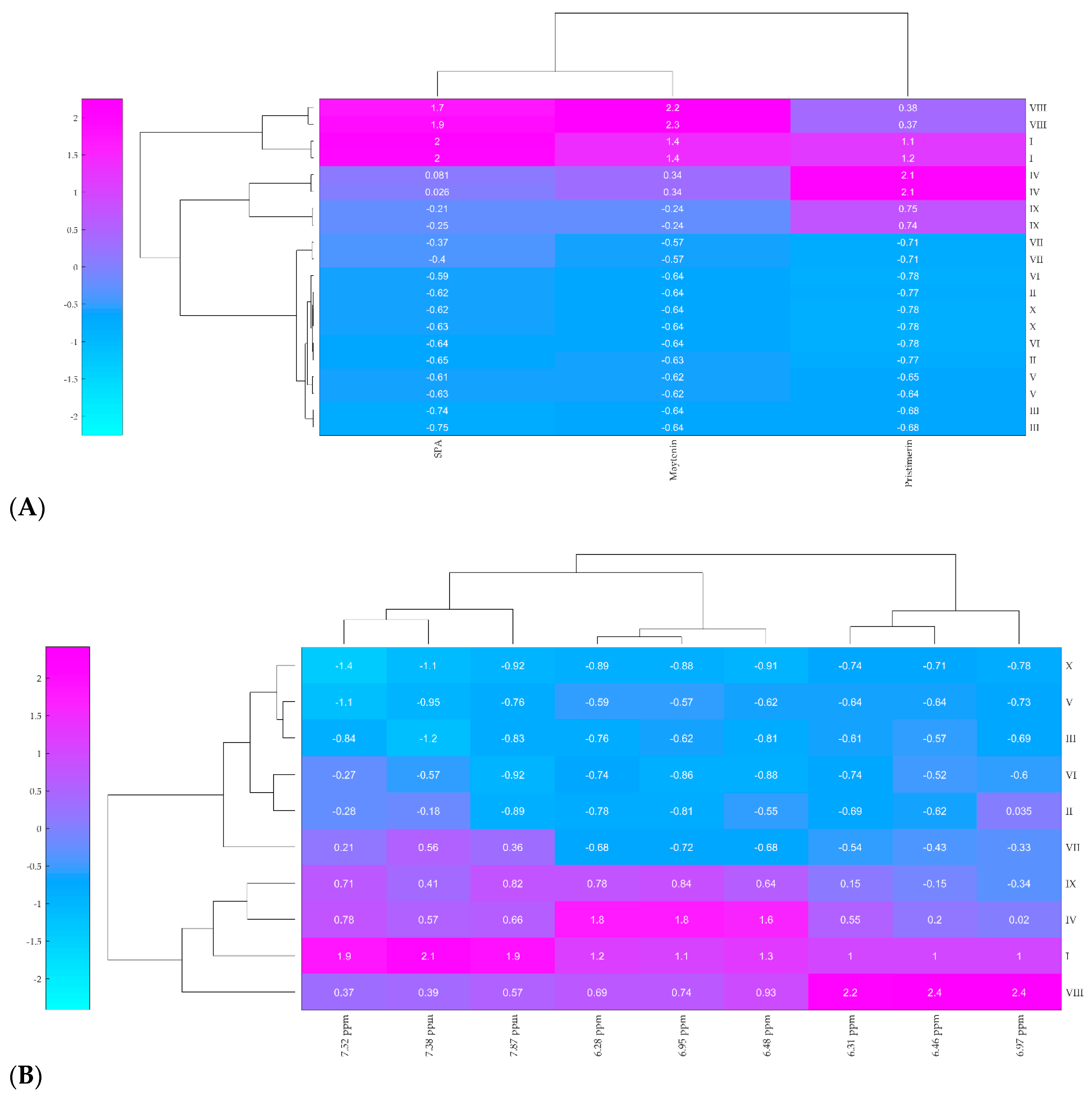
2.2. Hierarchical Clustering Analysis (HCA)
2.3. Discussion
3. Experimental
3.1. Sampling
3.2. Metabolite Extraction
3.3. General Analytical Procedures
3.4. Hierarchical Clustering Analysis (HCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lovelock, J. The Vanishing Face of Gaia, First Trade Paper ed.; Basic Books: New York, NY, USA, 2009; ISBN 978-0-465-01549-8. [Google Scholar]
- Crutzen, P.J.; Stoermer, E.F. The “Anthropocene”. Glob. Chang. Newsl. 2000, 41, 17–18. [Google Scholar]
- Pavarini, D.P.; Da Silva, D.B.; Carollo, C.A.; Portella, A.P.F.; Latansio-Aidar, S.R.; Cavalin, P.O.; Oliveira, V.C.; Rosado, B.H.P.; Aidar, M.P.M.; Bolzani, V.S.; et al. Application of MALDI-MS analysis of Rainforest chemodiversity: A keystone for biodiversity conservation and sustainable use. J. Mass Spectrom. 2012, 47, 1482–1485. [Google Scholar] [CrossRef]
- Joly, C.A.; Metzger, J.P.; Tabarelli, M. Experiences from the Brazilian Atlantic Forest: Ecological findings and conservation initiatives. New Phytol. 2014, 204, 459–473. [Google Scholar] [CrossRef]
- Joly, C.A.; Rodrigues, R.R.; Metzger, J.P.; Haddad, C.F.B.; Verdade, L.M.; Oliveira, M.C.; Bolzani, V.S. Biodiversity conservation research, training, and policy in São Paulo. Science 2010, 328, 1358–1359. [Google Scholar] [CrossRef]
- Carnevale Neto, F.; Pilon, A.C.; Selegato, D.M.; Freire, R.T.; Gu, H.; Raftery, D.; Lopes, N.P.; Castro-Gamboa, I. Dereplication of natural products using GC-TOF mass spectrometry: Improved metabolite identification by spectral deconvolution ratio analysis. Front. Mol. Biosci. 2016, 3, 59. [Google Scholar] [CrossRef]
- FAPESP. Knowledge and Sustainable Use of Brazilian Biodiversity: Biota-FAPESP Program/The State of São Paulo Research Foundation, Online Edition; Prol Editora Gráfica Ltd.: São Paulo, Brazil, 2008. [Google Scholar]
- Pilon, A.C.; Valli, M.; Dametto, A.C.; Pinto, M.E.F.; Freire, R.T.; Castro-Gamboa, I.; Andricopulo, A.D.; Bolzani, V.S. NuBBEDB: An updated database to uncover chemical and biological information from Brazilian biodiversity. Sci. Rep. 2017, 7, 7215. [Google Scholar] [CrossRef]
- Valli, M.; Dos Santos, R.N.; Figueira, L.D.; Nakajima, C.H.; Castro-Gamboa, I.; Andricopulo, A.D.; Bolzani, V.S. Development of a natural products database from the biodiversity of Brazil. J. Nat. Prod. 2013, 76, 439–444. [Google Scholar] [CrossRef]
- Mayrhofer, S.; Teuber, M.; Zimmer, I.; Louis, S.; Fischbach, R.J. Diurnal and Seasonal Variation of Isoprene Biosynthesis - Related Genes in Grey Poplar Leaves. Plant Physiol. 2005, 139, 474–484. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Inderjit, W.D.A.; Karban, R.; Callaway, R.M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evol. 2011, 26, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.M.; Aubourg, S.; Schouwey, M.B.; Daviet, L.; Schalk, M.; Toub, O.; Steven, T.L.; Bohlmann, J. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biol. 2010, 10, 226–248. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.J.; Green, S.A.; Chen, X.; Bailleul, E.J.D.; Matich, A.J.; Wang, M.Y.; Atkinson, R.G. Functional genomics reveals that a compact terpene synthase gene family can account for terpene volatile production in apple. Plant Physiol. 2013, 161, 787–804. [Google Scholar] [CrossRef]
- Bueno, P.C.P.; Pereira, F.M.V.; Torres, R.B.; Cavalheiro, A.J. Development of a comprehensive method for analyzing clerodane-type diterpenes and phenolic compounds from Casearia sylvestris Swartz (Salicaceae) based on ultra high performance liquid chromatography combined with chemometric tools. J. Sep. Sci. 2015, 38, 1–21. [Google Scholar] [CrossRef]
- Pavarini, D.P.; Pavarini, S.P.; Niehues, M.; Lopes, N.P. Exogenous influences on plant secondary metabolite levels. Anim. Feed Sci. Technol. 2012, 176, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Muntendam, R.; Happyana, N.; Erkelens, C.; Bruining, F.; Kayser, O. Time dependent metabolomics and transcriptional analysis of cannabinoid biosynthesis in Cannabis sativa var. Bedrobinol and Bediol grown under standardized condition and with genetic homogeneity. Online Int. J. Med. Plant Res. 2012, 1, 31–40. [Google Scholar]
- Happyana, N.; Kayser, O. Monitoring Metabolite Profiles of Cannabis sativa L. Trichomes during Flowering Period Using 1H NMR-Based Metabolomics and Real-Time PCR. Planta Med. 2016, 82, 1217–1223. [Google Scholar] [CrossRef]
- Alvarenga, N.; Ferro, E.A. Bioactive Triterpenes and Related Compounds from Celastraceae. Stud. Nat. Prod. Chem. 2006, 33, 239–307. [Google Scholar]
- Souza-Moreira, T.M.; Alves, T.B.; Pinheiro, K.A.; Felippe, L.G.; De Lima, G.M.A.; Watanabe, T.F.; Barbosa, C.C.; Santos, V.A.F.F.M.; Lopes, N.P.; Valentini, S.R.; et al. Friedelin Synthase from Maytenus ilicifolia: Leucine 482 Plays an Essential Role in the Production of the Most Rearranged Pentacyclic Triterpene. Sci. Rep. 2016, 6, 36858. [Google Scholar] [CrossRef]
- Carvalho, P.R.F.; Silva, D.H.S.; Bolzani, V.S.; Furlan, M. Antioxidant quinonemethide triterpenes from Salacia campestris. Chem. Biodivers 2005, 2, 367–372. [Google Scholar] [CrossRef]
- Jeller, A.H.; Silva, D.H.S.; Lião, L.M.; Bolzani, V.S.; Furlan, M. Antioxidant phenolic and quinonemethide triterpenes from Cheiloclinium cognatum. Phytochemistry 2004, 65, 1977–1982. [Google Scholar] [CrossRef]
- Dos Santos, V.A.F.F.M.; Santos, D.P.; Castro-Gamboa, I.; Zanoni, M.V.B.; Furlan, M. Evaluation of Antioxidant Capacity and Synergistic Associations of Quinonemethide Triterpenes and Phenolic Substances from Maytenus ilicifolia (Celastraceae). Molecules 2010, 15, 6956–6973. [Google Scholar]
- Costa, P.M.; Ferreira, P.M.; Bolzani, V.S.; Furlan, M.; dos Santos, V.A.F.F.M.; Corsino, J.; de Moraes, M.O.; Costa-Lotufo, L.V.; Montenegro, R.C.; Pessoa, C. Antiproliferative activity of pristimerin isolated from Maytenus ilicifolia (Celastraceae) in human HL-60 cells. Toxicol. In Vitro 2008, 22, 854–863. [Google Scholar] [CrossRef]
- Paz, T.A.; dos Santos, V.A.F.F.M.; Inácio, M.C.; Pina, E.S.; Pereira, M.A.S.; Furlan, M. Production of the Quinone-Methide Triterpene Maytenin by In Vitro Adventitious Roots of Peritassa campestris (Cambess.) A.C.Sm. (Celastraceae) and Rapid Detection and Identification by APCI-IT-MS/MS. Biomed. Res. Int. 2013, 2013, 485837. [Google Scholar] [CrossRef]
- Queiroga, C.L.; Silva, G.F.; Dias, P.C.; Possenti, A.; Carvalho, J.E. Evaluation of the antiulcerogenic activity of friedelan-3β-ol and friedelin isolated from Maytenus ilicifolia (Celastraceae). J. Ethnopharmacol. 2000, 72, 465–468. [Google Scholar] [CrossRef]
- Souza-Formigoni, M.L.; Oliveira, M.G.; Monteiro, M.G.; da Silveira-Filho, N.G.; Braz, S.; Carlini, E.A. Antiulcerogenic effects of two Maytenus species in laboratory animals. J. Ethnopharmacol. 1991, 34, 21–27. [Google Scholar] [CrossRef]
- Liu, J.K.; Jia, Z.J.; Wu, D.G.; Zhou, J.; Wang, Q.G. Insect antifeeding agents: Sesquiterpene alkaloids from Celastrus angulatus. Phytochemistry 1990, 29, 2503–2506. [Google Scholar]
- Núñez, M.J.; Guadaño, A.; Jimenez, I.A.; Ravelo, A.G.; Gonzalez-Coloma, A.; Bazzocchi, I.L. Insecticidal Sesquiterpene Pyridine Alkaloids from Maytenus chiapensis. J. Nat. Prod. 2004, 67, 14–18. [Google Scholar]
- Whitson, E.L.; Mala, S.M.V.D.; Veltri, C.A.; Bugni, T.S.; Silva, E.D.; Ireland, C.M. Oppositines A and B, Sesquiterpene Pyridine Alkaloids from a Sri Lankan Pleurostylia opposita. J. Nat. Prod. 2006, 69, 1833–1835. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, V.A.F.F.M.; Regasini, L.O.; Nogueira, C.R.; Passerini, G.D.; Martinez, I.; Bolzani, V.S.; Graminha, M.A.S.; Cicarelli, R.M.B.; Furlan, M. Antiprotozoal Sesquiterpene Pyridine Alkaloids from Maytenus ilicifolia. J. Nat. Prod. 2012, 75, 991–995. [Google Scholar] [CrossRef]
- Jardim, A.C.G.; Igloi, Z.; Shimizu, J.F.; Santos, V.A.; Felippe, L.G.; Mazzeu, B.F.; Amako, Y.; Furlan, M.; Harris, M.; Rahal, P. Natural compounds isolated from Brazilian plants are potent inhibitors of hepatitis C virus replication in vitro. Antivir. Res. 2014, 115, 39–47. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Tincusi, B.M.; Bazzocchi, I.L.; Tokuda, H.; Nishino, H.; Konoshima, T.; Jimenez, I.A.; Ravelo, A.G. Anti-tumor promoting effects of sesquiterpenes from Maytenus cuzcoina (Celastraceae). Bioorg. Med. Chem. 2000, 8, 1773–1778. [Google Scholar] [CrossRef]
- Jorge, R.M.; Leite, J.P.V.; Oliveira, A.B.; Tagliati, C.A. Evaluation of antinociceptive, anti-inflammatory and antiulcerogenic activities of Maytenus ilicifolia. J. Ethnopharmacol. 2004, 94, 93–100. [Google Scholar] [CrossRef]
- Field, B.; Osbourn, A.E. Metabolic diversification-independent assembly of operon-like gene clusters in different plants. Science 2008, 320, 543–547. [Google Scholar] [CrossRef]
- Corsino, J.; Bolzani, V.S.; Pereira, A.M.S.; França, S.C.; Furlan, M. Bioactive sesquiterpene pyridine alkaloids from Maytenus aquifolium. Phytochemistry 1998, 48, 137–140. [Google Scholar] [CrossRef]
- Dos Santos, V.A.F.F.M. Metabolomic, Biological and Proteomic Aspects of Maytenus ilicifolia and Salacia campestris (Celastraceae). Ph.D. Thesis, São Paulo State University (UNESP), Araraquara, Brazil, 2010. [Google Scholar]
- Kim, Y.S.; Cho, J.H.; Park, S.; Han, J.Y.; Back, K.; Choi, Y.E. Gene regulation patterns in triterpene biosynthetic pathway driven by overexpression of squalene synthase and methyl jasmonate elicitation in Bupleurum falcatum. Planta 2011, 233, 343–355. [Google Scholar] [CrossRef]
- Achnine, L.; Huhman, D.V.; Farag, M.A.; Sumner, L.W.; Blount, J.W.; Dixon, R.A. Genomics-based selection and functional characterization of triterpene glycosyltransferases from the model legume Medicago truncatula. Plant J. 2005, 41, 875–887. [Google Scholar] [CrossRef]
- Estevam, C.S.; Cavalcanti, A.M.; Cambui, E.V.F.; Araújo Neto, V.; Leopoldo, P.T.G.; Fernandes, R.P.M.; Araujo, B.S.; Porfírio, Z.; Sant’Ana, A.E.G. Phytochemistry and microbiological assay of the bark extracts of Maytenus rigida Mart. (Celastraceae). Braz. J. Microbiol. 2009, 19, 299–303. [Google Scholar]
- Monks, T.J.; Jones, D.C. The metabolism and toxicity of quinones, quinonimines, quinone methides, and quinone-thioethers. Curr. Drug Metab. 2002, 3, 425–438. [Google Scholar] [CrossRef]
- Gaudêncio, S.P.; Pereira, F. Dereplication: Racing to speed up the natural products discovery process. Nat. Prod. Rep. 2015, 32, 779–810. [Google Scholar] [CrossRef]
- Buedenbender, L.; Habener, L.J.; Grkovic, T.; Kurtböke, D.I.; Duffy, S.; Avery, V.M.; Carroll, A.R. HSQC–TOCSY fingerprinting for prioritization of polyketide-and peptide-producing microbial isolates. J. Nat. Prod. 2018, 81, 957–965. [Google Scholar] [CrossRef]
- Depke, T.; Franke, R.; Brönstrup, M. Clustering of MS2 spectra using unsupervised methods to aid the identification of secondary metabolites from Pseudomonas aeruginosa. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1071, 19–28. [Google Scholar] [CrossRef]
- Selegato, D.M.; Freire, R.T.; Tannús, A.; Castro-Gamboa, I. New Dereplication Method Applied to NMR-Based Metabolomics on Different. J. Braz. Chem. Soc. 2016, 27, 1421–1431. [Google Scholar]
- Shawky, E. Multivariate analyses of NP-TLC chromatographic retention data for grouping of structurally-related plant secondary metabolites. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1029–1030, 10–15. [Google Scholar] [CrossRef]
- Nosengo, N. New tricks for old drugs. Nature 2016, 534, 314–316. [Google Scholar] [CrossRef] [Green Version]
- Peng, B.; Xu, L.; Cao, F.; Wei, T.; Yang, C.; Uzan, G.; Zhang, D. HSP90 inhibitor, celastrol, arrests human monocytic leukemia cell U937 at G0/G1 in thiol-containing agents reversible way. Mol. Cancer 2010, 9, 79–92. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Sample | Physiognomy | Type |
|---|---|---|
| I | Adult | Individuals |
| II | Young | Individuals |
| III | Adult | Clone |
| IV | Adult | Individuals |
| V | Young | Individuals |
| VI | Adult | Clone |
| VII | Adult | Clone |
| VIII | Adult | Clone |
| IX | Adult | Individuals |
| X | Young | Individuals |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavarini, D.P.; Selegato, D.M.; Castro-Gamboa, I.; do Sacramento, L.V.S.; Furlan, M. Ecological Insights to Track Cytotoxic Compounds among Maytenus ilicifolia Living Individuals and Clones of an Ex Situ Collection. Molecules 2019, 24, 1160. https://doi.org/10.3390/molecules24061160
Pavarini DP, Selegato DM, Castro-Gamboa I, do Sacramento LVS, Furlan M. Ecological Insights to Track Cytotoxic Compounds among Maytenus ilicifolia Living Individuals and Clones of an Ex Situ Collection. Molecules. 2019; 24(6):1160. https://doi.org/10.3390/molecules24061160
Chicago/Turabian StylePavarini, Daniel Petinatti, Denise Medeiros Selegato, Ian Castro-Gamboa, Luiz Vitor Silva do Sacramento, and Maysa Furlan. 2019. "Ecological Insights to Track Cytotoxic Compounds among Maytenus ilicifolia Living Individuals and Clones of an Ex Situ Collection" Molecules 24, no. 6: 1160. https://doi.org/10.3390/molecules24061160