Bioactive 2-(Methyldithio)Pyridine-3-Carbonitrile from Persian Shallot (Allium stipitatum Regel.) Exerts Broad-Spectrum Antimicrobial Activity
Abstract
:1. Introduction
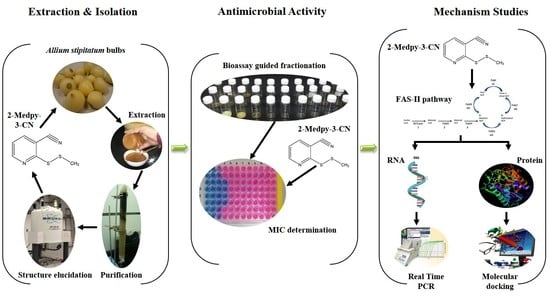
2. Results
2.1. Bioassay-Guided Fractionation
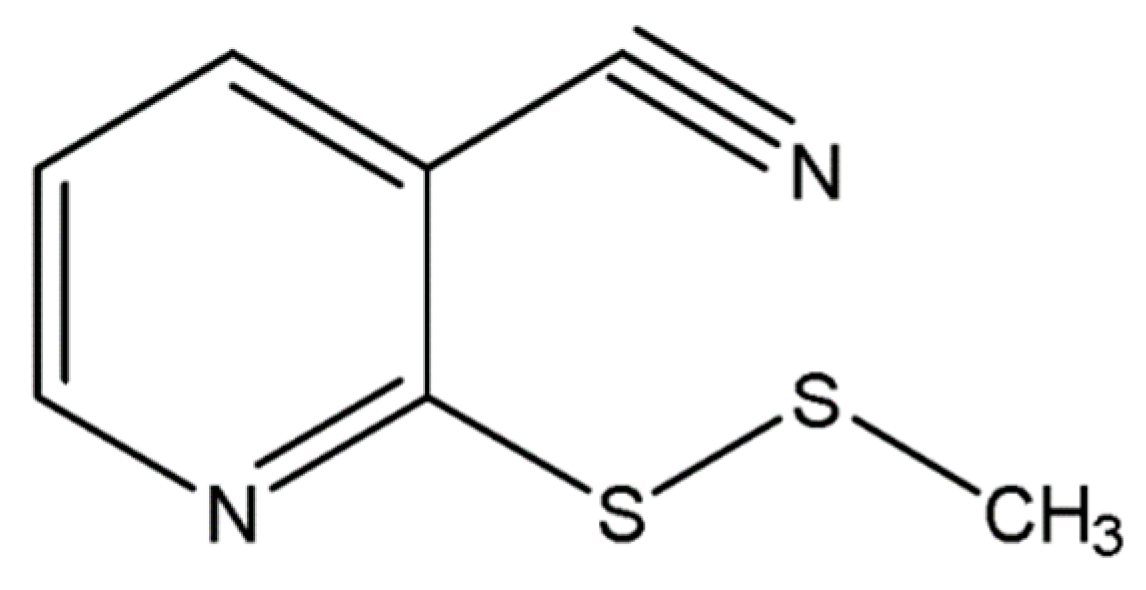
2.2. Structure Elucidation and Identification
2.3. Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), and Minimum Fungicidal Concentration (MFC) of 2-Medpy-3-CN
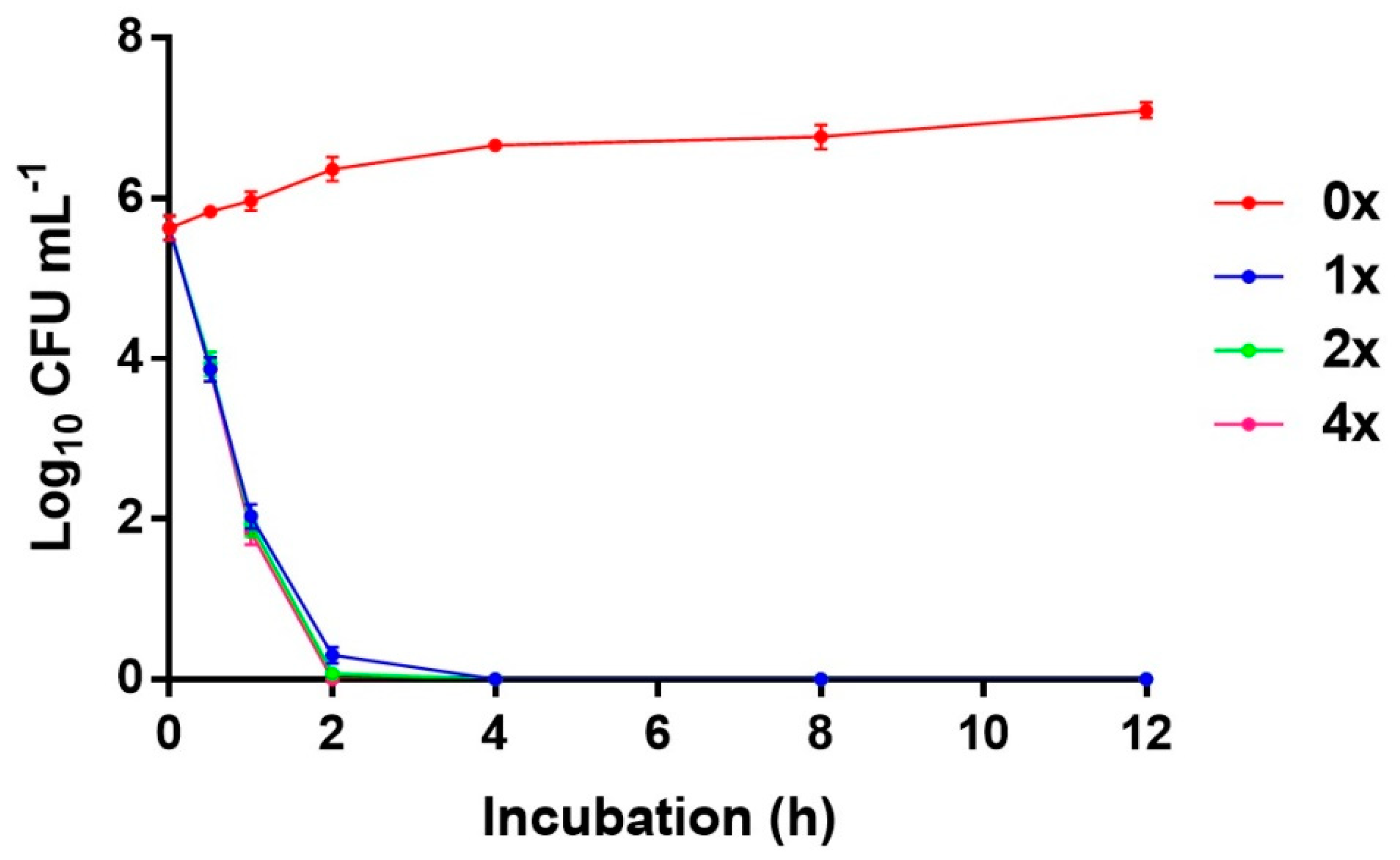
2.4. Time-to-Kill Assay
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Plant Material and Preparation of Extracts
4.1.2. General Experimental Procedures
4.1.3. Isolation of Potential Antimicrobial Compounds
4.2. Biological Evaluation with Methicillin-Resistant S. aureus (MRSA)
4.2.1. Test Microorganisms
4.2.2. MIC, MBC, and MFC Measurements
4.2.3. Time-To-Kill Assay for Detecting the Bactericidal Effect of the Compound for MRSA
4.3. Prediction of ADME Properties
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pfeltz, R.F.; Wilkinson, B.J. The escalating challenge of vancomycin resistance in Staphylococcus Aureus. Curr. Drug Targets 2004, 4, 273–294. [Google Scholar] [CrossRef]
- Bonten, M.J.; Willems, R.; Weinstein, R.A. Vancomycin-resistant enterococci: Why are they here, and where do they come from? Lancet Infect. Dis. 2001, 15, 314–325. [Google Scholar] [CrossRef]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter Baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Neela, V.; Rankouhi, S.Z.R.; van Belkum, A.; Goering, R.V.; Hamat, R.A. Stenotrophomonas maltophilia in Malaysia: Molecular epidemiology and trimethoprim-sulfamethoxazole resistance. Int. J. Infect. Dis. 2012, 16, e603–e607. [Google Scholar] [CrossRef]
- Horn, D.L.; Neofytos, D.; Anaissie, E.J.; Fishman, J.A.; Steinbach, W.J.; Olyaei, A.J.; Marr, K.A.; Pfaller, M.A.; Chang, C.H.; Webster, K.M. Epidemiology and outcomes of candidemia in 2019 patients: Data from the prospective antifungal therapy alliance registry. Clin. Infect. Dis. 2009, 48, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Moet, G.J.; Jones, R.N.; Castanheira, M. Candida bloodstream infections: Comparison of species distribution and resistance to echinocandin and azole antifungal agents in Intensive Care Unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008–2009). Int. J. Antimicrob. Agents 2011, 38, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef]
- Reed, S.D.; Friedman, J.Y.; Engemann, J.J.; Griffiths, R.I.; Anstrom, K.J.; Kaye, K.S.; Stryjewski, M.E.; Szczech, L.A.; Reller, L.B.; Corey, G.R.; et al. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect. Control Hosp. Epidemiol. 2005, 26, 175–183. [Google Scholar] [CrossRef]
- McHugh, C.G.; Riley, L.W. Risk factors and costs associated with methicillin-resistant Staphylococcus aureus bloodstream infections. Infect. Control Hosp. Epidemiol. 2004, 25, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.E.; Qi, Y.; Kaye, K.S.; Harbarth, S.; Karchmer, A.W.; Carmeli, Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: Mortality, length of stay, and hospital charges. Infect. Control Hosp. Epidemiol. 2005, 26, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.G. Natural Products as a Source for Novel Antibiotics. Trends Pharmacol. Sci. 2016, 37, 689–701. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.D.; Birdi, T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef]
- Petrovska, B.B.; Cekovska, S. Extracts from the history and medical properties of garlic. Pharmacogn. Rev. 2010, 4, 106–110. [Google Scholar] [CrossRef]
- Sivam, G.P. Protection against Helicobacter pylori and other bacterial infections by garlic. J. Nutr. 2001, 131, 1106S–1108S. [Google Scholar] [CrossRef]
- Li, G.; Ma, X.; Deng, L.; Zhao, X.; Wei, Y.; Gao, Z.; Jia, J.; Xu, J.; Sun, C. Fresh Garlic Extract Enhances the Antimicrobial Activities of Antibiotics on Resistant Strains in Vitro. Jundishapur J. Microbiol. 2015, 8, e14814. [Google Scholar] [CrossRef]
- Palaksha, M.N.; Ahmed, M.; Das, S. Antibacterial activity of garlic extract on streptomycin-resistant Staphylococcus aureus and Escherichia coli solely and in synergism with streptomycin. J. Nat. Sci. Biol. Med. 2010, 1, 12–15. [Google Scholar] [CrossRef]
- Reiter, J.; Levina, N.; van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef]
- Asili, A.; Behravan, J.; Reza Naghavi, M.; Asili, J. Genetic diversity of persian shallot (Allium hirtifolium) ecotypes based on morphological traits, allicin content and RAPD markers. Open Access J. Med. Aromat. Plants 2010, 1, 1–6. [Google Scholar]
- Ebrahimi, R.; Zamani, Z.; Kashi, A. Genetic diversity evaluation of wild Persian shallot (Allium hirtifolium Boiss.) using morphological and RAPD markers. Sci. Hortic. 2009, 119, 345–351. [Google Scholar] [CrossRef]
- Karunanidhi, A.; Ghaznavi-Rad, E.; Jeevajothi Nathan, J.; Abba, Y.; van Belkum, A.; Neela, V. Allium stipitatum extract exhibits in vivo antibacterial activity against methicillin-resistant Staphylococcus aureus and accelerates burn wound healing in a full-thickness murine burn model. Evid. Based Complement. Alternat. Med. 2017, 2017, 1914732. [Google Scholar] [CrossRef] [PubMed]
- Karunanidhi, A.; Ghaznavi-Rad, E.; Hamat, R.A.; Pichika, M.R.; Lung, L.T.T.; Mohd Fauzi, F.; Chigurupati, S.; van Belkum, A.; Neela, V. Antibacterial and Antibiofilm Activities of Nonpolar Extracts of Allium stipitatum Regel. against Multidrug Resistant Bacteria. Biomed Res. Int. 2018, 2018, 9845075. [Google Scholar] [CrossRef] [PubMed]
- Karunanidhi, A.; Ghaznavi-Rad, E.; Jeevajothi Nathan, J.; Mohd Fauzi, F.; Lung, L.T.T.; Hamat, R.A.; Neela, V. Antifungal and antibiofilm activity of Persian shallot (Allium stipitatum Regel.) against clinically significant Candida spp. Trop. Biomed. 2018, 35, 1–11. [Google Scholar]
- Danquah, C.A.; Kakagianni, E.; Khondkar, P.; Maitra, A.; Rahman, M.; Evangelopoulos, D.; McHugh, T.D.; Stapleton, P.; Malkinson, J.; Bhakta, S.; et al. Analogues of Disulfides from Allium stipitatum Demonstrate Potent Anti-tubercular Activities through Drug Efflux Pump and Biofilm Inhibition. Sci. Rep. 2018, 8, 1150. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, G.; Poeschl, R.; Zimhony, O.; Gunaratnam, M.; Moreira, J.B.C.; Neidle, S.; Evangelopoulos, D.; Bhakta, S.; Malkinson, J.P.; Boshoff, H.I.; et al. Bioactive pyridine-N-oxide disulfides from Allium stipitatum. J. Nat. Prod. 2009, 72, 360–365. [Google Scholar] [CrossRef]
- Jinbo, Z.; Mingan, W.; Wenjun, W.; Zhiqing, J.; Zhaonong, H. Insecticidal sesquiterpene pyridine alkaloids from Euonymus species. Phytochemistry 2002, 61, 699–704. [Google Scholar] [CrossRef]
- Krejčová, P.; Kučerová, P.; Stafford, G.I.; Jäger, A.K.; Kubec, R. Antiinflammatory and neurological activity of pyrithione and related sulfur-containing pyridine N-oxides from Persian shallot (Allium stipitatum). J. Ethnopharmacol. 2014, 154, 176–182. [Google Scholar] [CrossRef]
- Miron, T.; Shin, I.; Feigenblat, G.; Weiner, L.; Mirelman, D.; Wilchek, M.; Rabinkov, A. A spectrophotometric assay for allicin, alliin, and alliinase (Alliin lyase) with a chromogenic thiol: Reaction of 4-mercaptopyridine with thiosulfinates. Anal. Biochem. 2002, 307, 76–83. [Google Scholar] [CrossRef]
- Salina, E.G.; Ryabova, O.; Vocat, A.; Nikonenko, B.; Cole, S.T.; Makarov, V. New 1-hydroxy-2-thiopyridine derivatives active against both replicating and dormant Mycobacterium tuberculosis. J. Infect. Chemother. 2017, 23, 794–797. [Google Scholar] [CrossRef]
- Salina, E.; Ryabova, O.; Kaprelyants, A.; Makarov, V. New 2-thiopyridines as potential candidates for killing both actively growing and dormant Mycobacterium tuberculosis cells. Antimicrob. Agents Chemother. 2014, 58, 55–60. [Google Scholar] [CrossRef]
- Kotb, E.R.; Anwar, M.M.; Abbas, H.-A.S.; Abd El-Moez, S.I. A concise synthesis and antimicrobial activity of a novel series of naphthylpyridine-3-carbonitrile compounds. Acta Pol. Pharm. 2013, 70, 667–679. [Google Scholar]
- Alam, M.M.; Akhter, M.; Husain, A.; Marella, A.; Tanwar, O.P.; Ali, R.; Hasan, S.M.; Kumar, H.; Haider, R.; Shaquiquzzaman, M. Anti-inflammatory and antimicrobial activity of 4,5-dihydropyrimidine-5-carbonitrile derivatives: Their synthesis and spectral elucidation. Acta Pol. Pharm. 2012, 69, 1077–1085. [Google Scholar]
- Sayed, H.H.; Abbas, H.-A.S.; Morsi, E.M.H.; Amr, A.E.-G.E.; Abdelwahad, N.A.M. Antimicrobial activity of some synthesized glucopyranosyl-pyrimidine carbonitrile and fused pyrimidine systems. Acta Pharm. 2010, 60, 479–491. [Google Scholar] [CrossRef] [Green Version]
- Al-Abdullah, E.S.; Al-Turkistani, A.A.; Al-Deeb, O.A.; El-Brollosy, N.R.; Habib, E.E.; El-Emam, A.A. Pyrimidine-5-carbonitriles II: Synthesis and antimicrobial activity of novel 6-alkyl-2,4-disubstituted pyrimidine-5-carbonitriles. Drug Res. 2014, 64, 31–39. [Google Scholar] [CrossRef]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Macro- and microdilution methods of antimicrobial susceptibility testing. In Antimicrobial Susceptibility Testing Protocols; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement; Clinical Laboratory Standards Institutes: Wayne, PA, USA, 2012; Volume 32, ISBN 1562387855.
- Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; Clinical Laboratory Standards Institutes: Wayne, PA, USA, 2015; ISBN 1562388657.
- Schrödinger. QikProp, version 3.4; Schrödinger LLC: New York, NY, USA, 2013. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
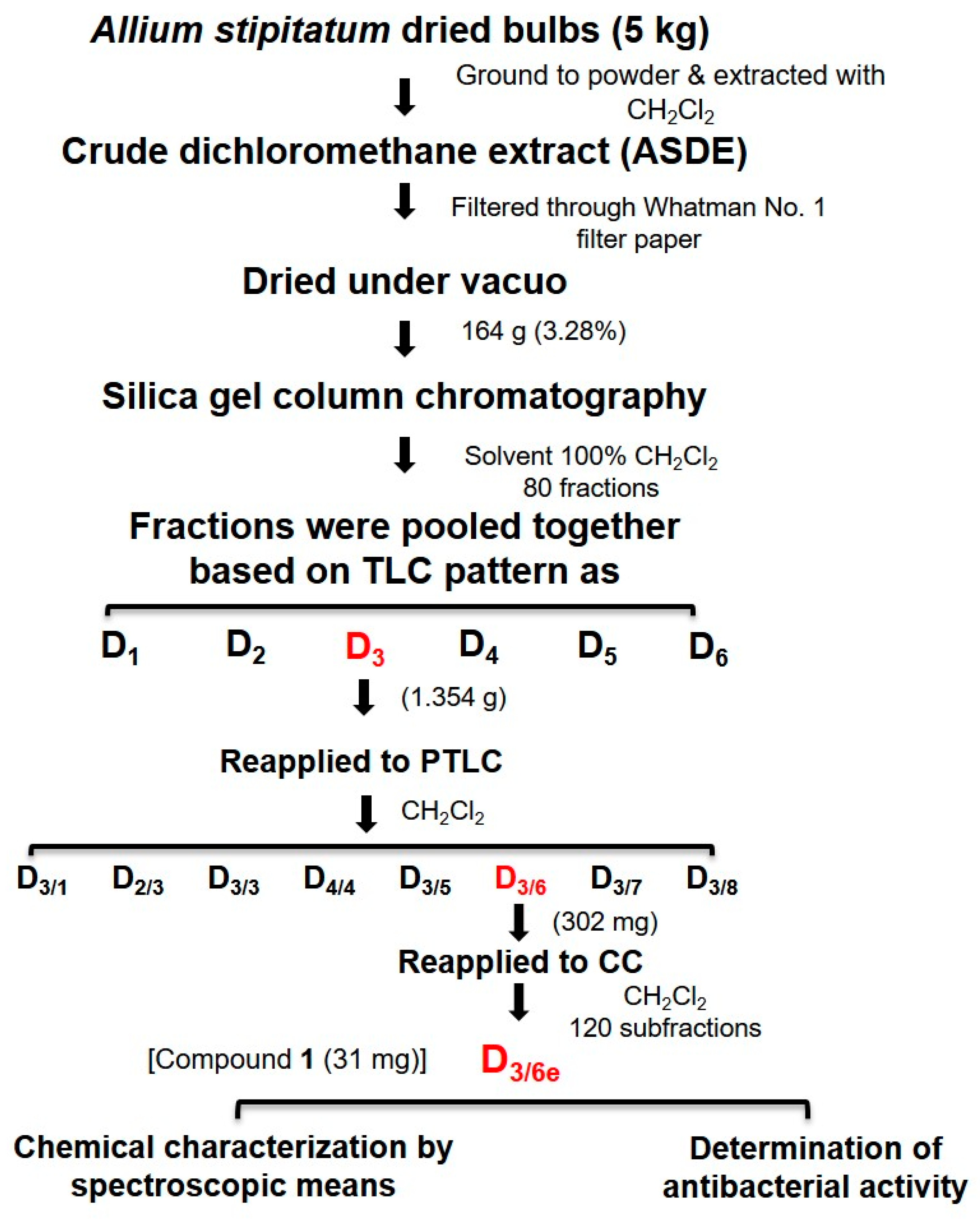
| Fraction | Weight (g) | Physical Appearance | MIC for MRSA (µg·mL−1) |
|---|---|---|---|
| D1 | 0.636 | Yellow liquid | No activity |
| D2 | 1.055 | Brown solid | 128 |
| D3 | 1.354 | Brown gum | 32 |
| D4 | 1.875 | Brown gum | 256 |
| D5 | 1.206 | Dark-yellow solid | No activity |
| D6 | 0.749 | Yellow solid | No activity |
| Fraction | Weight (g) | Physical Appearance | MIC for MRSA (µg·mL−1) |
|---|---|---|---|
| D3/1 | 0.153 | Yellow gum | No activity |
| D3/2 | 0.114 | Brown solid | No activity |
| D3/3 | 0.196 | Dark brown solid | No activity |
| D3/4 | 0.115 | Brown gum | 32 |
| D3/5 | 0.087 | Brown gum | 16 |
| D3/6 | 0.302 | Brown gum | 16 |
| D3/7 | 0.180 | White solid | 32 |
| D3/8 | 0.242 | White solid | No activity |
| Fraction | Weight (mg) | Physical Appearance | MIC for MRSA (µg·mL−1) |
|---|---|---|---|
| D3/6a | 17 | Brown gum and white solid | No activity |
| D3/6b | 35 | Brown gum and white solid | No activity |
| D3/6c | 48 | Brown gum and yellow solid | No activity |
| D3/6d | 65 | Yellow liquid | 32 |
| D3/6e | 31 | Yellow liquid | 4 |
| D3/6f | 28 | Pale-yellow solid | No activity |
| Position | δH (J in Hz) | δC, Type | DEPT 90 | DEPT 135 | COSY | NOESY | HMBC | HSQC |
|---|---|---|---|---|---|---|---|---|
| 2 | 162.18, C | 4,6 | ||||||
| 3 | 107.87, C | 4,5,6 | ||||||
| 4 | 7.89, 1H, dd (1.5, 7.5 Hz) | 141.02, CH | 141.04, CH | 141.04, CH | 5,6 | 5 | 5,6 | 4 |
| 5 | 7.26, 1H, dd (4.5, 7.5 Hz) | 120.58, CH | 120.58, CH | 120.58, CH | 4,6 | 4,6 | 4,6 | 5 |
| 6 | 8.77, 1H, dd (1.5, 4.5 Hz) | 152.87, CH | 152.88, CH | 152.89, CH | 4,5 | 5 | 4,5 | 6 |
| –CN | 114.96, C | 4,5,6 | ||||||
| –CH3 | 2.60, 3H, s | 23.23, CH3 | 23.23 | 7 | 6 | –CH3 |
| Microorganisms | Antimicrobial Activity | |||
|---|---|---|---|---|
| 2-Medpy-3-CN | Antibiotic (µg·mL−1) | DMSO (10%) (20 µL Per Disk) | ||
| MIC (µg·mL−1) a | MBC (µg·mL−1) b | |||
| Bacteria | ||||
| Acinetobacter baumannii | 4 | 8 | ≤4 (IMP) | - |
| Acinetobacter iwoffii | 0.5 | 1 | ≤4 (IMP) | - |
| Escherichia coli | 32 | 64 | 0.5–2 (TET) | - |
| Methicillin-resistant Staphylococcus aureus | 4 | 8 | 0.5–2 (VAN) | - |
| Methicillin-sensitive S. aureus | 4 | 16 | ≤8 (VAN) | - |
| Pseudomonas aeruginosa | >64 | >64 | >64 (TMP) | - |
| Salmonella typhi | >64 | >64 | <1 (CIP) | - |
| Shigella dysenteriae | >64 | >64 | ≤8/4 (AM/CL) | - |
| Stenotrophomonas maltophilia | 32 | >64 | ≥2–38 (TP/SX) | - |
| Yeast | ||||
| Candida albicans | 0.5 | 1 | 0.12 (FLU) c | - |
| Candida glabrata | 0.25 | 1 | 16 (FLU) c | - |
| Candida krusei | 1 | 4 | 1 (FLU) c | - |
| Candida parapsilosis | 0.5 | 2 | 0.5 (FLU) c | - |
| Candida tropicalis | 2 | 4 | 32 (FLU) c | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunanidhi, A.; Ghaznavi-Rad, E.; Jeevajothi Nathan, J.; Joseph, N.; Chigurupati, S.; Mohd Fauzi, F.; Pichika, M.R.; Hamat, R.A.; Lung, L.T.T.; van Belkum, A.; et al. Bioactive 2-(Methyldithio)Pyridine-3-Carbonitrile from Persian Shallot (Allium stipitatum Regel.) Exerts Broad-Spectrum Antimicrobial Activity. Molecules 2019, 24, 1003. https://doi.org/10.3390/molecules24061003
Karunanidhi A, Ghaznavi-Rad E, Jeevajothi Nathan J, Joseph N, Chigurupati S, Mohd Fauzi F, Pichika MR, Hamat RA, Lung LTT, van Belkum A, et al. Bioactive 2-(Methyldithio)Pyridine-3-Carbonitrile from Persian Shallot (Allium stipitatum Regel.) Exerts Broad-Spectrum Antimicrobial Activity. Molecules. 2019; 24(6):1003. https://doi.org/10.3390/molecules24061003
Chicago/Turabian StyleKarunanidhi, Arunkumar, Ehsanollah Ghaznavi-Rad, Jayakayatri Jeevajothi Nathan, Narcisse Joseph, Sridevi Chigurupati, Fazlin Mohd Fauzi, Mallikarjuna Rao Pichika, Rukman Awang Hamat, Leslie Than Thian Lung, Alex van Belkum, and et al. 2019. "Bioactive 2-(Methyldithio)Pyridine-3-Carbonitrile from Persian Shallot (Allium stipitatum Regel.) Exerts Broad-Spectrum Antimicrobial Activity" Molecules 24, no. 6: 1003. https://doi.org/10.3390/molecules24061003