Isoflavones
Abstract
:1. History
2. Isoflavone Classification and Chemical Structure
3. Isoflavone Role in Plants
4. Isoflavone Occurrence Relevant for Animals and People
5. Isoflavone Metabolism in Animals
6. Biological and Health Effects of Isoflavones in Animals
6.1. Estrogen Activity
6.1.1. Sheep
6.1.2. Cattle
6.2. Isoflavone Effect on Health and Productivity of Farm Animals
7. Isoflavone Metabolism in Humans
8. Biological and Health Effects of Isoflavones in Humans
8.1. Menopausal Symptoms and Estrogenic Activity
8.2. Cardiovascular Diseases
8.3. Bone Health
8.4. Breast Cancer
8.5. Uterine Cancer
8.6. Prostate Cancer
8.7. Thyroid Function
8.8. Isoflavones as Prophylactics Against Irradiation
8.9. Antioxidant Activity
9. Isoflavone Utilization for Functional Food Production
10. Potential Risks for Humans and Environment Related to Isoflavones
10.1. Risks for Children
10.2. Estrogen Activity of Animal Farming Waste
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kurzer, M.S.; Xu, X. Dietary phytoestrogens. Annu. Rev. Nutr. 1997, 17, 353–381. [Google Scholar] [CrossRef] [PubMed]
- Bennetts, H.W.; Uuderwood, E.J.; Shier, F.L. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust. Vet. J. 1946, 22, 2–12. [Google Scholar] [CrossRef]
- Mustonen, E.; Taponen, S.; Andersson, M.; Sukura, A.; Katila, T.; Taponen, J. Fertility and growth of nulliparous ewes after feeding red clover silage with high phyto-oestrogen concentrations. Animal 2014, 8, 1699–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lightfoot, R.J.; Croker, K.P.; Neil, H.G. Failure of sperm transport in relation to ewe infertility following prolonged grazing on oestrogenic pastures. Aust. J. Agric. Res. 1968, 18, 755–765. [Google Scholar] [CrossRef]
- Rossiter, R.C.; Beck, A.B. Physiological and ecological studies on the estrogenic isoflavones in subterranean clover (Trifolium subterraneum) I. Effects of temperature. Aust. J. Agric. Res. 1966, 17, 29–37. [Google Scholar] [CrossRef]
- Braden, A.; Hart, N.; Lamberton, J. The estrogenic activity and metabolism of certain isoflavones in sheep. Aust. J. Agric. Res. 1967, 18, 335–348. [Google Scholar] [CrossRef]
- Nottle, M.C. Composition of some urinary calculi of ruminants in Western Australia. Res. Vet. Sci. 1976, 21, 309–317. [Google Scholar] [CrossRef]
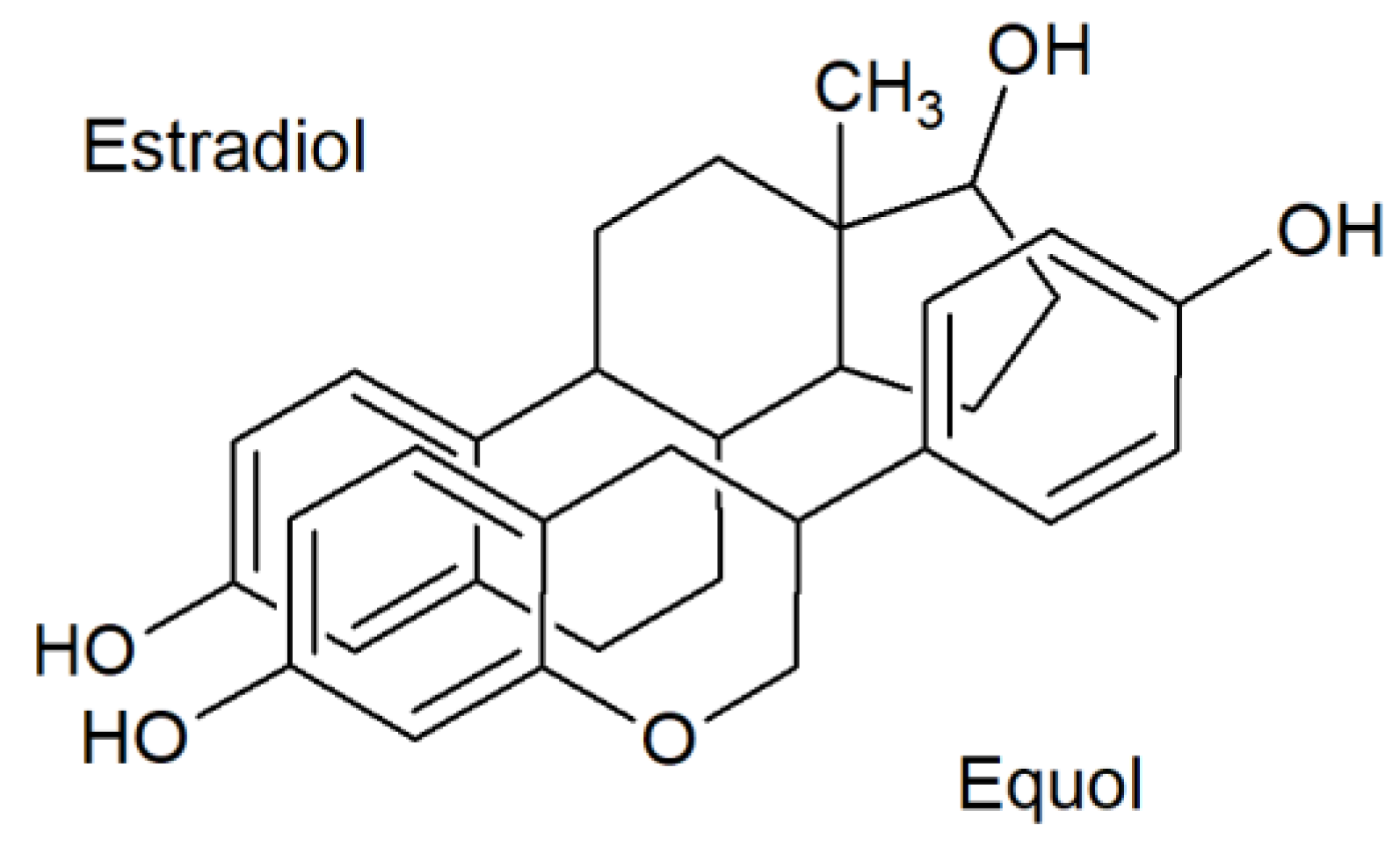
- Marrian, G.F.; Haslewood, G.A. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares’ urine. Biochem. J. 1932, 26, 1227–1232. [Google Scholar] [CrossRef]
- Marrian, G.F.; Beall, D. The constitution of equol. Biochem. J. 1935, 29, 1586–1589. [Google Scholar] [CrossRef]
- Shutt, D.; Braden, A. The significance of equol in relation to the oestrogenic responses in sheep ingesting clover with a high formononetin content. Aust. J. Agric. Res. 1968, 19, 545. [Google Scholar] [CrossRef]
- Shutt, D.A.; Weston, R.H.; Hogan, J.P. Quantitative aspects of phytoestrogen metabolism in sheep fed on subterranean clover (Trifolium subterraneum, cultivar Clare) or red clover (Trifolium pratense). Aust. J. Agric. Res. 1970, 21, 714–722. [Google Scholar] [CrossRef]
- Lundh, T. Metabolism of Estrogenic Isoflavones in Domestic Animals. Proc. Soc. Exp. Biol. Med. 1995, 208, 33–39. [Google Scholar] [CrossRef]
- Klyne, W.; Wright, A.A. Steroids and other lipids of pregnant cow’s urine. J. Endocrinol. 1959, 18, 32–45. [Google Scholar] [CrossRef]
- Chang, H.H.-S.; Robinson, A.R.; Common, R.H. Excretion of Radioactive Daidzein and Equol as Monosulfates and Disulfates in the Urine of the Laying Hen. Can. J. Biochem. 1975, 53, 223–230. [Google Scholar] [CrossRef]
- Blair, R.M.; Appt, S.E.; Franke, A.A.; Clarkson, T.B. Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (Macaca fascicularis). J. Nutr. 2003, 133, 2262–2267. [Google Scholar] [CrossRef]
- Brown, N.M.; Setchell, K.D. Animal models impacted by phytoestrogens in commercial chow: Implications for pathways influenced by hormones. Lab. Investig. 2001, 81, 735–747. [Google Scholar] [CrossRef]
- Juniewicz, P.E.; Morell, S.P.; Moser, A.; Ewing, L.L. Identification of phytoestrogens in the urine of male dogs. J. Steroid Biochem. 1988, 31, 987–994. [Google Scholar] [CrossRef]
- Axelson, M.; Kirk, D.N.; Farrant, R.D.; Cooley, G.; Lawson, A.M.; Setchell, K.D. The identification of the weak oestrogen equol [7-hydroxy-3-(4’-hydroxyphenyl)chroman] in human urine. Biochem. J. 1982, 201, 353–357. [Google Scholar] [CrossRef]
- Committee on Toxicity. Phytoestrogens and Health: COT Report. 2003. Available online: https://cot.food.gov.uk/sites/default/files/cot/phytoreport0503.pdf (accessed on 25 July 2008).
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef]
- Dixon, R.A. Legume Natural Products: Understanding and Manipulating Complex Pathways for Human and Animal Health. Plant Physiol. 2003, 131, 878–885. [Google Scholar] [CrossRef] [Green Version]
- Ko, K.-P. Isoflavones: Chemistry, Analysis, Functions and Effects on Health and Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 7001–7010. [Google Scholar] [CrossRef] [PubMed]
- Coward, L.; Barnes, N.C.; Setchell, K.D.R.; Barnes, S. Genistein, daidzein, and their β-glycoside conjugates: Antitumor isoflavones in soybean foods from American and Asian diets. J. Agric. Food Chem. 1993, 41, 1961–1967. [Google Scholar] [CrossRef]
- Bingham, S.A.; Atkinson, C.; Liggins, J.; Bluck, L.; Coward, A. Phyto-oestrogens: Where are we now? Br. J. Nutr. 1998, 79, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Daems, F.; Romnee, J.-M.; Heuskin, S.; Froidmont, É.; Lognay, G. Analytical methods used to quantify isoflavones in cow’s milk: A review. Dairy Sci. Technol. 2016, 96, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Dakora, F.D.; Phillips, D.A. Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol. Mol. Plant Pathol. 1996, 49, 1–20. [Google Scholar] [CrossRef]
- Bellou, S.; Karali, E.; Bagli, E.; Al-Maharik, N.; Morbidelli, L.; Ziche, M.; Adlercreutz, H.; Murphy, C.; Fotsis, T. The isoflavone metabolite 6-methoxyequol inhibits angiogenesis and suppresses tumor growth. Mol. Cancer 2012, 11, 35. [Google Scholar] [CrossRef]
- Rípodas, C.; Via, V.D.; Aguilar, O.M.; Zanetti, M.E.; Blanco, F.A. Knock-down of a member of the isoflavone reductase gene family impairs plant growth and nodulation in Phaseolus vulgaris. Plant Physiol. Biochem. 2013, 68, 81–89. [Google Scholar] [CrossRef]
- Subramanian, S.; Stacey, G.; Yu, O. Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. Plant J. 2006, 48, 261–273. [Google Scholar] [CrossRef]
- Sukumaran, A.; McDowell, T.; Chen, L.; Renaud, J.; Dhaubhadel, S. Isoflavonoid-specific prenyltransferase gene family in soybean: GmPT01, a pterocarpan 2-dimethylallyltransferase involved in glyceollin biosynthesis. Plant J. 2018, 96, 966–981. [Google Scholar] [CrossRef]
- Liu, Y.; Hassan, S.; Kidd, B.N.; Garg, G.; Mathesius, U.; Singh, K.B.; Anderson, J.P. Ethylene Signaling Is Important for Isoflavonoid-Mediated Resistance to Rhizoctonia solani in Roots of Medicago truncatula. Mol. Plant-Microbe Interact. 2017, 30, 691–700. [Google Scholar] [CrossRef]
- Hasanah, Y.; Nisa, T.C.; Armidin, H.; Hanum, H. Isoflavone content of soybean [Glycine max (L). Merr.] cultivars with different nitrogen sources and growing season under dry land conditions. JAEID 2015, 109, 5–17. [Google Scholar] [CrossRef]
- Adler, S.A.; Purup, S.; Hansen-Møller, J.; Thuen, E.; Steinshamn, H. Phytoestrogens and Their Metabolites in Bulk-Tank Milk: Effects of Farm Management and Season. PLoS ONE 2015, 10, e0127187. [Google Scholar] [CrossRef] [PubMed]
- Saloniemi, H.; Wähälä, K.; Nykanen-Kurki, P.; Kallela, K.; Saastamoinen, I. Phytoestrogen Content and Estrogenic Effect of Legume Fodder. Exp. Biol. Med. 1995, 208, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Steinshamn, H.; Purup, S.; Thuen, E.; Hansen-Møller, J. Effects of Clover-Grass Silages and Concentrate Supplementation on the Content of Phytoestrogens in Dairy Cow Milk. J. Dairy Sci. 2008, 91, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Butkutė, B.; Padarauskas, A.; Cesevičienė, J.; Taujenis, L.; Norkevičienė, E. Phytochemical composition of temperate perennial legumes. Crop Pasture Sci. 2018, 69, 1020. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Rangel, M.; Delgado-Zamarreño, M.M.; Pérez-Martín, L.; Rodríguez-Gonzalo, E.; Domínguez-Álvarez, J. Analysis of Isoflavones in Foods: Analysis of isoflavones in foods…. Compr. Rev. Food Sci. Food Saf. 2018, 17, 391–411. [Google Scholar] [CrossRef]
- Frankenfeld, C.L. Dairy consumption is a significant correlate of urinary equol concentration in a representative sample of US adults. Am. J. Clin. Nutr. 2011, 93, 1109–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andres, S.; Hansen, U.; Niemann, B.; Palavinskas, R.; Lampen, A. Determination of the isoflavone composition and estrogenic activity of commercial dietary supplements based on soy or red clover. Food Funct. 2015, 6, 2017–2025. [Google Scholar] [CrossRef] [Green Version]
- Klyne, W.; Wright, A.A. Steroids and other lipids of pregnant goat’s urine. Biochem. J. 1957, 66, 92–101. [Google Scholar] [CrossRef]
- Miksicek, R.J. Estrogenic Flavonoids: Structural Requirements for Biological Activity. Exp. Biol. Med. 1995, 208, 44–50. [Google Scholar] [CrossRef]
- Nilsson, A.; Hill, J.L.; Davies, H.L. An in vitro study of formononetin and biochanin A in rumen fluid from sheep. Biochim. Biophys. Acta 1967, 148, 92–98. [Google Scholar] [CrossRef]
- Dickinson, J.M.; Smith, G.R.; Randel, R.D.; Pemberton, I.J. In vitro metabolism of formononetin and biochanin A in bovine rumen fluid. J. Anim. Sci. 1988, 66, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Wocławek-Potocka, I.; Mannelli, C.; Boruszewska, D.; Kowalczyk-Zieba, I.; Waśniewski, T.; Skarżyński, D.J. Diverse Effects of Phytoestrogens on the Reproductive Performance: Cow as a Model. Inter. J. Endocrinol. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.-H. The antioxidant activity of daidzein metabolites, O-desmethylangolensin and equol, in HepG2 cells. Mol. Med. Rep. 2014, 9, 328–332. [Google Scholar] [CrossRef]
- Njåstad, K.M.; Adler, S.A.; Hansen-Møller, J.; Thuen, E.; Gustavsson, A.-M.; Steinshamn, H. Gastrointestinal metabolism of phytoestrogens in lactating dairy cows fed silages with different botanical composition. J. Dairy Sci. 2014, 97, 7735–7750. [Google Scholar] [CrossRef] [PubMed]
- Adams, N.R. Detection of the effects of phytoestrogens on sheep and cattle. J. Anim. Sci. 1995, 73, 1509–1515. [Google Scholar] [CrossRef]
- Trnková, A.; Šancová, K.; Zapletalová, M.; Kašparovská, J.; Dadáková, K.; Křížová, L.; Lochman, J.; Hadrová, S.; Ihnatová, I.; Kašparovský, T. Determination of in vitro isoflavone degradation in rumen fluid. J. Dairy Sci. 2018, 101, 5134–5144. [Google Scholar] [CrossRef]
- Lundh, T.J.O.; Pettersson, H.I.; Martinsson, K.A. Comparative levels of free and conjugated plant estrogens in blood plasma of sheep and cattle fed estrogenic silage. J. Agric. Food Chem. 1990, 38, 1530–1534. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Morand, C.; Besson, C.; Kraft, G.; Viala, D.; Scalbert, A.; Besle, J.-M.; Manach, C. Tissue distribution of isoflavones in ewes after consumption of red clover silage. Arch. Biochem. Biophys. 2008, 476, 205–210. [Google Scholar] [CrossRef]
- Tucker, H.A.; Knowlton, K.F.; Meyer, M.T.; Khunjar, W.O.; Love, N.G. Effect of diet on fecal and urinary estrogenic activity. J. Dairy Sci. 2010, 93, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Třináctý, J.; Křížová, L.; Schulzová, V.; Hajšlová, J.; Hanuš, O. The effect of feeding soybean-derived phytoestogens on their concentration in plasma and milk of lactating dairy cows. Arch. Anim. Nutr. 2009, 63, 219–229. [Google Scholar] [CrossRef]
- Mustonen, E.A.; Tuori, M.; Saastamoinen, I.; Taponen, J.; Wähälä, K.; Saloniemi, H.; Vanhatalo, A. Equol in milk of dairy cows is derived from forage legumes such as red clover. Br. J. Nutr. 2009, 102, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- King, R.A.; Mano, M.M.; Head, R.J. Assessment of isoflavonoid concentrations in Australian bovine milk samples. J. Dairy Res. 1998, 65, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Höjer, A.; Adler, S.; Purup, S.; Hansen-Møller, J.; Martinsson, K.; Steinshamn, H.; Gustavsson, A.-M. Effects of feeding dairy cows different legume-grass silages on milk phytoestrogen concentration. J. Dairy Sci. 2012, 95, 4526–4540. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H.; Viala, D.; Doreau, M.; Besle, J.-M. Clover isoflavones move to cows’ milk. In Proceedings of the 1st International Conference on Polyphenols and Health, Vichy, France, 18–21 November 2004; p. 296. [Google Scholar]
- Flachowsky, G.; Hünerberg, M.; Meyer, U.; Kammerer, D.R.; Carle, R.; Goerke, M.; Eklund, M. Isoflavone concentration of soybean meal from various origins and transfer of isoflavones into milk of dairy cows. J. Verbrauch. Lebensm. 2011, 6, 449–456. [Google Scholar] [CrossRef]
- Křížová, L.; Třináctý, J.; Hajšlová, J.; Havlíková, Š. The Effect of Technological Processing on the Content of Isoflavones in Bovine Milk and Dairy Products. In Soybean—Applications and Technology; Ng, T.-B., Ed.; InTech: Rijeka, Croatia, 2011; pp. 95–110. ISBN 978-953-307-207-4. [Google Scholar]
- Křížová, L.; Veselý, A.; Třináctý, J.; Schulzová, V.; Hurajová, A.; Hajšlová, J.; Kvasničková, E.; Havlíková, Š. Changes in isoflavones concentrations in cheese during processing and ripening. Acta Univ. Agric. Silvic. Mendel. Brun. 2011, 59, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Kasparovska, J.; Pecinkova, M.; Dadakova, K.; Krizova, L.; Hadrova, S.; Lexa, M.; Lochman, J.; Kasparovsky, T. Effects of Isoflavone-Enriched Feed on the Rumen Microbiota in Dairy Cows. PLoS ONE 2016, 11, e0154642. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.; Weisbjerg, M.R.; Hansen-Møller, J.; Sejrsen, K. Effect of forage on the content of phyto-oestrogens in bovine milk. Animal 2009, 3, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.W. Estrogen Content of Colostrum and Milk of Dairy Cattle. J. Dairy Sci. 1958, 41, 630–640. [Google Scholar] [CrossRef]
- Shennan, D.B.; Peaker, M. Transport of milk constituents by the mammary gland. Physiol. Rev. 2000, 80, 925–951. [Google Scholar] [CrossRef] [PubMed]
- Schwen, R.J.; Nguyen, L.; Jackson, R.L. Elucidation of the metabolic pathway of S-equol in rat, monkey and man. Food Chem. Toxicol. 2012, 50, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse-Tedd, K.M.; Cave, N.J.; Ugarte, C.E.; Waldron, L.A.; Prasain, J.K.; Arabshahi, A.; Barnes, S.; Thomas, D.G. Dietary isoflavone absorption, excretion, and metabolism in captive cheetahs (Acinonyx jubatus). J. Zoo Wildl. Med. 2011, 42, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Marshall, T. Clover disease: What do we know and what can we do. J. Dep. Agric. West. Aust. Ser. 4 1973, 14, 2. [Google Scholar]
- Sakakibara, H.; Viala, D.; Ollier, A.; Combeau, A.; Besle, J.-M. Isoflavones in several clover species and in milk from goats fed clovers. Biofactors 2004, 22, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Woclawek-Potocka, I.; Bah, M.M.; Korzekwa, A.; Piskula, M.K.; Wiczkowski, W.; Depta, A.; Skarzynski, D.J. Soybean-derived phytoestrogens regulate prostaglandin secretion in endometrium during cattle estrous cycle and early pregnancy. Exp. Biol. Med. 2005, 230, 189–199. [Google Scholar] [CrossRef]
- Woclawek-Potocka, I.; Borkowski, K.; Korzekwa, A.; Okuda, K.; Skarzynski, D.J. Phyto- and endogenous estrogens differently activate intracellular calcium ion mobilization in bovine endometrial cells. J. Reprod. Dev. 2006, 52, 731–740. [Google Scholar] [CrossRef]
- Woclawek-Potocka, I.; Piskula, M.K.; Bah, M.; Siemieniuch, M.J.; Korzekwa, A.; Brzezicka, E.; Skarzynski, D.J. Concentrations of isoflavones and their metabolites in the blood of pregnant and non-pregnant heifers fed soy bean. J. Reprod. Dev. 2008, 54, 358–363. [Google Scholar] [CrossRef]
- Piotrowska, K.K.; Woclawek-Potocka, I.; Bah, M.M.; Piskula, M.K.; Pilawski, W.; Bober, A.; Skarzynski, D.J. Phytoestrogens and their metabolites inhibit the sensitivity of the bovine corpus luteum to luteotropic factors. J. Reprod. Dev. 2006, 52, 33–41. [Google Scholar] [CrossRef]
- Watzková, J.; Křížová, L.; Pavlík, A.; Schulzová, V.; Hajšlová, J.; Lojza, J. The Effect of Soybean-Derived Phytoestrogens on Concentrations of Plasma Isoflavones, 15-keto-13,14-dihydroprostaglandin F2α and Progesterone in Dairy Cows. Acta Vet. Brno 2010, 79, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Goff, A.K. Steroid hormone modulation of prostaglandin secretion in the ruminant endometrium during the estrous cycle. Biol. Reprod. 2004, 71, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Asselin, E.; Goff, A.K.; Bergeron, H.; Fortier, M.A. Influence of sex steroids on the production of prostaglandins F2α and E2 and response to oxytocin in cultured epithelial and stromal cells of the bovine endometrium. Biol. Reprod. 1996, 54, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Miyamoto, Y.; Skarzynski, D.J. Regulation of endometrial prostaglandin F(2α) synthesis during luteolysis and early pregnancy in cattle. Domest. Anim. Endocrinol. 2002, 23, 255–264. [Google Scholar] [CrossRef]
- Woclawek-Potocka, I.; Bober, A.; Korzekwa, A.; Okuda, K.; Skarzynski, D.J. Equol and para-ethyl-phenol stimulate prostaglandin F2α secretion in bovine corpus luteum: Intracellular mechanisms of action. Prostaglandins Other Lipid Mediat. 2006, 79, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Shore, L.S.; Rios, C.; Marcus, S.; Bernstein, M.; Shemesh, M. Relationship between peripheral estrogen concentrations at insemination and subsequent fetal loss in cattle. Theriogenology 1998, 50, 101–107. [Google Scholar] [CrossRef]
- Kowalczyk-Zieba, I.; Woclawek-Potocka, I.; Piskula, M.K.; Piotrowska-Tomala, K.K.; Boruszewska, D.; Bah, M.M.; Siemieniuch, M.J.; Skarzynski, D.J. Experimentally induced mastitis and metritis modulate soy bean derived isoflavone biotransformation in dairy cows. Theriogenology 2011, 76, 1744–1755. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, I.; Senapati, M.R.; Jena, D.; Behera, P.C. Ethnoveterinary importance of herbal galactogogues—A review. Vet. World 2014, 7, 325–330. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Fisher, W.J.; Tweed, J.K.S.; Wilkins, R.J. Comparison of grass and legume silages for milk production. 1. Production responses with different levels of concentrate. J. Dairy Sci. 2003, 86, 2598–2611. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Kuoppala, K.; Toivonen, V.; Shingfield, K.J. Effects of forage species and stage of maturity on bovine milk fatty acid composition. Eur. J. Lipid Sci. Technol. 2007, 109, 856–867. [Google Scholar] [CrossRef]
- Liu, D.-Y.; He, S.-J.; Jin, E.-H.; Liu, S.-Q.; Tang, Y.-G.; Li, S.-H.; Zhong, L.-T. Effect of daidzein on production performance and serum antioxidative function in late lactation cows under heat stress. Livest. Sci. 2013, 152, 16–20. [Google Scholar] [CrossRef]
- Moorby, J.M.; Fraser, M.D.; Theobald, V.J.; Wood, J.D.; Haresign, W. The effect of red clover formononetin content on live-weight gain, carcass characteristics and muscle equol content of finishing lambs. Anim. Sci. 2004, 79, 303–313. [Google Scholar] [CrossRef]
- Speijers, M.H.M.; Fraser, M.D.; Theobald, V.J.; Haresign, W. Effects of ensiled forage legumes on performance of store finishing lambs. Anim. Feed Sci. Technol. 2005, 120, 203–216. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhang, C.Q. Effects of daidzein on messenger ribonucleic Acid expression of gonadotropin receptors in chicken ovarian follicles. Poult. Sci. 2008, 87, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Guo-zhen, J.; Li, W. Effect of Daidzein on Ileum Microflora Biodiversity in Hy-Line Variety Brown Layers. J. Northeast Agric. Univ. 2014, 21, 31–36. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of Polyphenols and Polyphenol-Rich Dietary Sources on Gut Microbiota Composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of Pure Isoflavones in Healthy Humans and Analysis of Commercial Soy Isoflavone Supplements. J. Nutr. 2001, 131, 1362S–1375S. [Google Scholar] [CrossRef] [Green Version]
- Heinonen, S.; Wähälä, K.; Adlercreutz, H. Identification of Isoflavone Metabolites Dihydrodaidzein, Dihydrogenistein, 6′-OH-O-dma, and cis-4-OH-equol in Human Urine by Gas Chromatography–Mass Spectroscopy Using Authentic Reference Compounds. Anal. Biochem. 1999, 274, 211–219. [Google Scholar] [CrossRef]
- Sfakianos, J.; Coward, L.; Kirk, M.; Barnes, S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J. Nutr. 1997, 127, 1260–1268. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Faughnan, M.S.; Avades, T.; Zimmer-Nechemias, L.; Brown, N.B.; Wolfe, B.; Brashear, W.T.; Desai, P.; Oldfield, M.F.; Botting, N.P.; et al. Comparing the pharmacokinetics of daidzein and genistein using 13C-labeled tracers in premenopausal women. Am. J. Clin. Nutr. 2003, 77, 411–419. [Google Scholar] [CrossRef]
- Hur, H.-G.; Lay, J.O., Jr.; Beger, R.D.; Freeman, J.P.; Rafii, F. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch. Microbiol. 2000, 174, 422–428. [Google Scholar] [CrossRef]
- Yerramsetty, V.; Gallaher, D.D.; Ismail, B. Malonylglucoside Conjugates of Isoflavones Are Much Less Bioavailable Compared with Unconjugated β-Glucosidic Forms in Rats. J. Nutr. 2014, 144, 631–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubik, L.; Meydani, M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am. J. Clin. Nutr. 2003, 77, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.I.; Williamson, G.; Swallow, D.L.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Decroos, K.; Vanhemmens, S.; Cattoir, S.; Boon, N.; Verstraete, W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch. Microbiol. 2005, 183, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.; Little, J.M.; Barone, G.W.; Chen, G.; Radominska-Pandya, A.; Badger, T.M. Sulfation of the isoflavones genistein and daidzein in human and rat liver and gastrointestinal tract. J. Med. Food 2006, 9, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, K.; Furuta, T.; Yokokawa, A.; Ishii, K. Identification and quantification of daidzein-7-glucuronide-4’-sulfate, genistein-7-glucuronide-4’-sulfate and genistein-4’,7-diglucuronide as major metabolites in human plasma after administration of kinako. Anal. Bioanal. Chem. 2010, 397, 1563–1572. [Google Scholar] [CrossRef]
- Barnes, S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef]
- Setchell, K.D.; Cassidy, A. Dietary isoflavones: Biological effects and relevance to human health. J. Nutr. 1999, 129, 758S–767S. [Google Scholar] [CrossRef]
- Gaya, P.; Medina, M.; Sánchez-Jiménez, A.; Landete, J. Phytoestrogen Metabolism by Adult Human Gut Microbiota. Molecules 2016, 21, 1034. [Google Scholar] [CrossRef]
- Setchell, K.D.R. Equol—Origins, actions, and clinical relevance of this specific soy isoflavone metabolite. J. Nutr. 2004, 134, 1235S–1236S. [Google Scholar]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Helferich, W.G.; Katzenellenbogen, J.A. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors α and β. Bioorg. Med. Chem. 2004, 12, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Toro-Funes, N.; Morales-Gutiérrez, F.J.; Veciana-Nogués, M.T.; Vidal-Carou, M.C.; Spencer, J.P.E.; Rodriguez-Mateos, A. The intracellular metabolism of isoflavones in endothelial cells. Food Funct. 2015, 6, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.M.; Hoikkala, A.; Wähälä, K.; Adlercreutz, H. Metabolism of the soy isoflavones daidzein, genistein and glycitein in human subjects. Identification of new metabolites having an intact isoflavonoid skeleton. J. Steroid Biochem. Mol. Biol. 2003, 87, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Axelson, M.; Setchell, K.D. The excretion of lignans in rats—Evidence for an intestinal bacterial source for this new group of compounds. FEBS Lett. 1981, 123, 337–342. [Google Scholar] [CrossRef]
- Setchell, K.D.; Zimmer-Nechemias, L.; Cai, J.; Heubi, J.E. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet 1997, 350, 23–27. [Google Scholar] [CrossRef]
- Setchell, K.D.; Zimmer-Nechemias, L.; Cai, J.; Heubi, J.E. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am. J. Clin. Nutr. 1998, 68, 1453S–1461S. [Google Scholar] [CrossRef]
- Rowland, I.R.; Wiseman, H.; Sanders, T.A.; Adlercreutz, H.; Bowey, E.A. Interindividual variation in metabolism of soy isoflavones and lignans: Influence of habitual diet on equol production by the gut microflora. Nutr. Cancer 2000, 36, 27–32. [Google Scholar] [CrossRef]
- Braune, A.; Blaut, M. Evaluation of inter-individual differences in gut bacterial isoflavone bioactivation in humans by PCR-based targeting of genes involved in equol formation. J. Appl. Microbiol. 2018, 124, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Oka, J.; Ezaki, J.; Ohtomo, T.; Ueno, T.; Uchiyama, S.; Toda, T.; Uehara, M.; Ishimi, Y. Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal Japanese women: A double-blind, randomized, controlled trial. Menopause 2007, 14, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L.; Atkinson, C.; Thomas, W.K.; Gonzalez, A.; Jokela, T.; Wähälä, K.; Schwartz, S.M.; Li, S.S.; Lampe, J.W. High concordance of daidzein-metabolizing phenotypes in individuals measured 1 to 3 years apart. Br. J. Nutr. 2005, 94, 873–876. [Google Scholar] [CrossRef] [Green Version]
- Akaza, H.; Miyanaga, N.; Takashima, N.; Naito, S.; Hirao, Y.; Tsukamoto, T.; Fujioka, T.; Mori, M.; Kim, W.-J.; Song, J.M.; et al. Comparisons of percent equol producers between prostate cancer patients and controls: Case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn. J. Clin. Oncol. 2004, 34, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.A.; Lai, J.F.; Halm, B.M.; Pagano, I.; Kono, N.; Mack, W.J.; Hodis, H.N. Equol production changes over time in postmenopausal women. J. Nutr. Biochem. 2012, 23, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.A.; Lai, J.F.; Halm, B.M. Absorption, distribution, metabolism, and excretion of isoflavonoids after soy intake. Arch. Biochem. Biophys. 2014, 559, 24–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankenfeld, C.L.; McTiernan, A.; Tworoger, S.S.; Atkinson, C.; Thomas, W.K.; Stanczyk, F.Z.; Marcovina, S.M.; Weigle, D.S.; Weiss, N.S.; Holt, V.L.; et al. Serum steroid hormones, sex hormone-binding globulin concentrations, and urinary hydroxylated estrogen metabolites in post-menopausal women in relation to daidzein-metabolizing phenotypes. J. Steroid Biochem. Mol. Biol. 2004, 88, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qin, L.; Liu, A.; Uchiyama, S.; Ueno, T.; Li, X.; Wang, P. Prevalence of the equol-producer phenotype and its relationship with dietary isoflavone and serum lipids in healthy Chinese adults. J. Epidemiol. 2010, 20, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Cole, S.J. Method of defining equol-producer status and its frequency among vegetarians. J. Nutr. 2006, 136, 2188–2193. [Google Scholar] [CrossRef]
- Redruello, B.; Guadamuro, L.; Cuesta, I.; Álvarez-Buylla, J.R.; Mayo, B.; Delgado, S. A novel UHPLC method for the rapid and simultaneous determination of daidzein, genistein and equol in human urine. J. Chromatogr. B 2015, 1005, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassidy, A. Plant Oestrogens and Their Relation to Hormonal Status in Women; Cambridge University: Cambridge, UK, 1991. [Google Scholar]
- Lipovac, M.; Pfitscher, A.; Hobiger, S.; Laschitz, T.; Imhof, M.; Chedraui, P.; Jungbauer, A. Red clover isoflavone metabolite bioavailability is decreased after fructooligosaccharide supplementation. Fitoterapia 2015, 105, 93–101. [Google Scholar] [CrossRef]
- Nielsen, I.L.F.; Williamson, G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr. Cancer 2007, 57, 1–10. [Google Scholar] [CrossRef]
- Cohen, L.A.; Crespin, J.S.; Wolper, C.; Zang, E.A.; Pittman, B.; Zhao, Z.; Holt, P.R. Soy isoflavone intake and estrogen excretion patterns in young women: Effect of probiotic administration. In Vivo 2007, 21, 507–512. [Google Scholar]
- Elghali, S.; Mustafa, S.; Amid, M.; Manap, M.Y.A.B.D.; Ismail, A.; Abas, F. Bioconversion of daidzein to equol by Bifidobacterium breve 15700 and Bifidobacterium longum BB536. J. Funct. Foods 2012, 4, 736–745. [Google Scholar] [CrossRef]
- Shimada, Y.; Yasuda, S.; Takahashi, M.; Hayashi, T.; Morihiro, M.; Sato, I.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Cloning and expression of a novel NADP(H)-dependent daidzein reductase, an enzyme involved in the metabolism of daidzein, from equol-producing Lactococcus strain 20–92. Appl. Environ. Microbiol. 2010, 76, 5892–5901. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.-I.; Han, J.; Wang, X.-L.; Song, D.-G.; Kim, S.-U. Stereospecific Biotransformation of Dihydrodaidzein into (3S)-Equol by the Human Intestinal Bacterium Eggerthella Strain Julong 732. Appl. Environ. Microbiol. 2009, 75, 3062–3068. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Han, J. Deglycosylation of isoflavone C-glycosides by newly isolated human intestinal bacteria. J. Sci. Food Agric. 2015, 95, 1925–1931. [Google Scholar] [CrossRef]
- Tamura, M.; Tsushida, T.; Shinohara, K. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe 2007, 13, 32–35. [Google Scholar] [CrossRef]
- Schoefer, L.; Mohan, R.; Braune, A.; Birringer, M.; Blaut, M. Anaerobic C-ring cleavage of genistein and daidzein by Eubacterium ramulus. FEMS Microbiol. Lett. 2002, 208, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-L.; Kim, H.-J.; Kang, S.-I.; Kim, S.-I.; Hur, H.-G. Production of phytoestrogen S-equol from daidzein in mixed culture of two anaerobic bacteria. Arch. Microbiol. 2007, 187, 155–160. [Google Scholar] [CrossRef]
- Ueno, T.; Uchiyama, S. Identification of the specific intestinal bacteria capable of metabolising soy isoflavone to equol. (Abs.). Ann. Nutr. Metab. 2001, 45, 114. [Google Scholar]
- Baber, R.J. Phytoestrogens in health: The role of isoflavones. In Isoflavones: Chemistry, Analysis, Function and Effects; Preedy, V.R., Ed.; Food and Nutritional Components in Focus; RCS Publishing: Cambridge, UK, 2013; pp. 3–13. ISBN 978-1-84973-419-6. [Google Scholar]
- Messina, M.; Kucuk, O.; Lampe, J.W. An overview of the health effects of isoflavones with an emphasis on prostate cancer risk and prostate-specific antigen levels. J. AOAC Int. 2006, 89, 1121–1134. [Google Scholar]
- Messina, M.; Hilakivi-Clarke, L. Early intake appears to be the key to the proposed protective effects of soy intake against breast cancer. Nutr. Cancer 2009, 61, 792–798. [Google Scholar] [CrossRef]
- Shu, X.O.; Zheng, Y.; Cai, H.; Gu, K.; Chen, Z.; Zheng, W.; Lu, W. Soy food intake and breast cancer survival. JAMA 2009, 302, 2437–2443. [Google Scholar] [CrossRef]
- Carroll, K.K. Review of clinical studies on cholesterol-lowering response to soy protein. J. Am. Diet. Assoc. 1991, 91, 820–827. [Google Scholar]
- Teede, H.J.; Dalais, F.S.; Kotsopoulos, D.; Liang, Y.L.; Davis, S.; McGrath, B.P. Dietary soy has both beneficial and potentially adverse cardiovascular effects: A placebo-controlled study in men and postmenopausal women. J. Clin. Endocrinol. Metab. 2001, 86, 3053–3060. [Google Scholar] [CrossRef]
- Hoie, L.H.; Guldstrand, M.; Sjoholm, A.; Graubaum, H.J.; Gruenwald, J.; Zunft, H.J.F.; Lueder, W. Cholesterol-lowering effects of a new isolated soy protein with high levels of nondenaturated protein in hypercholesterolemic patients. Adv. Ther. 2007, 24, 439–447. [Google Scholar] [CrossRef]
- Ye, Y.-B.; Tang, X.-Y.; Verbruggen, M.A.; Su, Y.-X. Soy isoflavones attenuate bone loss in early postmenopausal Chinese women: A single-blind randomized, placebo-controlled trial. Eur. J. Nutr. 2006, 45, 327–334. [Google Scholar] [CrossRef]
- Lethaby, A.E.; Brown, J.; Marjoribanks, J.; Kronenberg, F.; Roberts, H.; Eden, J. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst. Rev. 2007, CD001395. [Google Scholar] [CrossRef]
- Farquhar, C.M.; Marjoribanks, J.; Lethaby, A.; Lamberts, Q.; Suckling, J.A.; Cochrane HT Study Group. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst. Rev. 2005, 2015, CD004143. [Google Scholar] [CrossRef]
- Turner, R.; Baron, T.; Wolffram, S.; Minihane, A.M.; Cassidy, A.; Rimbach, G.; Weinberg, P.D. Effect of circulating forms of soy isoflavones on the oxidation of low density lipoprotein. Free Radic. Res. 2004, 38, 209–216. [Google Scholar] [CrossRef]
- Lund, T.D.; Munson, D.J.; Haldy, M.E.; Setchell, K.D.R.; Lephart, E.D.; Handa, R.J. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol. Reprod. 2004, 70, 1188–1195. [Google Scholar] [CrossRef]
- Nagel, S.C.; vom Saal, F.S.; Welshons, W.V. Developmental effects of estrogenic chemicals are predicted by an in vitro assay incorporating modification of cell uptake by serum. J. Steroid Biochem. Mol. Biol. 1999, 69, 343–357. [Google Scholar] [CrossRef]
- Hilakivi-Clarke, L.; de Assis, S. Fetal origins of breast cancer. Trends Endocrinol. Metab. 2006, 17, 340–348. [Google Scholar] [CrossRef]
- Wang, Y.; Man Gho, W.; Chan, F.L.; Chen, S.; Leung, L.K. The red clover (Trifolium pratense) isoflavone biochanin A inhibits aromatase activity and expression. Br. J. Nutr. 2008, 99, 303–310. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Evers, N.M.; van de Klundert, T.M.C.; van Aesch, Y.M.; Wang, S.; de Roos, W.K.; Romano, A.; de Haan, L.H.J.; Murk, A.J.; Ederveen, A.G.H.; Rietjens, I.M.C.M.; et al. Human T47D-ERβ breast cancer cells with tetracycline-dependent ERβ expression reflect ERα/ERβ ratios in rat and human breast tissue. Toxicol. In Vitro 2013, 27, 1753–1761. [Google Scholar] [CrossRef]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor α (ERα) and ERβ in human cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef]
- Krebs, E.E.; Ensrud, K.E.; MacDonald, R.; Wilt, T.J. Phytoestrogens for treatment of menopausal symptoms: A systematic review. Obstet. Gynecol. 2004, 104, 824–836. [Google Scholar] [CrossRef]
- Howes, L.G.; Howes, J.B.; Knight, D.C. Isoflavone therapy for menopausal flushes: A systematic review and meta-analysis. Maturitas 2006, 55, 203–211. [Google Scholar] [CrossRef]
- Jou, H.-J.; Wu, S.-C.; Chang, F.-W.; Ling, P.-Y.; Chu, K.S.; Wu, W.-H. Effect of intestinal production of equol on menopausal symptoms in women treated with soy isoflavones. Int. J. Gynaecol. Obstet. 2008, 102, 44–49. [Google Scholar] [CrossRef]
- Chandrareddy, A.; Muneyyirci-Delale, O.; McFarlane, S.I.; Murad, O.M. Adverse effects of phytoestrogens on reproductive health: A report of three cases. Complement. Ther. Clin. Pract. 2008, 14, 132–135. [Google Scholar] [CrossRef]
- Anthony, M.S.; Clarkson, T.B.; Hughes, C.L.; Morgan, T.M.; Burke, G.L. Soybean isoflavones improve cardiovascular risk factors without affecting the reproductive system of peripubertal rhesus monkeys. J. Nutr. 1996, 126, 43–50. [Google Scholar] [CrossRef]
- Zhan, S.; Ho, S.C. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am. J. Clin. Nutr. 2005, 81, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Sacks, F.M.; Lichtenstein, A.; Van Horn, L.; Harris, W.; Kris-Etherton, P.; Winston, M.; American Heart Association Nutrition Committee. Soy protein, isoflavones, and cardiovascular health: An American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006, 113, 1034–1044. [Google Scholar] [CrossRef]
- Reynolds, K.; Chin, A.; Lees, K.A.; Nguyen, A.; Bujnowski, D.; He, J. A meta-analysis of the effect of soy protein supplementation on serum lipids. Am. J. Cardiol. 2006, 98, 633–640. [Google Scholar] [CrossRef]
- Landmesser, U.; Hornig, B.; Drexler, H. Endothelial function: A critical determinant in atherosclerosis? Circulation 2004, 109, II27–II33. [Google Scholar] [CrossRef]
- Mäkelä, S.; Savolainen, H.; Aavik, E.; Myllärniemi, M.; Strauss, L.; Taskinen, E.; Gustafsson, J.A.; Häyry, P. Differentiation between vasculoprotective and uterotrophic effects of ligands with different binding affinities to estrogen receptors α and β. Proc. Natl. Acad. Sci. USA 1999, 96, 7077–7082. [Google Scholar] [CrossRef]
- Katz, D.L.; Evans, M.A.; Njike, V.Y.; Hoxley, M.L.; Nawaz, H.; Comerford, B.P.; Sarrel, P.M. Raloxifene, soy phytoestrogens and endothelial function in postmenopausal women. Climacteric 2007, 10, 500–507. [Google Scholar] [CrossRef]
- Evans, M.; Njike, V.Y.; Hoxley, M.; Pearson, M.; Katz, D.L. Effect of soy isoflavone protein and soy lecithin on endothelial function in healthy postmenopausal women. Menopause 2007, 14, 141–149. [Google Scholar] [CrossRef]
- Teede, H.J.; Giannopoulos, D.; Dalais, F.S.; Hodgson, J.; McGrath, B.P. Randomised, controlled, cross-over trial of soy protein with isoflavones on blood pressure and arterial function in hypertensive subjects. J. Am. Coll. Nutr. 2006, 25, 533–540. [Google Scholar] [CrossRef]
- Liu, X.X.; Li, S.H.; Chen, J.Z.; Sun, K.; Wang, X.J.; Wang, X.G.; Hui, R.T. Effect of soy isoflavones on blood pressure: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 463–470. [Google Scholar] [CrossRef]
- Levine, J.P. Effective strategies to identify postmenopausal women at risk for osteoporosis. Geriatrics 2007, 62, 22–30. [Google Scholar]
- Onoe, Y.; Miyaura, C.; Ohta, H.; Nozawa, S.; Suda, T. Expression of estrogen receptor β in rat bone. Endocrinology 1997, 138, 4509–4512. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Giustina, A.; Bilezikian, J.P. Mechanisms of anabolic therapies for osteoporosis. N. Engl. J. Med. 2007, 357, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.M.K.; Martin, B.R.; Jackson, G.S.; Elmore, D.; McCabe, G.P.; Nolan, J.R.; Barnes, S.; Peacock, M.; Weaver, C.M. Soy isoflavones do not affect bone resorption in postmenopausal women: A dose-response study using a novel approach with 41Ca. J. Clin. Endocrinol. Metab. 2007, 92, 577–582. [Google Scholar] [CrossRef]
- Alekel, D.L.; Germain, A.S.; Peterson, C.T.; Hanson, K.B.; Stewart, J.W.; Toda, T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am. J. Clin. Nutr. 2000, 72, 844–852. [Google Scholar] [CrossRef] [Green Version]
- Arjmandi, B.H.; Lucas, E.A.; Khalil, D.A.; Devareddy, L.; Smith, B.J.; McDonald, J.; Arquitt, A.B.; Payton, M.E.; Mason, C. One year soy protein supplementation has positive effects on bone formation markers but not bone density in postmenopausal women. Nutr. J. 2005, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Ho, S.C.; Lam, S.S.H.; Ho, S.S.S.; Woo, J.L.F. Beneficial effect of soy isoflavones on bone mineral content was modified by years since menopause, body weight, and calcium intake: A double-blind, randomized, controlled trial. Menopause 2004, 11, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Yang, H.-P.; Yang, H.-T.; Yang, T.-C.; Shieh, M.-J.; Huang, S.-Y. One-year soy isoflavone supplementation prevents early postmenopausal bone loss but without a dose-dependent effect. J. Nutr. Biochem. 2006, 17, 509–517. [Google Scholar] [CrossRef]
- Ishimi, Y. Dietary equol and bone metabolism in postmenopausal Japanese women and osteoporotic mice. J. Nutr. 2010, 140, 1373S–1376S. [Google Scholar] [CrossRef] [PubMed]
- Taku, K.; Melby, M.K.; Nishi, N.; Omori, T.; Kurzer, M.S. Soy isoflavones for osteoporosis: An evidence-based approach. Maturitas 2011, 70, 333–338. [Google Scholar] [CrossRef]
- Tousen, Y.; Ezaki, J.; Fujii, Y.; Ueno, T.; Nishimuta, M.; Ishimi, Y. Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: A pilot randomized, placebo-controlled trial. Menopause 2011, 18, 563–574. [Google Scholar] [CrossRef]
- Tousen, Y.; Ishiwata, H.; Ishimi, Y.; Ikegami, S. Equol, a Metabolite of Daidzein, Is More Efficient than Daidzein for Bone Formation in Growing Female Rats. Phytother. Res. 2015, 29, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, M.; Uehara, M.; Wu, J.; Adlercreutz, H.; Suzuki, K.; Kanazawa, K.; Takeda, K.; Yamada, K.; Ishimi, Y. Equol, a metabolite of daidzein, inhibits bone loss in ovariectomized mice. J. Nutr. 2004, 134, 2623–2627. [Google Scholar] [CrossRef] [PubMed]
- Youlden, D.R.; Cramb, S.M.; Dunn, N.A.M.; Muller, J.M.; Pyke, C.M.; Baade, P.D. The descriptive epidemiology of female breast cancer: An international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012, 36, 237–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Erp-Baart, M.-A.J.; Brants, H.A.M.; Kiely, M.; Mulligan, A.; Turrini, A.; Sermoneta, C.; Kilkkinen, A.; Valsta, L.M. Isoflavone intake in four different European countries: The VENUS approach. Br. J. Nutr. 2003, 89 (Suppl. 1), S25–S30. [Google Scholar] [CrossRef]
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar] [CrossRef]
- Messina, M.J.; Wood, C.E. Soy isoflavones, estrogen therapy, and breast cancer risk: Analysis and commentary. Nutr. J. 2008, 7, 17. [Google Scholar] [CrossRef]
- Shin, H.-R.; Joubert, C.; Boniol, M.; Hery, C.; Ahn, S.H.; Won, Y.-J.; Nishino, Y.; Sobue, T.; Chen, C.-J.; You, S.-L.; et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Cause Control 2010, 21, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Bardin, A.; Boulle, N.; Lazennec, G.; Vignon, F.; Pujol, P. Loss of ERβ expression as a common step in estrogen-dependent tumor progression. Endocr. Relat. Cancer 2004, 11, 537–551. [Google Scholar] [CrossRef] [Green Version]
- Lazennec, G.; Bresson, D.; Lucas, A.; Chauveau, C.; Vignon, F. ERβ inhibits proliferation and invasion of breast cancer cells. Endocrinology 2001, 142, 4120–4130. [Google Scholar] [CrossRef] [Green Version]
- Sotoca Covaleda, A.M.; van den Berg, H.; Vervoort, J.; van der Saag, P.; Ström, A.; Gustafsson, J.-A.; Rietjens, I.; Murk, A.J. Influence of cellular ERα/ERβ ratio on the ERα-agonist induced proliferation of human T47D breast cancer cells. Toxicol. Sci. 2008, 105, 303–311. [Google Scholar] [CrossRef]
- Islam, M.A.; Bekele, R.; Vanden Berg, J.H.J.; Kuswanti, Y.; Thapa, O.; Soltani, S.; van Leeuwen, F.X.R.; Rietjens, I.M.C.M.; Murk, A.J. Deconjugation of soy isoflavone glucuronides needed for estrogenic activity. Toxicol. In Vitro 2015, 29, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Horn-Ross, P.L.; John, E.M.; Canchola, A.J.; Stewart, S.L.; Lee, M.M. Phytoestrogen intake and endometrial cancer risk. J. Natl. Cancer Inst. 2003, 95, 1158–1164. [Google Scholar] [CrossRef]
- Xu, W.H.; Zheng, W.; Xiang, Y.B.; Ruan, Z.X.; Cheng, J.R.; Dai, Q.; Gao, Y.T.; Shu, X.O. Soya food intake and risk of endometrial cancer among Chinese women in Shanghai: Population based case-control study. BMJ 2004, 328, 1285. [Google Scholar] [CrossRef]
- Murray, M.J.; Meyer, W.R.; Lessey, B.A.; Oi, R.H.; DeWire, R.E.; Fritz, M.A. Soy protein isolate with isoflavones does not prevent estradiol-induced endometrial hyperplasia in postmenopausal women: A pilot trial. Menopause 2003, 10, 456–464. [Google Scholar] [CrossRef]
- Messina, M.J. Emerging evidence on the role of soy in reducing prostate cancer risk. Nutr. Rev. 2003, 61, 117–131. [Google Scholar] [CrossRef]
- Lund, T.D.; Blake, C.; Bu, L.; Hamaker, A.N.; Lephart, E.D. Equol an isoflavonoid: Potential for improved prostate health, in vitro and in vivo evidence. Reprod. Biol. Endocrinol. 2011, 9, 4. [Google Scholar] [CrossRef]
- Adams, K.F.; Chen, C.; Newton, K.M.; Potter, J.D.; Lampe, J.W. Soy isoflavones do not modulate prostate-specific antigen concentrations in older men in a randomized controlled trial. Cancer Epidemiol. Biomark. Prev. 2004, 13, 644–648. [Google Scholar]
- Fischer, L.; Mahoney, C.; Jeffcoat, A.R.; Koch, M.A.; Thomas, B.E.; Valentine, J.L.; Stinchcombe, T.; Boan, J.; Crowell, J.A.; Zeisel, S.H. Clinical characteristics and pharmacokinetics of purified soy isoflavones: Multiple-dose administration to men with prostate neoplasia. Nutr. Cancer 2004, 48, 160–170. [Google Scholar] [CrossRef]
- Yan, L.; Spitznagel, E.L. Soy consumption and prostate cancer risk in men: A revisit of a meta-analysis. Am. J. Clin. Nutr. 2009, 89, 1155–1163. [Google Scholar] [CrossRef]
- Chang, H.C.; Doerge, D.R. Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicol. Appl. Pharmacol. 2000, 168, 244–252. [Google Scholar] [CrossRef]
- Messina, M.; Redmond, G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: A review of the relevant literature. Thyroid 2006, 16, 249–258. [Google Scholar] [CrossRef]
- Chorazy, P.A.; Himelhoch, S.; Hopwood, N.J.; Greger, N.G.; Postellon, D.C. Persistent hypothyroidism in an infant receiving a soy formula: Case report and review of the literature. Pediatrics 1995, 96, 148–150. [Google Scholar]
- Dillingham, B.L.; McVeigh, B.L.; Lampe, J.W.; Duncan, A.M. Soy protein isolates of varied isoflavone content do not influence serum thyroid hormones in healthy young men. Thyroid 2007, 17, 131–137. [Google Scholar] [CrossRef]
- Radović, B.; Mentrup, B.; Köhrle, J. Genistein and other soya isoflavones are potent ligands for transthyretin in serum and cerebrospinal fluid. Br. J. Nutr. 2006, 95, 1171–1176. [Google Scholar] [CrossRef] [Green Version]
- Hagen, G.A.; Solberg, L.A. Brain and cerebrospinal fluid permeability to intravenous thyroid hormones. Endocrinology 1974, 95, 1398–1410. [Google Scholar] [CrossRef]
- Köhrle, J.; Fang, S.L.; Yang, Y.; Irmscher, K.; Hesch, R.D.; Pino, S.; Alex, S.; Braverman, L.E. Rapid effects of the flavonoid EMD 21388 on serum thyroid hormone binding and thyrotropin regulation in the rat. Endocrinology 1989, 125, 532–537. [Google Scholar] [CrossRef]
- Hillman, G.G.; Singh-Gupta, V.; Hoogstra, D.J.; Abernathy, L.; Rakowski, J.; Yunker, C.K.; Rothstein, S.E.; Sarkar, F.H.; Gadgeel, S.; Konski, A.A.; et al. Differential effect of soy isoflavones in enhancing high intensity radiotherapy and protecting lung tissue in a pre-clinical model of lung carcinoma. Radiother. Oncol. 2013, 109, 117–125. [Google Scholar] [CrossRef]
- Moosmann, B.; Behl, C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc. Natl. Acad. Sci. USA 1999, 96, 8867–8872. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Larrea, M.B.; Mohan, A.R.; Paganga, G.; Miller, N.J.; Bolwell, G.P.; Rice-Evans, C.A. Antioxidant activity of phytoestrogenic isoflavones. Free Radic. Res. 1997, 26, 63–70. [Google Scholar] [CrossRef]
- Amigo-Benavent, M.; Silván, J.M.; Moreno, F.J.; Villamiel, M.; Del Castillo, M.D. Protein quality, antigenicity, and antioxidant activity of soy-based foodstuffs. J. Agric. Food Chem. 2008, 56, 6498–6505. [Google Scholar] [CrossRef]
- Yoon, G.-A.; Park, S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014, 8, 618–624. [Google Scholar] [CrossRef] [Green Version]
- Wiseman, H.; O’Reilly, J.D.; Adlercreutz, H.; Mallet, A.I.; Bowey, E.A.; Rowland, I.R.; Sanders, T.A. Isoflavone phytoestrogens consumed in soy decrease F(2)-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am. J. Clin. Nutr. 2000, 72, 395–400. [Google Scholar] [CrossRef]
- Djuric, Z.; Chen, G.; Doerge, D.R.; Heilbrun, L.K.; Kucuk, O. Effect of soy isoflavone supplementation on markers of oxidative stress in men and women. Cancer Lett. 2001, 172, 1–6. [Google Scholar] [CrossRef]
- Monteiro, N.E.S.; Queirós, L.D.; Lopes, D.B.; Pedro, A.O.; Macedo, G.A. Impact of microbiota on the use and effects of isoflavones in the relief of climacteric symptoms in menopausal women – A review. J. Funct. Foods 2018, 41, 100–111. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Zhao, X.; Shoaf, S.E.; Ragland, K. The pharmacokinetics of S-(-)equol administered as SE5-OH tablets to healthy postmenopausal women. J. Nutr. 2009, 139, 2037–2043. [Google Scholar] [CrossRef]
- Andersen, C.; Nielsen, T.S.; Purup, S.; Kristensen, T.; Eriksen, J.; Søegaard, K.; Sørensen, J.; Fretté, X.C. Phyto-oestrogens in herbage and milk from cows grazing white clover, red clover, lucerne or chicory-rich pastures. Animal 2009, 3, 1189–1195. [Google Scholar] [CrossRef]
- Nielsen, T.S.; Nørgaard, J.V.; Purup, S.; Fretté, X.C.; Bonefeld-Jørgensen, E.C. Estrogenic activity of bovine milk high or low in equol using immature mouse uterotrophic responses and an estrogen receptor transactivation assay. Cancer Epidemiol. 2009, 33, 61–68. [Google Scholar] [CrossRef]
- Antignac, J.; Cariou, R.; LeBizec, B.; André, F. New data regarding phytoestrogens content in bovine milk. Food Chem. 2004, 87, 275–281. [Google Scholar] [CrossRef]
- Krajčová, A.; Schulzová, V.; Lojza, J.; Křížová, L.; Hajšlová, J. Phytoestrogens in bovine plasma and milk—LC-MS/MS analysis. Czech J. Food Sci. 2010, 28, 264–274. [Google Scholar] [CrossRef]
- Daems, F.; Jasselette, C.; Romnee, J.-M.; Planchon, V.; Lognay, G.; Froidmont, É. Validating the use of an ultra-performance liquid chromatography with tandem mass spectrometry method to quantify equol in cow’s milk. Dairy Sci. Technol. 2015, 95, 303–319. [Google Scholar] [CrossRef] [Green Version]
- Kuhnle, G.G.C.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of foods of animal origin: Dairy products, eggs, meat, fish, and seafood. J. Agric. Food Chem. 2008, 56, 10099–10104. [Google Scholar] [CrossRef] [PubMed]
- Kašparovská, J.; Dadáková, K.; Lochman, J.; Hadrová, S.; Křížová, L.; Kašparovský, T. Changes in equol and major soybean isoflavone contents during processing and storage of yogurts made from control or isoflavone-enriched bovine milk determined using LC-MS (TOF) analysis. Food Chem. 2017, 222, 67–73. [Google Scholar] [CrossRef]
- Atanassova, N.; McKinnell, C.; Fisher, J.; Sharpe, R.M. Neonatal treatment of rats with diethylstilboestrol (DES) induces stromal-epithelial abnormalities of the vas deferens and cauda epididymis in adulthood following delayed basal cell development. Reproduction 2005, 129, 589–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, A.A.; Custer, L.J. Daidzein and genistein concentrations in human milk after soy consumption. Clin. Chem. 1996, 42, 955–964. [Google Scholar] [PubMed]
- Balakrishnan, B.; Thorstensen, E.B.; Ponnampalam, A.P.; Mitchell, M.D. Transplacental transfer and biotransformation of genistein in human placenta. Placenta 2010, 31, 506–511. [Google Scholar] [CrossRef]
- Franke, A.A.; Custer, L.J.; Wang, W.; Shi, C.Y. HPLC analysis of isoflavonoids and other phenolic agents from foods and from human fluids. Proc. Soc. Exp. Biol. Med. 1998, 217, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Irvine, C.H.; Shand, N.; Fitzpatrick, M.G.; Alexander, S.L. Daily intake and urinary excretion of genistein and daidzein by infants fed soy- or dairy-based infant formulas. Am. J. Clin. Nutr. 1998, 68, 1462S–1465S. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.J.; Grady, J.J.; Marshall, M.V.; Ramanujam, V.M.; Anderson, K.E. Altered time course of urinary daidzein and genistein excretion during chronic soya diet in healthy male subjects. Nutr. Cancer 1995, 24, 311–323. [Google Scholar] [CrossRef]
- Olea, N.; Olea-Serrano, F.; Lardelli-Claret, P.; Rivas, A.; Barba-Navarro, A. Inadvertent exposure to xenoestrogens in children. Toxicol. Ind. Health 1999, 15, 151–158. [Google Scholar] [CrossRef]
- Talsness, C.; Grote, K.; Kuriyama, S.; Presibella, K.; Sterner-Kock, A.; Poça, K.; Chahoud, I. Prenatal Exposure to the Phytoestrogen Daidzein Resulted in Persistent Changes in Ovarian Surface Epithelial Cell Height, Folliculogenesis, and Estrus Phase Length in Adult Sprague-Dawley Rat Offspring. J. Toxicol. Environ. Health Part A 2015, 78, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Degen, G.H.; Janning, P.; Diel, P.; Michna, H.; Bolt, H.M. Transplacental transfer of the phytoestrogen daidzein in DA/Han rats. Arch. Toxicol. 2002, 76, 23–29. [Google Scholar] [CrossRef]
- Dinsdale, E.C.; Chen, J.; Ward, W.E. Early life exposure to isoflavones adversely affects reproductive health in first but not second generation female CD-1 mice. J. Nutr. 2011, 141, 1996–2002. [Google Scholar] [CrossRef]
- Jefferson, W.N.; Patisaul, H.B.; Williams, C.J. Reproductive consequences of developmental phytoestrogen exposure. Reproduction 2012, 143, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Molzberger, A.F.; Vollmer, G.; Hertrampf, T.; Möller, F.J.; Kulling, S.; Diel, P. In utero and postnatal exposure to isoflavones results in a reduced responsivity of the mammary gland towards estradiol. Mol. Nutr. Food Res. 2012, 56, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Kaludjerovic, J.; Chen, J.; Ward, W.E. Early life exposure to genistein and daidzein disrupts structural development of reproductive organs in female mice. J. Toxicol. Environ. Health Part A 2012, 75, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Greathouse, K.L.; Bredfeldt, T.; Everitt, J.I.; Lin, K.; Berry, T.; Kannan, K.; Mittelstadt, M.L.; Ho, S.; Walker, C.L. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol. Cancer Res. 2012, 10, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, K.; Baranowska-Bosiacka, I.; Marchlewicz, M.; Gutowska, I.; Noceń, I.; Zawiślak, M.; Chlubek, D.; Wiszniewska, B. Changes in male reproductive system and mineral metabolism induced by soy isoflavones administered to rats from prenatal life until sexual maturity. Nutrition 2011, 27, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, W.; Liu, J.; Sun, Y.; Li, Y.; Li, H.; Xiao, S.; Shen, X. Metabolomic changes in follicular fluid induced by soy isoflavones administered to rats from weaning until sexual maturity. Toxicol. Appl. Pharmacol. 2013, 269, 280–289. [Google Scholar] [CrossRef]
- Fukaya, T.; Funayama, Y.; Muakami, T.; Sugawara, J.; Yajima, A. Does apoptosis contribute follicular atresia and luteal regression in human ovary? Horm. Res. 1997, 48 (Suppl. 3), 20–26. [Google Scholar] [CrossRef]
- Verdin, E.; Hirschey, M.D.; Finley, L.W.S.; Haigis, M.C. Sirtuin regulation of mitochondria: Energy production, apoptosis, and signaling. Trends Biochem. Sci. 2010, 35, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Rajah, T.T.; Peine, K.J.; Du, N.; Serret, C.A.; Drews, N.R. Physiological concentrations of genistein and 17β-estradiol inhibit MDA-MB-231 breast cancer cell growth by increasing BAX/BCL-2 and reducing pERK1/2. Anticancer Res. 2012, 32, 1181–1191. [Google Scholar] [PubMed]
- Tang, S.; Hu, J.; Meng, Q.; Dong, X.; Wang, K.; Qi, Y.; Chu, C.; Zhang, X.; Hou, L. Daidzein induced apoptosis via down-regulation of Bcl-2/Bax and triggering of the mitochondrial pathway in BGC-823 cells. Cell Biochem. Biophys. 2013, 65, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Wang, B.; Shu, F.R.; Chen, K.; Mi, M.T. Equol promotes rat osteoblast proliferation and differentiation through activating estrogen receptor. Genet. Mol. Res. 2014, 13, 5055–5063. [Google Scholar] [CrossRef] [PubMed]
- Strom, B.L.; Schinnar, R.; Ziegler, E.E.; Barnhart, K.T.; Sammel, M.D.; Macones, G.A.; Stallings, V.A.; Drulis, J.M.; Nelson, S.E.; Hanson, S.A. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA 2001, 286, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Churella, H.R.; Borschel, M.W.; Thomas, M.R.; Breen, M.; Jacobs, J. Growth and protein status of term infants fed soy protein formulas differing in protein content. J. Am. Coll. Nutr. 1994, 13, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, J.B.; Ostrom, K.M.; Jacobs, J.R.; Blatter, M.M.; Ndife, L.I.; Gooch, W.M.; Cho, S. Growth of newborn, term infants fed soy formulas for 1 year. Clin. Pediatr. 1999, 38, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, J.M.; Moore, M.B.; Andres, A.; Estroff, J.A.; Badger, T.M. Ultrasonographic patterns of reproductive organs in infants fed soy formula: Comparisons to infants fed breast milk and milk formula. J. Pediatr. 2010, 156, 215–220. [Google Scholar] [CrossRef]
- Raman, D.R.; Williams, E.L.; Layton, A.C.; Burns, R.T.; Easter, J.P.; Daugherty, A.S.; Mullen, M.D.; Sayler, G.S. Estrogen content of dairy and swine wastes. Environ. Sci. Technol. 2004, 38, 3567–3573. [Google Scholar] [CrossRef]
- Hutchins, S.R.; White, M.V.; Hudson, F.M.; Fine, D.D. Analysis of lagoon samples from different concentrated animal feeding operations for estrogens and estrogen conjugates. Environ. Sci. Technol. 2007, 41, 738–744. [Google Scholar] [CrossRef]
- Dragomirescu, A.; Andoni, M.; Craina, M. Endocrine disrupting compounds in environment—A review. J. Food. Agric. Environ. 2015, 13, 117–119. [Google Scholar] [CrossRef]
- Hoerger, C.C.; Wettstein, F.E.; Bachmann, H.J.; Hungerbühler, K.; Bucheli, T.D. Occurrence and Mass Balance of Isoflavones on an Experimental Grassland Field. Environ. Sci. Technol. 2011, 45, 6752–6760. [Google Scholar] [CrossRef]
- Hoerger, C.C.; Wettstein, F.E.; Hungerbühler, K.; Bucheli, T.D. Occurrence and Origin of Estrogenic Isoflavones in Swiss River Waters. Environ. Sci. Technol. 2009, 43, 6151–6157. [Google Scholar] [CrossRef]
- Kuster, M.; Azevedo, D.A.; López de Alda, M.J.; Aquino Neto, F.R.; Barceló, D. Analysis of phytoestrogens, progestogens and estrogens in environmental waters from Rio de Janeiro (Brazil). Environ. Int. 2009, 35, 997–1003. [Google Scholar] [CrossRef]
| Aglycon | 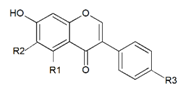 | R1 | R2 | R3 | |
| Daidzein | H | H | OH | ||
| Genistein | OH | H | OH | ||
| Glycitein | H | OCH3 | OH | ||
| Formononetin | H | H | OCH3 | ||
| Biochanin A | OH | H | OCH3 | ||
| Glucoside | 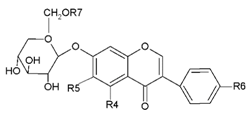 | R4 | R5 | R6 | R7 |
| Daidzin | H | H | OH | H | |
| Genistin | H | H | OH | H | |
| Glycitin | H | OCH3 | OH | H | |
| Ononin | H | H | OCH3 | H | |
| Sissotrin | OH | H | OCH3 | H | |
| Acetyldaidzin | H | H | OH | COCH3 | |
| Acetylgenistin | OH | H | OH | COCH3 | |
| Acetylglycitin | H | OCH3 | OH | COCH3 | |
| Malonyldaidzin | H | H | OH | COCH2COOH | |
| Malonylgenistin | OH | H | OH | COCH2COOH | |
| Malonylglycitin | H | OCH3 | OH | COCH2COOH | |
| Malonylononin | H | H | OCH3 | COCH2COOH | |
| Malonylsissotrin | OH | H | OCH3 | COCH2COOH |
| Analyte | Values | Isoflavone Source | Reference | |
|---|---|---|---|---|
| Daidzein | µg/L | 0.3–1.9 | C | [211] |
| 0.8–2.1 | C | [62] | ||
| 0.3–5.7 | C | [212] | ||
| 0.2–7.7 | C | [35] | ||
| 0.0–9.6 | U | [213] | ||
| 12.5–15.6 | S | [53] | ||
| 10–19 | S | [214] | ||
| 36.5–40.3 | S | [59] | ||
| 5.6–112.4 | S | [61] | ||
| Equol | µg/L | 3.6–15.6 | S | [59] |
| 3.5–54.8 | S | [53] | ||
| 4–76 | S | [214] | ||
| 14.5–61.1 * | S | [58] | ||
| 11–130 | U | [215] | ||
| 171–287 | C | [54] | ||
| 14.1–293.0 | U | [213] | ||
| 34.6–340.2 | S | [61] | ||
| 35–345 | C | [55] | ||
| 18.8–355.4 | C | [211] | ||
| 52–364 | C | [35] | ||
| 57–1003 | C + A | [212] | ||
| Genistein | µg/L | 1.7–2.2 | C | [62] |
| 1.9–3.0 | C | [35] | ||
| 0.0–5.8 | U | [213] | ||
| 2.4–31.3 | S | [61] | ||
| 3–37 | C | [55] | ||
| 34.4–37.3 | S | [53] | ||
| 170.6–175.8 | S | [59] | ||
| Glycitein | µg/L | 3.6–4.5 | C | [211] |
| 23.4–27.9 | S | [59] | ||
| 0.0–189.1 | S | [61] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. https://doi.org/10.3390/molecules24061076
Křížová L, Dadáková K, Kašparovská J, Kašparovský T. Isoflavones. Molecules. 2019; 24(6):1076. https://doi.org/10.3390/molecules24061076
Chicago/Turabian StyleKřížová, Ludmila, Kateřina Dadáková, Jitka Kašparovská, and Tomáš Kašparovský. 2019. "Isoflavones" Molecules 24, no. 6: 1076. https://doi.org/10.3390/molecules24061076
APA StyleKřížová, L., Dadáková, K., Kašparovská, J., & Kašparovský, T. (2019). Isoflavones. Molecules, 24(6), 1076. https://doi.org/10.3390/molecules24061076






