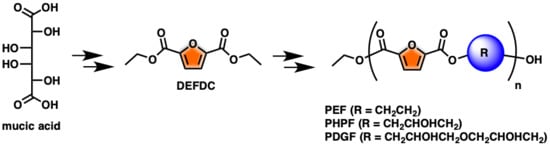
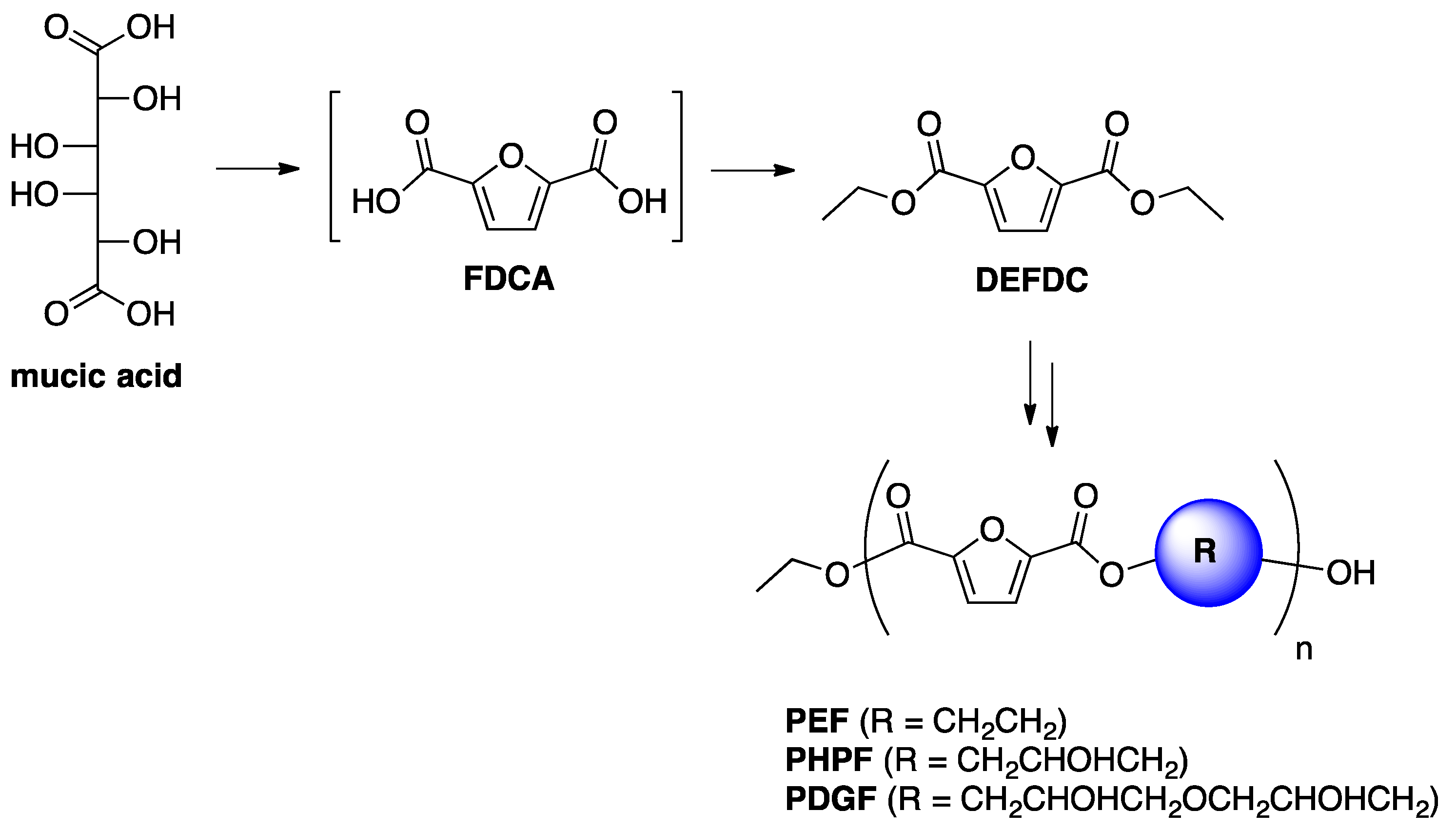
One-Pot FDCA Diester Synthesis from Mucic Acid and Their Solvent-Free Regioselective Polytransesterification for Production of Glycerol-Based Furanic Polyesters
Abstract
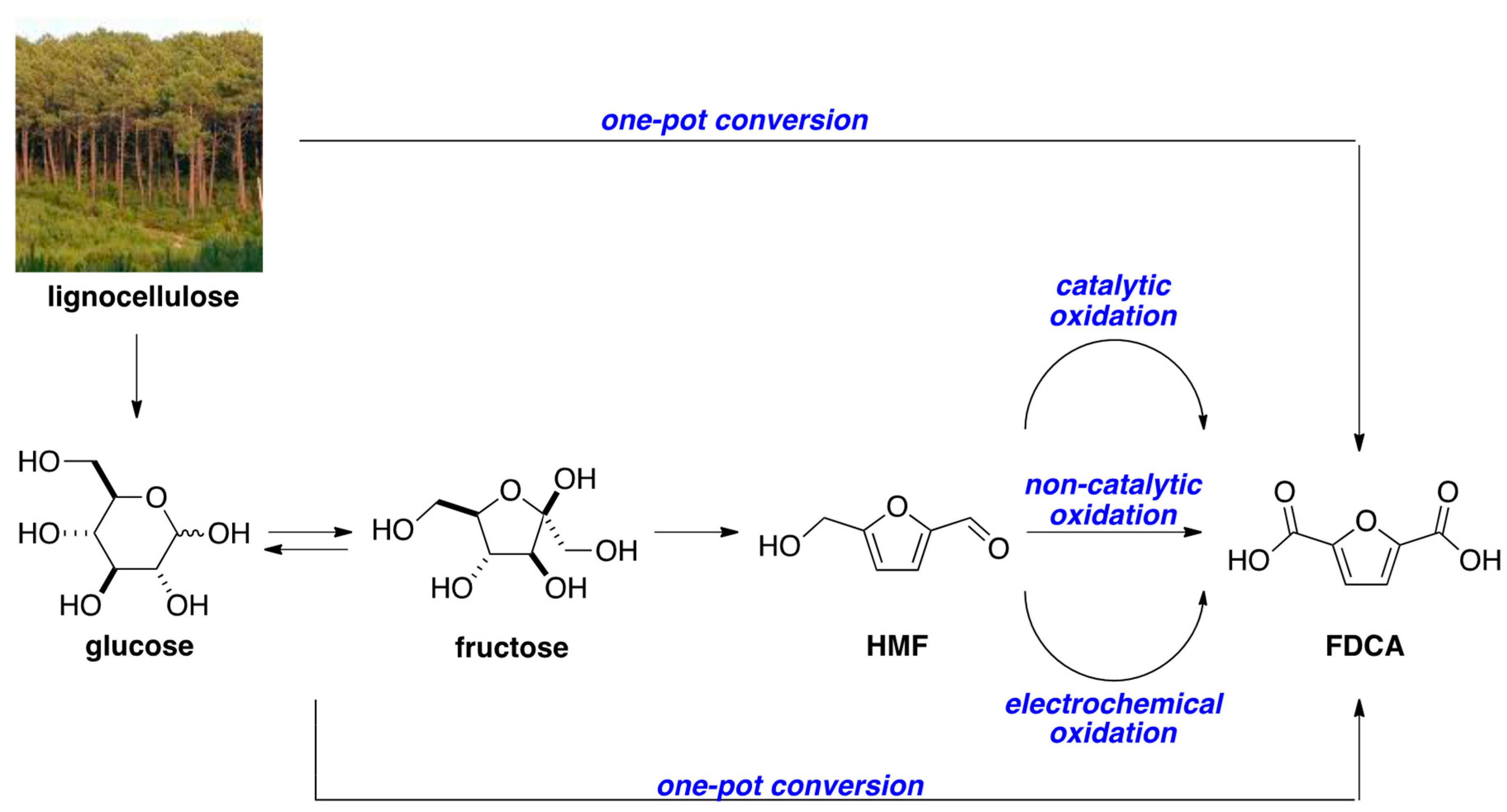
:1. Introduction
2. Results and Discussion
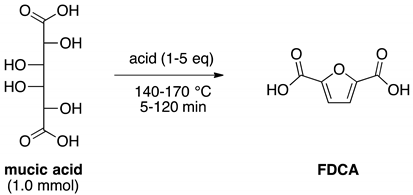
2.1. Optimization of DEFDC Synthesis
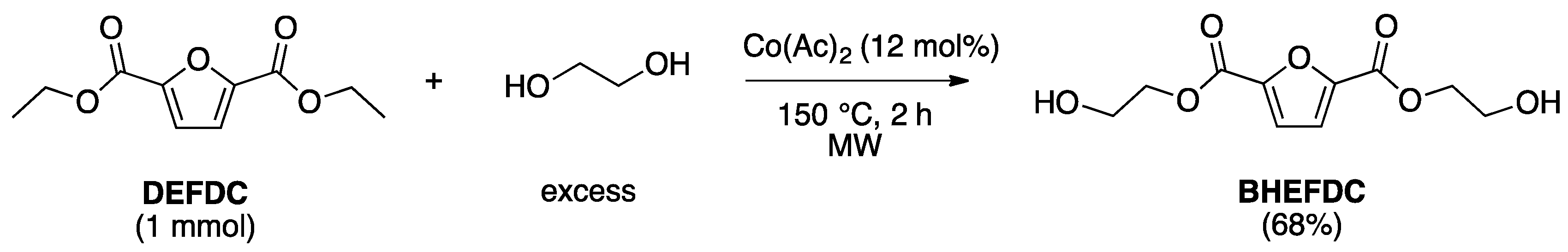
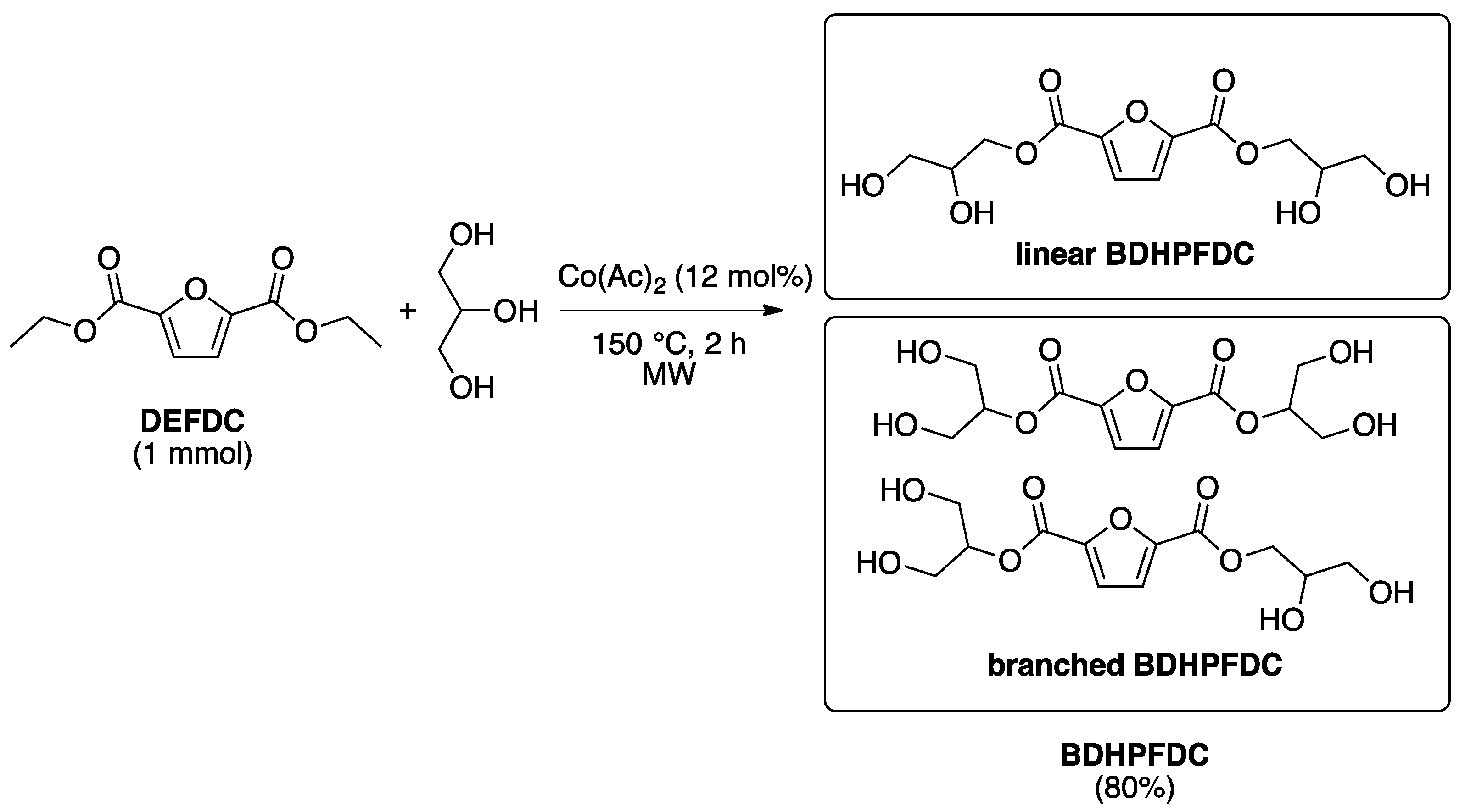
2.2. Optimization of the Prepolymers Synthesis and Additional Regioselective Acylation of Glycerol
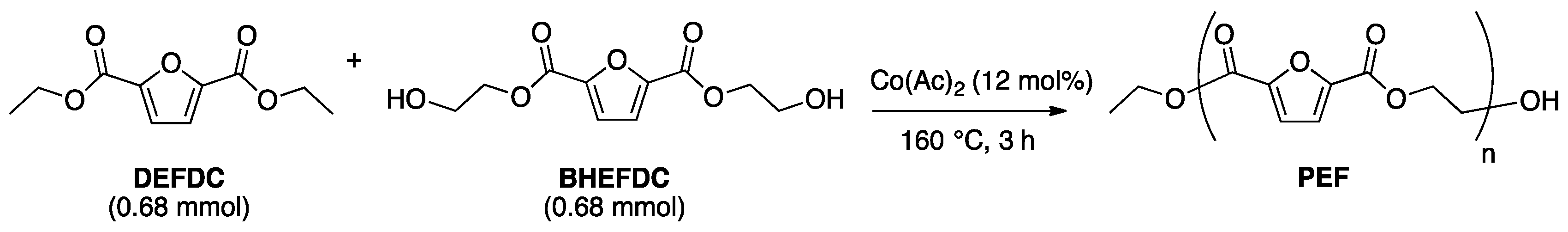
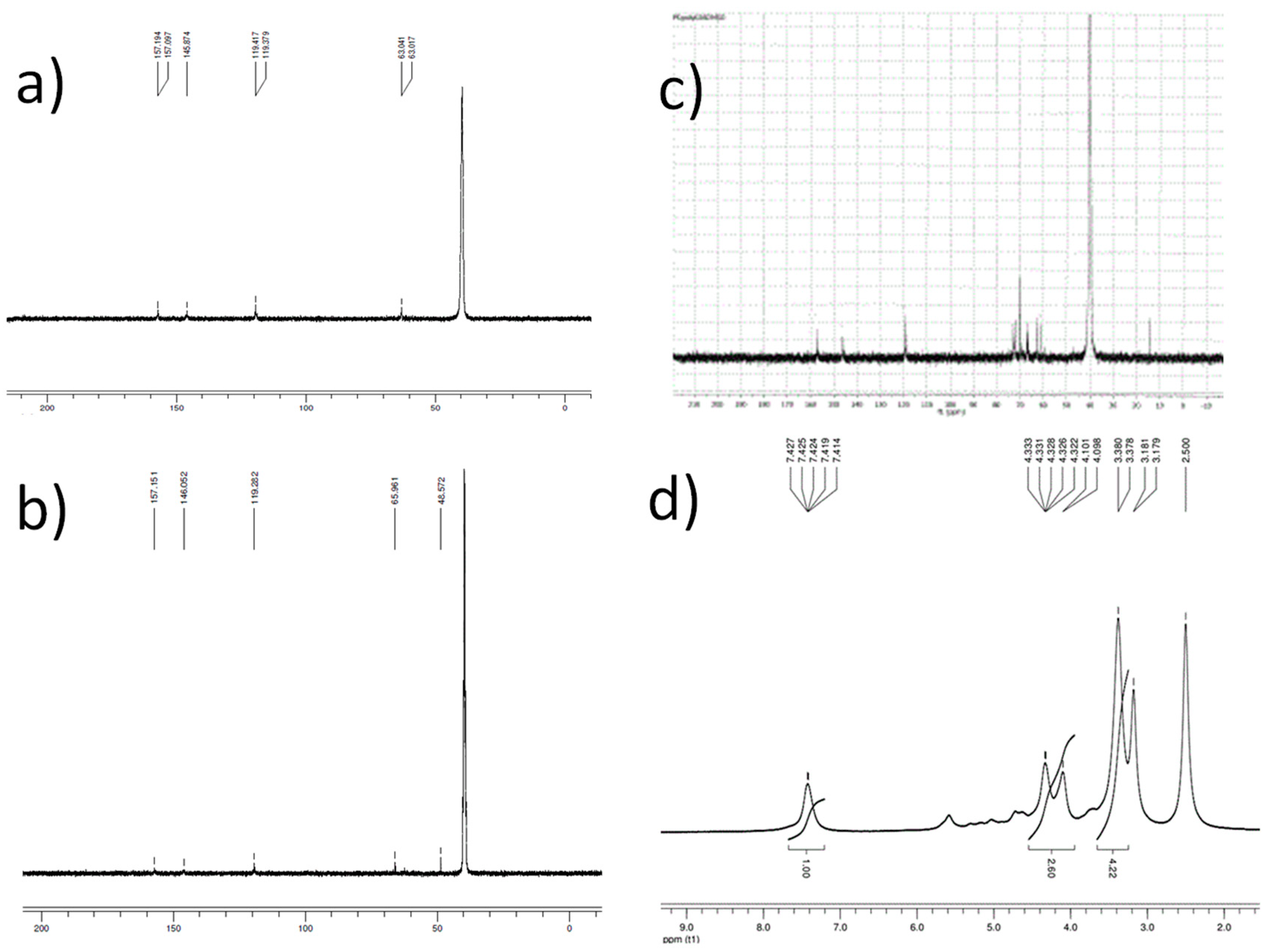
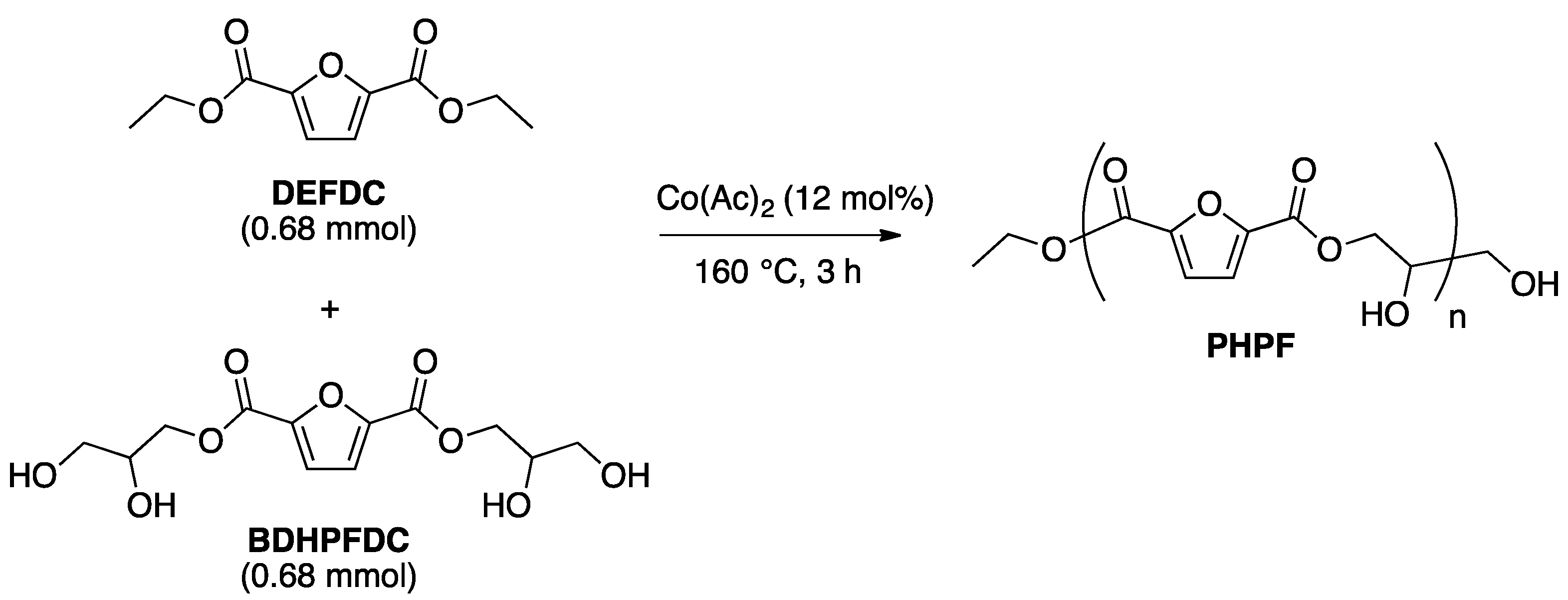
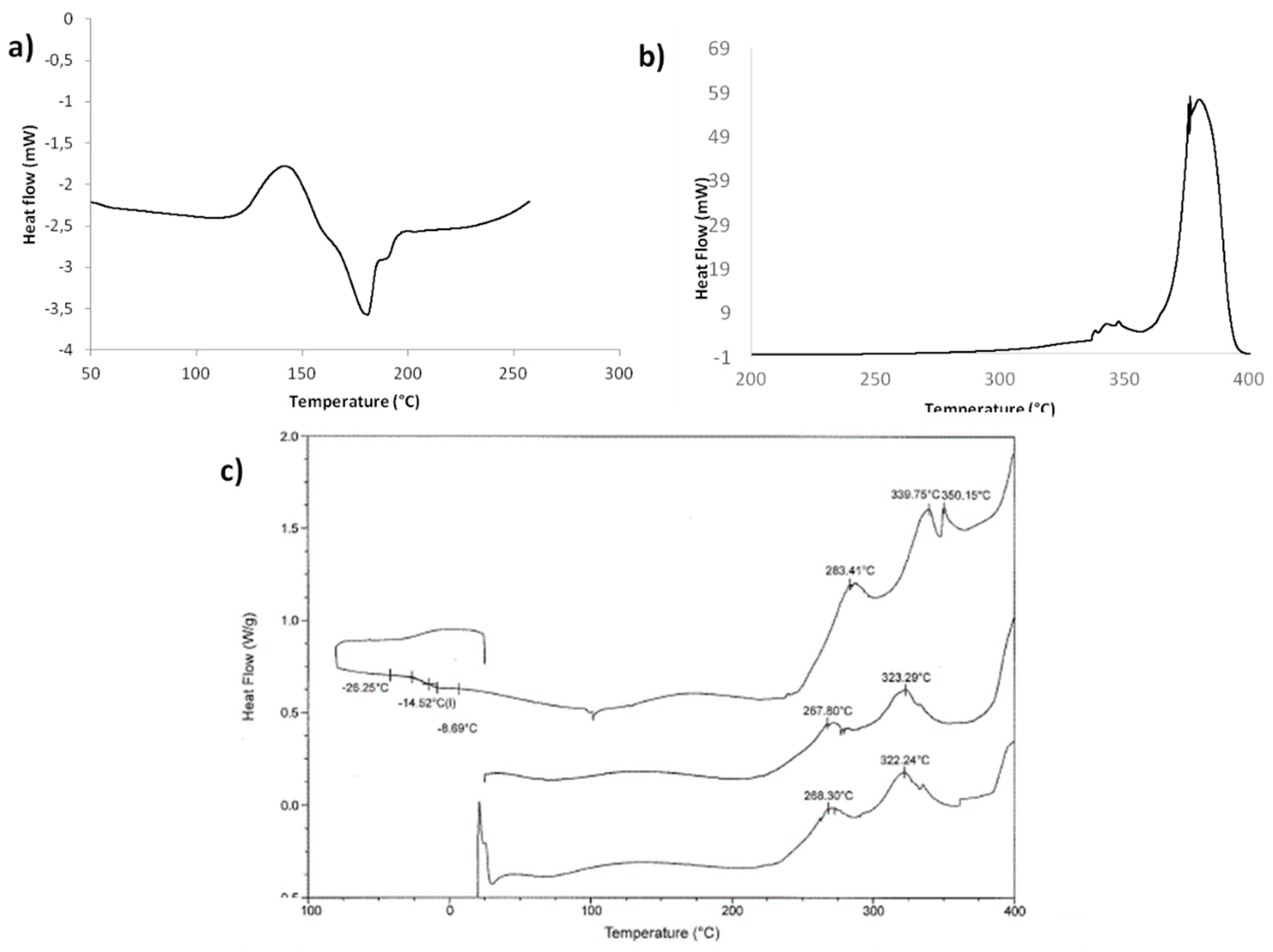
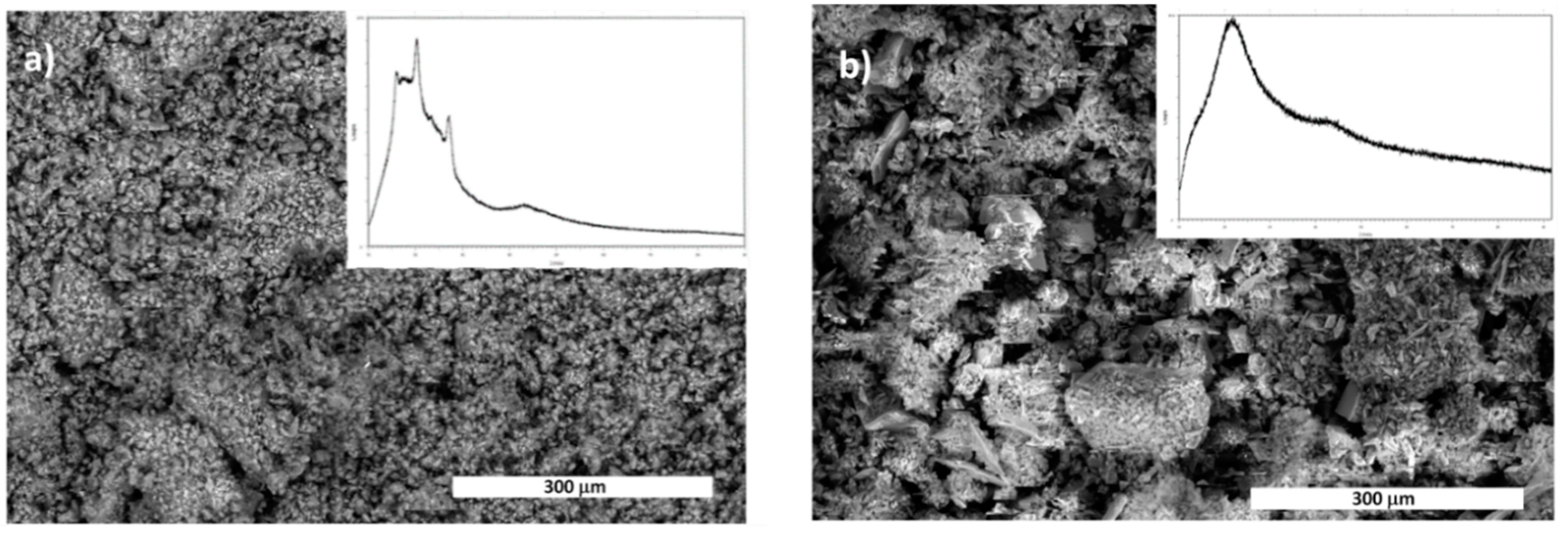
2.3. Optimization of Solvent-Free Poly Trans-Esterification and Solids Characterizations
3. Experimental Section
3.1. General Information
3.2. Synthesis and Characterization
3.2.1. Synthesis of Diethyl Furan-2,5-Dicarboxylate (DEFDC)
3.2.2. Synthesis of Bis(Hydroxyethyl)-2,5-Furandicarboxylate (BHEFDC)
3.2.3. Synthesis of Bis(2,3-Dihydropropyl)-2,5-Furandicarboxylate (BDHPFDC)
3.2.4. General Melt Polytransesterification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pal, P.; Saravanamurugan, S. Recent advances on development of 5-hydroxymethylfurfural oxidation with base (non-precious) metal-containing catalysts. ChemSusChem 2019, 12, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Kucherov, F.A.; Romashov, L.V.; Galkin, K.I.; Ananikov, V.P. Chemical transformations of biomass-derived C6-furanic platform chemicals for sustainable energy research, material science, and synthetic building blocks. ACS Sustain. Chem. Eng. 2018, 6, 8064–8092. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, G.W. Catalytic oxidation of carbohydrates into organic acids and furan chemicals. Chem. Soc. Rev. 2018, 47, 1351–1390. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Zhao, X.; Liu, D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, K. Recent advances in the catalytic synthesis of 2,5-furandicarboxylic acid and its derivatives. ACS Catal. 2015, 5, 6529–6544. [Google Scholar] [CrossRef]
- Partenheimer, W.; Grushin, V.V. Synthesis of 2,5-diformylfuran and furan-2,5-dicarboxylic acid by air-oxidation of 5-hydroxymethylfurfural. Unexpectedly selective aerobic oxidation of benzyl alcohol to benzaldehyde with metal=bromide catalysts. Adv. Synth. Catal. 2001, 343, 102–111. [Google Scholar] [CrossRef]
- Saha, B.; Dutta, S.; Abu-Omar, M.M. Aerobic oxidation of 5-hydroxymethylfurfural with homogeneous and nanoparticulate catalysts. Catal. Sci. Technol. 2012, 2, 79–81. [Google Scholar] [CrossRef]
- Lolli, A.; Albonetti, S.; Utili, L.; Amadori, R.; Ospitali, F.; Lucarelli, C.; Carani, F. Insights into the reaction mechanism for 5-hydroxymethylfurfural oxidation to FDCA on bimetallic Pd-Au nanoparticles. Appl. Catal. A Gen. 2015, 504, 408–419. [Google Scholar] [CrossRef]
- Abonetti, S.; Pasini, T.; Lolli, A.; Blosi, M.; Piccinini, M.; Dimitratos, N.; Lopez-Sanchez, J.A.; Morgan, D.J.; Carley, A.F.; Hutchings, G.J. Selective oxidation of 5-hydroxymethyl-2-furfural over TiO2-supported gold-copper catalysts prepared from preformed nanoparticles: Effect of Au/Cu ratio. Catal. Today 2012, 195, 120–126. [Google Scholar] [CrossRef]
- Rass, M.A.; Essayem, N.; Besson, M. Selective aerobic oxidation of 5-HMF into 2,5-furandicarboxylic acid with Pt catalysts supported on TiO2- and ZrO2-based supports. ChemSusChem 2015, 8, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Delbecq, F.; Wang, Y.; Len, C. Various carbohydrate precursors dehydration to 5-HMF in an acidic biphasic system under microwave heating using betaine as a co-catalyst. Mol. Catal. 2017, 434, 80–85. [Google Scholar] [CrossRef]
- Delbecq, F.; Len, C. Recent advances in the microave-assisted production of hydroxymethylfurfural by hydrolysis of cellulose derivatives—A review. Molecules 2018, 23, 1973. [Google Scholar] [CrossRef] [PubMed]
- Gaset, A.; Rigal, L.; Sene, B.; Ralainirina, R. 2,5-furan dicarboxylic ester preparation. France Patent FR 2723945B1.
- Lewkowski, J. Convenient synthesis of furan-2,5-dicarboxylic acid and its derivatives. Polish J. Chem. 2001, 75, 1943–1946. [Google Scholar]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.J.M.; Coehlo, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5982. [Google Scholar] [CrossRef]
- Hong, S.; Min, K.D.; Nam, B.U.; Park, O.O. High molecular weight bio furan-based co-polyesters for food packaging applications: Synthesis, characterization and solid-state polymerization. Green Chem. 2016, 18, 5142–5151. [Google Scholar] [CrossRef]
- Storbeck, R.; Ballauff, M. Synthesis and properties of polyesters based on 2,5-furandicarboxylic acid and 1,4:3,6-dianhydrohexitols. Polymer 1993, 34, 5003–5006. [Google Scholar] [CrossRef]
- Arnaud, S.P.; Wu, L.; Wong Chang, M.A.; Comerfod, J.W.; Farmer, T.J.; Schmid, M.; Chang, F.; Li, Z.; Mascal, M. New bio-based monomers: Tuneable polyester properties using branched diols from biomass. Faraday Discuss. 2017, 202, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulou, M.; Terzopoulou, Z.; Nerantzaki, M.; Tsagkalias, J.; Achilias, D.S.; Bikiaris, D.N.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Poly(ethylene furanoate-co-ethylene terephthalate) biobased copolymers: Synthesis, thermal properties and cocrystallization behavior. Eur. Polym. J. 2017, 89, 349–366. [Google Scholar] [CrossRef]
- Knoop, R.J.I.; Vogelzang, W.; van Haveren, J.; van Es, D.S. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Vogelzang, W.; Knoop, R.I.J.; Frissen, A.E.; van Haveren, J.; van Es, D.S. Biobased furandicarboxylic acids (FDCAs): Effects of isomeric substitution on polyester synthesis and properties. Green Chem. 2014, 16, 1957–1965. [Google Scholar] [CrossRef]
- Matos, M.; Sousa, A.F.; Fonseca, A.C.; Freire, C.S.R.; Coelho, J.F.J.; Silvestre, A.J.D. A new generation of furanic copolyesters with enhanced degradability: Poly(ethylene 2,5-furandicarboxylate)-co-poly(lactic acid) copolyesters. Macromol. Chem. Phys. 2014, 215, 2175–2184. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Martinez de Ilarduya, A.; Mufioz-Guerra, S. Poly(alkylene 2,5-furandicarboxylate)s (PEF and PBF) by ring opening polymerization. Polymer 2016, 87, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Sousa, A.F.; Matos, M.; Freire, C.S.R.; Silvestre, A.J.D.; Coehlo, J.F.J. New copolyesters derived from terephthalic and 2,5-furandicarboxylic acids: A step forward in the development of biobased polyesters. Polymer 2013, 54, 513–519. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhang, Y.; Liu, F.; Zhu, J. Modification of poly(ethylene 2,5-furandicarboxylate) with 1,4-cyclohexanedimethylene: Influence of composition on mechanical and barrier properties. Polymer 2016, 103, 1–8. [Google Scholar] [CrossRef]
- Gandini, A.; Sivestre, A.J.D.; Neto, C.P.; Sousa, A.F.; Gomes, M. The furan counterpart of poly(ethylene terephthalate): An alternative material based on renewable resources. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and characterization of poly(2,5-furan dicarboxylate)s based on a variety of diols. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Razzaq, A.; Bonham, P. Synthesis and characterization of all renewable resources based branched polyesters: Poly(2,5-furandicarboxylic acid-co-glycerol). ISRN Polym. Sci. 2013, 2013, 645169. [Google Scholar] [CrossRef]
- Gopalakrishnan, P.; Narayan-Sarathy, S.; Ghosh, T.; Mahajan, K.; Naceur Belgaum, M. Synthesis and characterization of bio-based furanic polyesters. J. Polym. Res. 2014, 21, 340. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jia, Z.; Liu, Y.; Sun, L.; Zhu, J. Synthesis of bio-based poly(ethylene 2,5-furandicarboxylate) copolyesters: Higher glass transition temperature, better transparency, and good barrier properties. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3298–3307. [Google Scholar] [CrossRef]
- Wu, J.; Xie, H.; Wu, L.; Li, B.G.; Dubois, P. DBU-catalyzed biobased poly(ethylene 2,5-furandicarboxylate) polyester with rapid melt crystallization: Synthesis, crystallization kinetics and melting behavior. RSC Adv. 2016, 6, 101578–101586. [Google Scholar] [CrossRef]
- Rosenboom, J.G.; De Roo, J.; Storti, G.; Morbidelli, M. Diffusion (DOSY) 1H NMR as an alternative method for molecular weight determination of poly(ethylene furanoate) (PEF) polyesters. Macromol. Chem. Phys. 2017, 218, 1600436. [Google Scholar] [CrossRef]
- Taguchi, Y.; Oishi, A.; Iida, H. One-step synthesis of dibutyl furandicarboxylates from galactaric acid. Chem. Lett. 2008, 37, 50–51. [Google Scholar] [CrossRef]
- Slavko, E.; Taylor, M.S. Catalyst-controlled polycondensation of glycerol with diacyl chlorides: Linear polyesters from a trifunctional monomer. Chem. Sci. 2017, 8, 7106–7111. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Billamboz, M.; Leonard, E.; Len, C.; Bottcher, C.; Prasad, A.K.; Haag, R.; Sharma, S.K. Self-assembly, photoresponsive behavior and transport potential of azobenzene grafted dendronized polymeric amphiphiles. RSC Adv. 2015, 5, 48301–48310. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, A.; Malhotra, S.; Mosurkal, R.; Dhawan, A.; Pandey, M.K.; Singh, B.K.; Kumar, R.; Prasad, A.K.; Sharma, S.K.; et al. Synthesis of macromolecular systems via lipase catalyzed biocatalytic reactions: Applications and future perspectives. Chem. Soc. Rev. 2016, 45, 6855–6887. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Loos, K. A biocatalytic approach towards sustainable furanic-aliphatic polyesters. Polym. Chem. 2015, 6, 5198–5211. [Google Scholar] [CrossRef]
- Van Aken, K.; Strekowski, L.; Patiny, L. Ecoscale, a semi-quantitative tool to select an organic preparation based on economical and acological parameters. Beilstein J. Org. Chem. 2006, 2, 3–9. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Entry | Acid | [Acid] [eq] | Temperature [°C] | Time [min] | Yield of FDCA [%] a |
|---|---|---|---|---|---|
| 1 | PTSA | 1 | 160 | 60 | 32 |
| 2 | PTSA | 2 | 160 | 60 | 41 |
| 3 | PTSA | 3 | 160 | 60 | 36 |
| 4 | PTSA | 4 | 160 | 60 | 34 |
| 5 | PTSA | 5 | 160 | 60 | 37 |
| 6 | MSA | 1 | 160 | 60 | 34 |
| 7 | MSA | 2 | 160 | 60 | 39 |
| 8 | MSA | 3 | 160 | 60 | 35 |
| 9 | MSA | 4 | 160 | 60 | 35 |
| 10 | MSA | 5 | 160 | 60 | 30 |
| 11 | CSA | 1 | 160 | 60 | 4 |
| 12 | CSA | 2 | 160 | 60 | 11 |
| 13 | CSA | 3 | 160 | 60 | 15 |
| 14 | CSA | 4 | 160 | 60 | 12 |
| 15 | CSA | 5 | 160 | 60 | 9 |
| 16 | PTSA | 2 | 140 | 60 | 12 |
| 17 | PTSA | 2 | 170 | 60 | 28 |
| 18 | PTSA | 2 | 160 | 5 | 8 |
| 19 | PTSA | 2 | 160 | 15 | 20 |
| 20 | PTSA | 2 | 160 | 30 | 30 |
| 21 | PTSA | 2 | 160 | 90 | 40 |
| 22 | PTSA | 2 | 160 | 120 | 37 |
| 23 | MSA | 2 | 140 | 60 | 15 |
| 24 | MSA | 2 | 170 | 60 | 25 |
| 25 | MSA | 2 | 160 | 5 | 8 |
| 26 | MSA | 2 | 160 | 15 | 30 |
| 27 | MSA | 2 | 160 | 30 | 39 |
| 28 | MSA | 2 | 160 | 90 | 35 |
| 29 | MSA | 2 | 160 | 120 | 32 |

| Entry | Temperature [°C] | Time [h] | Yield of DEFDC [%] a |
|---|---|---|---|
| 1 | 70 | 4 | 15 |
| 2 | 70 | 8 | 23 |
| 3 | 70 | 16 | 29 |
| 4 | 90 | 4 | 27 |
| 5 | 90 | 8 | 29 |
| 6 | 90 | 16 | 30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Delbecq, F.; Len, C. One-Pot FDCA Diester Synthesis from Mucic Acid and Their Solvent-Free Regioselective Polytransesterification for Production of Glycerol-Based Furanic Polyesters. Molecules 2019, 24, 1030. https://doi.org/10.3390/molecules24061030
Zhao D, Delbecq F, Len C. One-Pot FDCA Diester Synthesis from Mucic Acid and Their Solvent-Free Regioselective Polytransesterification for Production of Glycerol-Based Furanic Polyesters. Molecules. 2019; 24(6):1030. https://doi.org/10.3390/molecules24061030
Chicago/Turabian StyleZhao, Deyang, Frederic Delbecq, and Christophe Len. 2019. "One-Pot FDCA Diester Synthesis from Mucic Acid and Their Solvent-Free Regioselective Polytransesterification for Production of Glycerol-Based Furanic Polyesters" Molecules 24, no. 6: 1030. https://doi.org/10.3390/molecules24061030