Electric Properties of Molecule Zr2Fe Based on the Full Relativistic Theory
Abstract
:1. Introduction
2. Elemental Theory
3. Theoretical Calculations and Discussions
yy = 0.3247777245cos50.922 = 0.3247777245 × 0.69679214 = 0.226302565
yy = −0.3115686073cos50.922 = −0.3115686073 × 0.63037778 = −0.196405926
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, Z.Y.; Liu, C.X.; Song, J.F.; Liu, W.D. Hydrogenation and detritiation of Zr2Fe alloy in inert atmosphere. Chem. Eng. 2010, 38, 205–209. [Google Scholar]
- Li, A.; Wang, S.B.; Li, Z.N.; Jiang, L.J.; Liu, X.P. Disproportionation properties of Zr2Fe alloy. Chin. J. Rare Metals 2017, 41, 252–257. [Google Scholar]
- Ali, K.; Arya, A.; Ghosh, P.S.; Dey, G.K. A first principles study of cohesive, elastic and electronic properties of binary Fe–Zr intermetallics. Comp. Mater. Sci. 2016, 112, 52–66. [Google Scholar] [CrossRef]
- Tao, X.M.; Zhu, J.Q.; Guo, H.; Ouyang, Y.F.; Du, Y. Phase stability, thermodynamic and mechanical properties of AlZr2, FeZr2 and Al2FeZr6 from first-principles calculations. J. Nucl. Mater. 2013, 440, 6–10. [Google Scholar] [CrossRef]
- Yartysa, V.A.; Fjellvag, H.; Hauback, B.C.; Riabov, A.B. Neutron diffraction studies of Zr-containing intermetallic hydrides with ordered hydrogen sublattice. I. Crystal structure of Zr2FeD5. J. Alloys Compd. 1998, 274, 217–221. [Google Scholar] [CrossRef]
- Maynard, K.J.; Kherani, N.P.; Shmayda, W.T. Nitridation of Zr2Fe and Its Influence on Tritium Removal. Fusion. Sci. Technol. 1995, 28, 1546–1551. [Google Scholar] [CrossRef]
- Debye, P. Polar Molecules; Dover Publications, Inc.: New York, NY, USA, 1929; pp. 1036–1037. [Google Scholar]
- Wheatley, P.J. The Determination of Molecular Structure; Clarendon Press, Oxford University Press: London, UK, 1968; p. 265. [Google Scholar]
- Turkan, N.; Uluer, İ. An IBM(2) Investigation of Some Properties of 100Ru Nuclei. Math. Comput. Appl. 1997, 2, 79–84. [Google Scholar] [CrossRef]
- İnan, S.; Türkan, N.; Maraş, İ. The Investigation of 130,132Te by IBM-2. Math. Comput. Appl. 2012, 17, 48–55. [Google Scholar] [CrossRef]
- Silver, B.L. Irreducible Tensor Method; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Marszatek, M.; Saitovitch, H.; da Silva, P.R.J.; Hrynkiewicz, A.Z. Electric field gradient and its temperature variation at a probe in Zr2Fe and Zr2Co intermetallic compounds. J. Alloys Compd. 1995, 219, 124–127. [Google Scholar] [CrossRef]
- Gregoire, M.D.; Brooks, N.; Trubko, R.; Cronin, A.D. Analysis of Polarizability Measurements Made with Atom Interferometry. Atoms 2016, 4, 21. [Google Scholar] [CrossRef]
- Petrilli, H.M.; Pessoa, S.F. Electric-field gradient in Zr3Fe, Zr2Fe, and Zr2Cu. Phys. Rev. A 1991, 44, 10493–10501. [Google Scholar]
- Maroulis, G.; Xenides, D. Electric Multipole Moments and (Hyper)Polarizability of X–C≡C–X, X = F, Cl, Br and I. Int. J. Mol. Sci. 2003, 4, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Landau, L.D.; Lifshitz, F.M. Quantum Mechanics, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 1997. [Google Scholar]
- Luding, W.; Falter, C. Symmetries in Physics; Springer-Verlag Heidelberg: Berlin, Germany, 1996. [Google Scholar]
- Tinkham, M. Group Theory and Quantum Mechanics; McGraw-Hill: New York, NY, USA, 1964. [Google Scholar]
- Berengut, J.C.; Flambaum, V.V.; Kozlov, M.G. Calculation of isotope shifts and relativistic shifts in C I, C II, C III, and C IV. Phys. Rev. A. 2006, 73. [Google Scholar] [CrossRef]
- Zhu, Z.H. Atomic and Molecular Reaction Statics; Science Press: Beijing, China, 2007. [Google Scholar]
- Cornwell, J.F. Group Theory in Physics; Academic Press: London, UK, 1994. [Google Scholar]
- Saue, T.; Jensen, H.J.A. Quaternion symmetry in relativistic molecular calculations: The Dirac–Hartree–Fock method. J. Chem. Phys. 1999, 111, 6211–6222. [Google Scholar] [CrossRef]
- Saue, T.; Jensen, H.J.A. Linear response at the 4-component relativistic level: Application to the frequency-dependent dipole polarizabilities of the coinage metal dimers. J. Chem. Phys. 2003, 118, 522–536. [Google Scholar] [CrossRef]
- Fleig, T.; Visscher, L. Large-scale electron correlation calculations in the framework of the spin-free dirac formalism: The Au2 molecule revisited. Chem. Phys. 2005, 311, 113–120. [Google Scholar] [CrossRef]
- Charlie, H. Introduction to Mathematical Physics; Prentice_Hall, Inc.: Engleewood Cliffs, NJ, USA, 1976. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Basis Set | 3–21G |
|---|---|
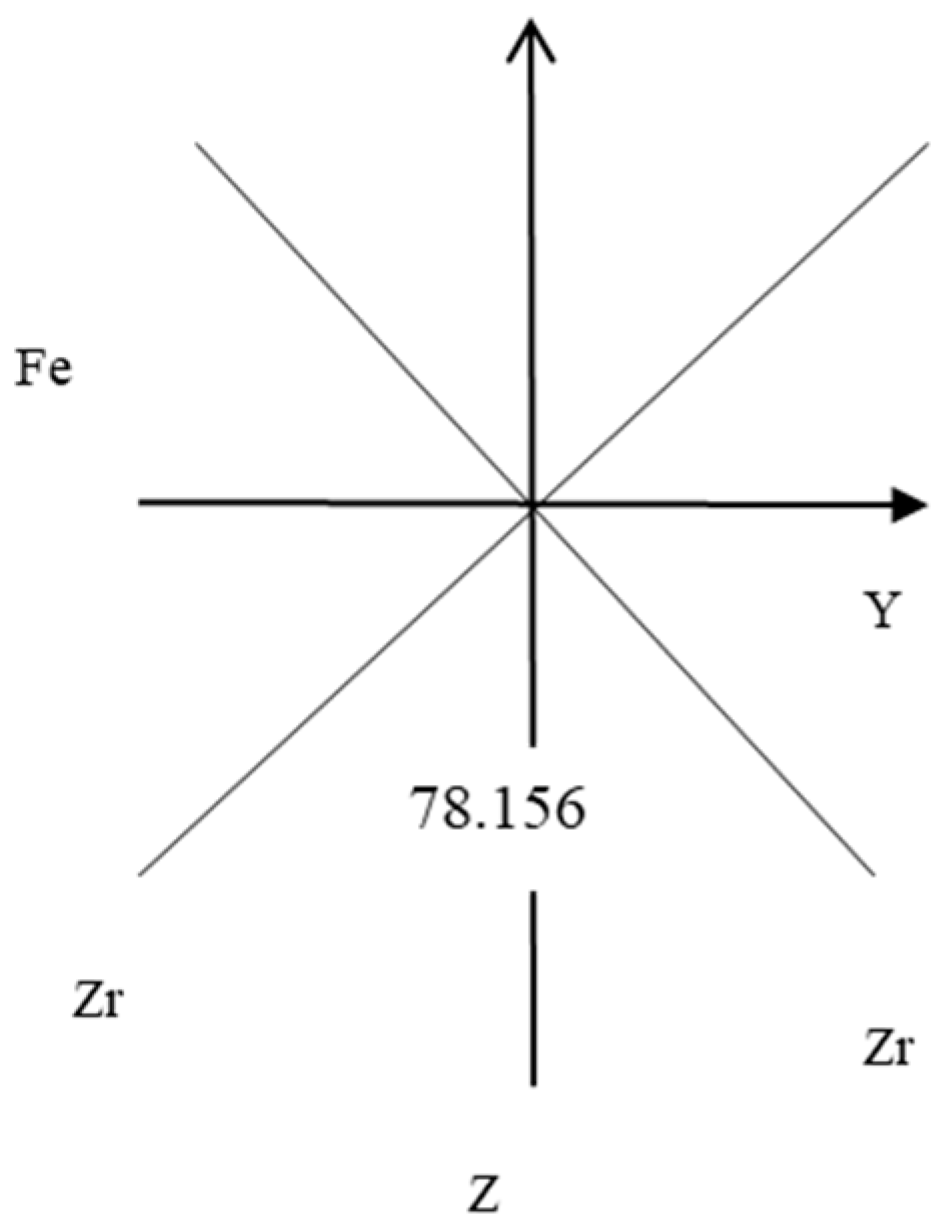
| bond length/Å | 2.1188 |
| bond angle/° | 78.156 |
| Energy/a.u. | −928.75326 |
| energy gap/a.u. | 4.03630384 |
| atomic charge | |
| Fe | −0.540605 |
| Zr | 0.270302 |
| Zr | 0.270302 |
| dipole moment/Debye | −6.39267291 |
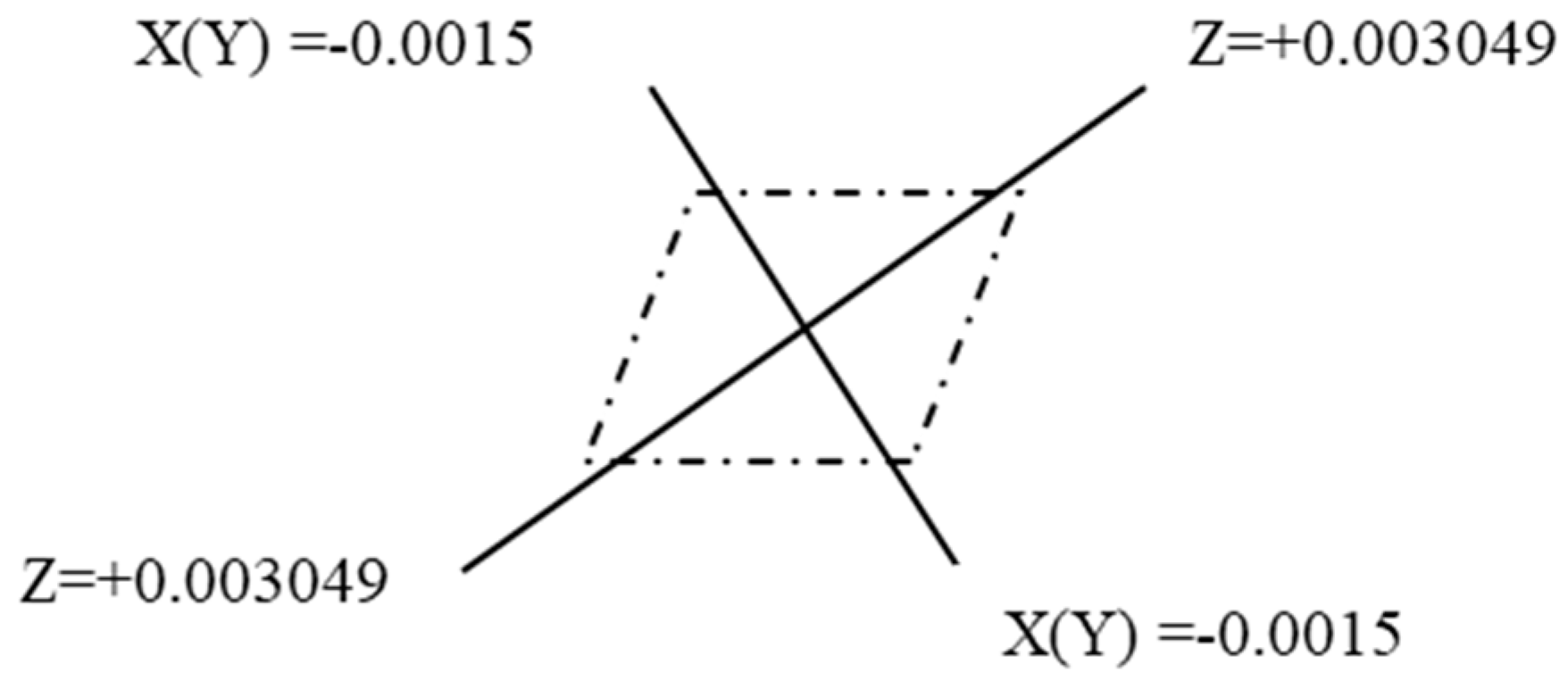
| quadrupole moment/a.u. | = −0.015036 |
| = −0.015036 | |
| = 0.0300738 | |
| (dipole moment:a.u. = 2.54177000 Debye) | |
| Frequency/a.u. | Polarizability/a.u. | Polarizability/Å3 | Electric Field Gradient/a.u. |
|---|---|---|---|
| frequency f = 0.00 | 0.01173850 | 0.0017395 | Nucleus: Zr |
| frequency f = 0.10 | 0.01174660 | 0.001740665 | = −0.311568607 |
| frequency f = 0.20 | 0.01177109 | 0.001744294 | = −0.311568607 |
| frequency f = 0.30 | 0.01181226 | 0.001750395 | = 0.6231372146 |
| frequency f = 0.40 | 0.01187045 | 0.0017590176 | Nucleus: Fe |
| frequency f = 0.50 | 0.01194617 | 0.0017702382 | = 0.3247777245 |
| frequency: | Polarizability: | = 0.3247777245 | |
| 1 a.u. = 2.3497 × 108 HZ | 1 a.u. = 0.1482 Å3 | = 0.6495554490 | |
| (electric field gradient: 1 a.u. = 9.71736 E + 21 V × m−2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Zhang, L.; Kong, X.; Hu, X.; Meng, D.; Zhu, Z. Electric Properties of Molecule Zr2Fe Based on the Full Relativistic Theory. Molecules 2019, 24, 1127. https://doi.org/10.3390/molecules24061127
Song J, Zhang L, Kong X, Hu X, Meng D, Zhu Z. Electric Properties of Molecule Zr2Fe Based on the Full Relativistic Theory. Molecules. 2019; 24(6):1127. https://doi.org/10.3390/molecules24061127
Chicago/Turabian StyleSong, Jiangfeng, Li Zhang, Xianggang Kong, Xiaoyu Hu, Daqiao Meng, and Zhenghe Zhu. 2019. "Electric Properties of Molecule Zr2Fe Based on the Full Relativistic Theory" Molecules 24, no. 6: 1127. https://doi.org/10.3390/molecules24061127





