Optimization and Formulation of Fucoxanthin-Loaded Microsphere (F-LM) Using Response Surface Methodology (RSM) and Analysis of Its Fucoxanthin Release Profile
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Microencapsulation Component by RSM
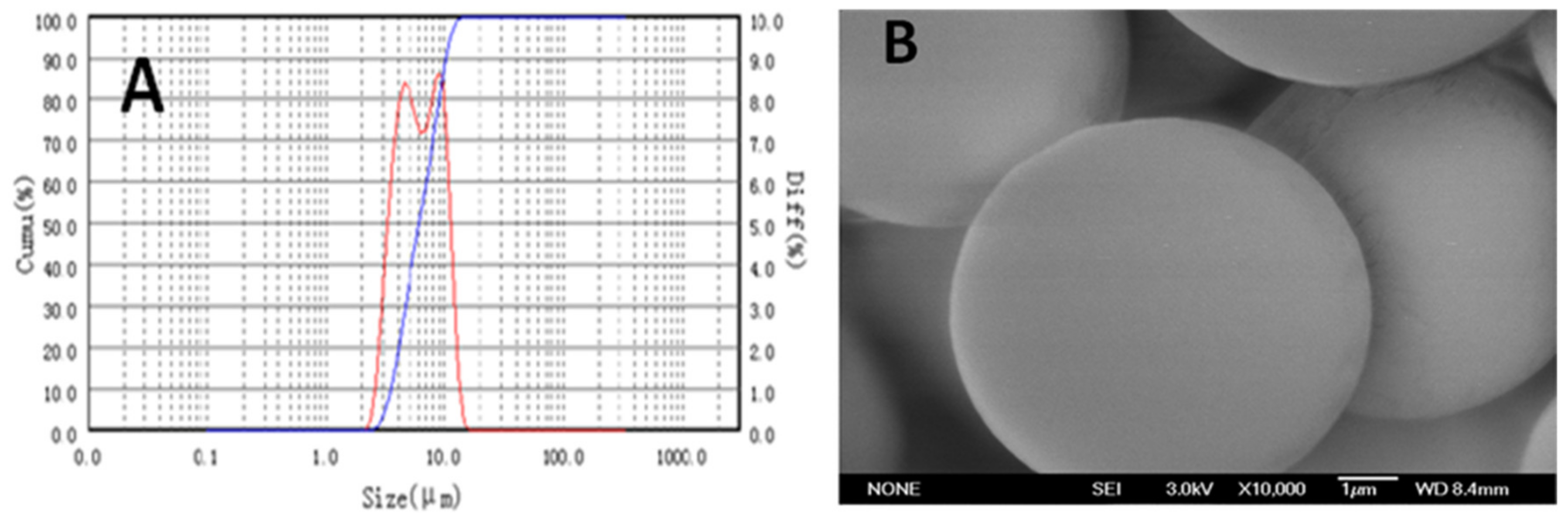
2.2. Particle Size (PS), Size Distribution, and External Morphology of F-LM
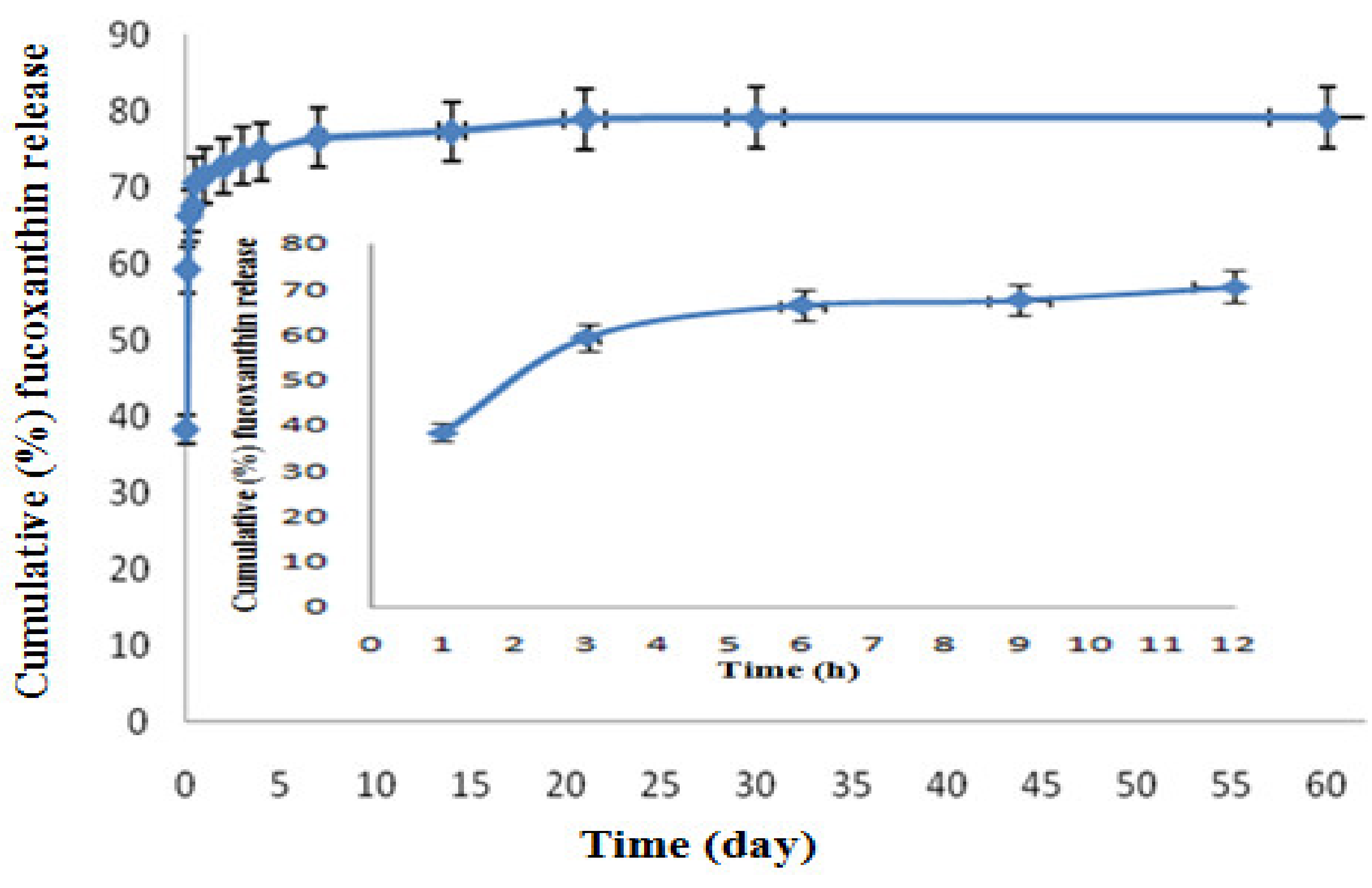
2.3. In Vitro Fucoxanthin Release Profile of F-LM
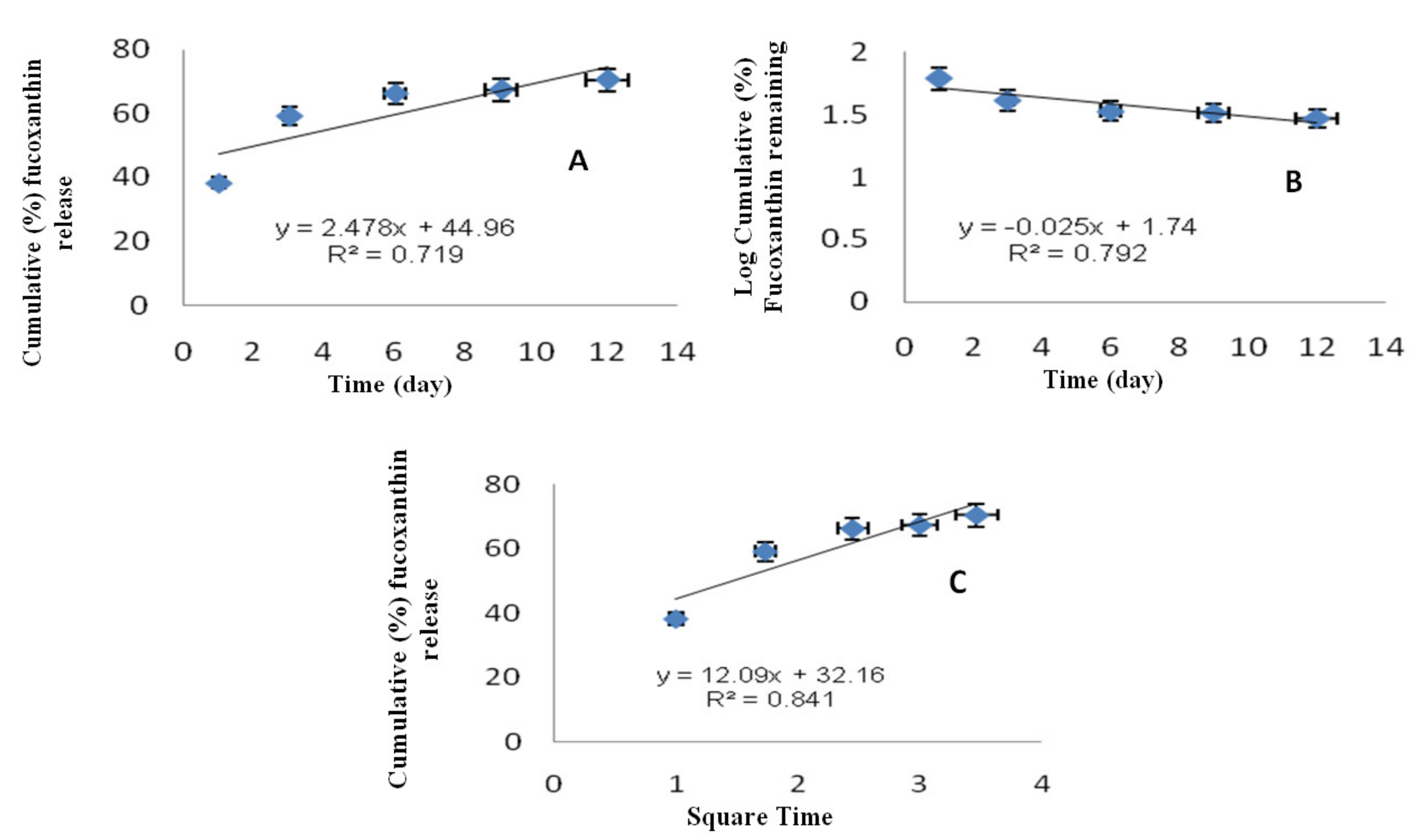
2.4. Release Kinetics of F-LM
2.5. Degradation Study of F-LM
3. Materials and Methods
3.1. Materials
3.2. Optimization of Medium Component by RSM
3.3. Fabrication of F-LM
3.4. EE of F-LM
3.5. Particle Size Analysis of F-LM
3.6. External Morphology of F-LM
3.7. In Vitro Fucoxanthin Release Profile
3.8. Evaluation of Release Kinetics
3.9. Degradation Study of F-LM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- AppaRao, B.; Shivalingam, M.R.; Reddy, Y.K.; Sunitha, N.; Jyothibasu, T.; Shyam, T. Design and evaluation of sustained release microcapsules containing diclofenac sodium. Int. J. Pharm. Biomed. Res. 2010, 1, 90–93. [Google Scholar]
- Kumar, S.R.; Hasokawa, M.; Miyashita, K. Fucoxanthin: A Marine Carotenoid Exerting Anti-Cancer Effects by Affecting Multiple Mechanism. Mar. Drugs 2013, 11, 5130–5147. [Google Scholar] [CrossRef] [PubMed]
- Satomi, Y. Antitumor and Cancer-preventative Function of Fucoxanthin: A Marine Carotenoid. Anticancer Res. 2017, 37, 1557–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tronino, D.; Offerta, A.; Ostacolo, C.; Russo, R.; De Caro, C.; Calignano, A.; Puglia, C.; Blasi, P. Nanoparticles prolong N-palmitoylethanolamide anti-inflammatory and analgesic effects in vivo. Colloid Surf. B 2016, 141, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Gaylen, M.Z. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J. Control. Release 2001, 72, 203–215. [Google Scholar]
- Lopes-Costa, E.; Abreu, M.; Gargiulo, D.; Rocha, E.; Ramos, A.A. Anticancer effects of seaweed compounds fucoxanthin and phloroglucinol, alone and in combination with 5-fluorouracil in colon cells. J. Toxicol. Environ. Health 2017, 80, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Hwang, S.J.; Park, J.B.; Park, H.J. Preparation and characterization of drug-loaded polymethacrylate microspheres by an emulsion solvent evaporation method. J. Microencapsul. 2002, 19, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Palaniyappan, M.; Vijayagopal, V.; Viswanathan, R.; Viruthagiri, T. Statistical optimization of substrate, carbon and nitrogen source by response surface methodology for pectinase production using Aspergillus fumigatus MTCC 870 in submerged fermentation. Afr. J. Biotechnol. 2009, 8, 6355–6363. [Google Scholar]
- Zhong, K.; Wang, Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohydr. Polym. 2010, 80, 19–25. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, S.W.; Tang, J.; Gu, X. Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology. Food Chem. 2007, 105, 1599–1605. [Google Scholar] [CrossRef]
- Torres-Lugo, M.; Peppas, N.A. Transmucosal delivery systems for calcitonin: A review. Biomaterials 2000, 21, 1191–1196. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Takenaga, M.; Kitagawa, A.; Ogawa, Y.; Mizushima, Y.; Igarashi, R. Insulin-loaded biodegradable PLGA microcapsules: Initial burst release controlled by hydrophilic additives. J. Control. Release 2002, 81, 235–249. [Google Scholar] [CrossRef]
- Maurus, P.B.; Kaeding, C.C. Bioabsorbable implant material review. Oper. Tech. Sports Med. 2004, 12, 158–160. [Google Scholar] [CrossRef]
- Dhana Lekshmi, U.M.; Poovi, G.; Kishore, N.; Reddy, P.N. In vitro characterization and in vivo toxicity study of repaglinide loaded poly (methyl methacrylate) nanoparticles. Int. J. Pharm. 2010, 396, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Lakshmana Prabu, S.; Shirwaikar, A.A.; Shirwaikar, A.; Kumar, A. Formulation and evaluation of sustained release microspheres of rosin containing aceclofenac. Ars Pharm. 2009, 50, 1–12. [Google Scholar]
- Dhakar, R.C. From Formulation Variables To Drug Entrapment Efficiency Of Microspheres: A Technical Review. J. Drug Deliv. Ther. 2012, 2, 128–133. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Gowda, D.V.; Rajesh, N.; Moin, A.; Shivakumar, H.G. Controlled Release Behaviour of Nifedipine from the Pellets of Gelucire/Microcrystalline Cellulose Blends. Int. J. Pharm. Tech. Res. 2010, 2, 1215–1226. [Google Scholar]
- Kushwaha, P.; Fareed, S.; Nanda, S.; Mishra, A. Design & fabrication of tramadol HCL loaded multiparticulate colon targeted drug delivery system. J. Chem. Pharm. Res. 2011, 3, 584–595. [Google Scholar]
- Ruan, G.; Feng, S.-S. Preparation and characterization of poly (lactic acid)-poly (ethylene glycol)-poly (lactic acid) (PLA-PEG-PLA) microspheres for controlled release of paclitaxel. Biomaterials 2003, 24, 5037–5044. [Google Scholar] [CrossRef]
- Wei, G.; Pettway, G.J.; McCauley, L.K.; Ma, P.X. The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres. Biomaterials 2004, 25, 345–352. [Google Scholar] [CrossRef]
- Li, P.; Xu, L.; Mou, Y.; Shan, T.; Mao, Z.; Lu, S.; Peng, Y.; Zhou, L. Medium optimization for exopolysaccharide production in liquid culture of endophytic fungus Berkleasmium sp. Dzf12. Int. J. Mol. Sci. 2012, 13, 11411–11426. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.T.; Baran, E.T.; Pinho, E.D.; Reis, R.L.; Neves, N.M. Performance of biodegradable microcapsules of poly(butylene succinate), poly(butylene succinate-co-adipate) and poly(butylene terephthalate-co-adipate) as drug encapsulation systems. Colloid Surf. B 2011, 84, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Gao, C.; Shi, Y.; Shen, J. Preparation of porous polylactide microspheres by emulsion-solvent evaporation based on solution induced phase separation. Polym. Adv. Technol. 2005, 16, 622–627. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Chung, T.S.; Ng, N.P. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials 2001, 22, 231–241. [Google Scholar] [CrossRef]
- Tsai, M.C.S. Biodegradable Paclitaxel-Loaded Plga Microspheres for Regional Treatment of Peritoneal Cancers. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2003. [Google Scholar]
- O’Hara, P.; Hickey, A.J. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: Manufacture and characterization. Pharm. Res. 2000, 17, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.F. Formulation and Evaluation of Polyester Microspheres by Solvent-Evaporation Method. Ph.D. Thesis, University of Strathclyde, Glasgow, Scotland, 2008. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Chia, H.H.; Chung, T.S. Effect of preparation temperature on the characteristics and release profiles of PLGA microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. J. Control. Release 2000, 69, 81–96. [Google Scholar] [CrossRef]
- Zhang, J.X.; Zhu, K.J. An improvement of double emulsion technique for preparing bovine serum albumin-loaded PLGA microspheres. J. Microencapsul. 2004, 21, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Igartua, M.; Hernández, R.M.; Esquisabel, A.; Gascón, A.R.; Calvo, M.B.; Pedraz, J.L. Enhanced immune response after subcutaneous and oral immunization with biodegradable PLGA microspheres. J. Control. Release 1998, 56, 63–73. [Google Scholar] [CrossRef]
- Lewis, D.H. Controlled Release of Bioactive Agents from Lactide/Glycolide Polymers. In Biodegradable Polymers as Drug Delivery Systems; Chasin, M., Langer, R., Eds.; Academic Press: New York, NY, USA, 1990; pp. 1–41. [Google Scholar]
- Mao, S.; Shi, Y.; Li, L.; Xu, J.; Schaper, A.; Kissel, T. Effects of process and formulation parameters on characteristics and internal morphology of poly(d,l-lactide-co-glycolide) microspheres formed by the solvent evaporation method. Eur. J. Pharm. Biopharm. 2008, 68, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.; Baek, N.; Park, K. Microencapsulation methods for delivery of protein drugs. Biotechnol. Bioprocess Eng. 2001, 6, 213–230. [Google Scholar] [CrossRef]
- Karatas, A.; Sonakin, O.; Kiliçarslan, M.; Baykara, T. Effects of Stirring Rate and Drug: Polymer Ratio on the Characteristics of Levobunolol HCL Loaded Poly (ε-Caprolactone) Microparticles. Turkish J. Pharm. Sci. 2010, 7, 225–236. [Google Scholar]
- Ghaderi, R.; Sturesson, C.; Carlfors, J. Effect of preparative parameters on the characteristics of poly (d,l-lactide-co-glycolide) microspheres made by the double emulsion method. Int. J. Pharm. 1996, 141, 205–216. [Google Scholar] [CrossRef]
- Ehtezazi, T.; Washington, C.; Melia, C.D. Determination of the internal morphology of poly(d,l-lactide) microspheres using stereological methods. J. Control. Release 1999, 57, 301–314. [Google Scholar] [CrossRef]
- Rahman, N.A. Development and Characterization of Double Walled Microspheres from poly(l-lactic) and poly(d-l-Lactide-co-glycolide) Blends. Ph.D. Dissertation, UMI No. 3087334. University of Rhode Island, Kingston, RI, USA, 2003; pp. 1–12. [Google Scholar]
- Emami, J.; Hamishehkar, H.; Najafabadi, A.R.; Gilani, K.; Minaiyan, M.; Mahdavi, H.; Nokhodchi, A. A novel approach to prepare insulin-loaded poly(lactic-co-glycolic acid) microcapsules and the protein stability study. J. Pharm. Sci. 2009, 98, 1712–1731. [Google Scholar] [CrossRef] [PubMed]
- Khang, G.; Seo, S.A.; Choi, H.S.; Rhee, J.M.; Lee, H.B. Evaluation of in vitro release profiles of fentanyl-loaded PLGA oligomer microspheres. Macromol. Res. 2002, 10, 246–252. [Google Scholar] [CrossRef]
- Vidyavathi, M.; Ramana, N.V. In vitro and In vivo studies on controlled release microspheres of Simvastatin. In Proceedings of the International Conference on Biology, Environment and Chemistry, Singapore, 15 July 2011. [Google Scholar]
- Middleton, J.C.; Tipton, A.J. Synthetic Biodegradable Polymers as Medical Devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Noviendri, D.; Jaswir, I.; Salleh, H.M.; Taher, M.; Miyashita, K.; Ramli, N. Fucoxanthin extraction and fatty acid analysis of Sargassum binderi and S. duplicatum. J. Med. Plants Res. 2011, 5, 2405–2412. [Google Scholar]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Miyashita, K. Fucoxanthin Extractions of Brown Seaweeds and Analysis of Their Lipid Fraction in Methanol. Food Sci. Technol. Res. 2012, 18, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Taher, M.; Miyashita, K.; Ramli, N. Analysis of fucoxanthin content and purification of all-trans-fucoxanthin from Turbinaria turbinata and Sargassum plagyophyllum by SiO2 open column chromatography and reversed phase-HPLC. J. Liquid Chrom. Rel. Tech. 2013, 36, 1340–1354. [Google Scholar]
- Averineni, R.L.; Shavi, G.V.; Gurram, A.K.; Deshpande, P.B.; Arumugam, K.; Maliyakkal, N.; Meka, S.R.; Nayanabhirama, U. PLGA 50:50 nanoparticles of paclitaxel: Development, in vitro anti-tumor activity in BT-549 cells and in vivo evaluation. Bull. Mater. Sci. 2012, 35, 319–326. [Google Scholar] [CrossRef]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the Major Antioxidant in Hijikia fusiformis, a Common Edible Seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Muntari, B.; Amid, A.; Mel, M.; Jami, M.S.; Salleh, H.M. Recombinant bromelain production in Escherichia coli: Process optimization in shake flask culture by response surface methodology. AMB Express 2012, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.N.; Alam, M.Z.; Muyibi, S.A.; Jamal, P.; Al-Mamun, A. Improvement of production of citric acid from oil palm empty fruit bunches: Optimization of media by statistical experimental designs. Bioresour. Technol. 2009, 100, 3113–3120. [Google Scholar] [CrossRef] [PubMed]
- Salihu, A.; Alam, M.Z.; Abdulkarim, M.I.; Salleh, H.M. Optimization of lipase production by Candida cylindracea in palm oil mill effluent based medium using statistical experimental design. J. Mol. Catal. B Enzym. 2011, 69, 66–73. [Google Scholar] [CrossRef]
- Mohamed, F.; van der Walle, C.F. PLGA microcapsules with novel dimpled surfaces for pulmonary delivery of DNA. Int. J. Pharm. 2006, 311, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Abdalmonemdoolaanea, A.F.; Awang, M.; Mohamed, F.; Ismail, A.F.H. High initial burst release of gentamicin formulated as PLGA microspheres implant for treating orthopaedic infection. Int. J. Pharm. Pharm. Sci. 2012, 4, 685–691. [Google Scholar]
- Arkendu, C.; Benoy, B.B.; Lait, K. Formulation, in vitro evaluation and stability of Prolong Release anti-HIV Bioadhesive Microencapsulated Vaginal Gel. J. Pharm. Res. 2010, 1, 28–37. [Google Scholar]
- Alfatama, M.; Ahmad, K.; Mohamed, F. Microencapsulation of cassia Alata: Fabrication and characterization. Int. J. Pharm. Pharm. Sci. 2012, 4, 219–224. [Google Scholar]
- Mori, K.; Ooi, T.; Hiraoka, M.; Oka, N.; Hamada, H.; Tamura, M.; Kusumi, T. Fucoxanthin and Its Metabolites in Edible Brown Algae Cultivated in Deep Seawater. Mar. Drugs 2004, 2, 63–72. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Takahashi, N.; Kawada, T.; Miyashita, K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Med. 2006, 18, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Sashima, T.; Hosokawa, M.; Miyashita, K. Comparative evaluation of growth inhibitory effect of stereoisomers of fucoxanthin in human cancer cell lines. J. Funct. Foods 2009, 1, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y. pH-sensitive and Targeted PLGA-Based Drug Delivery to Colorectal Cancer. Ph.D. Thesis, Deakin University, Victoria, Australia, 2012. [Google Scholar]
- Siepmann, J.; Faisant, N.; Akiki, J.; Richard, J.; Benoit, J.P. Effect of the size of biodegradable microparticles on drug release: Experiment and theory. J. Control. Release 2004, 96, 123–134. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds fucoxanthin are available from the authors. |
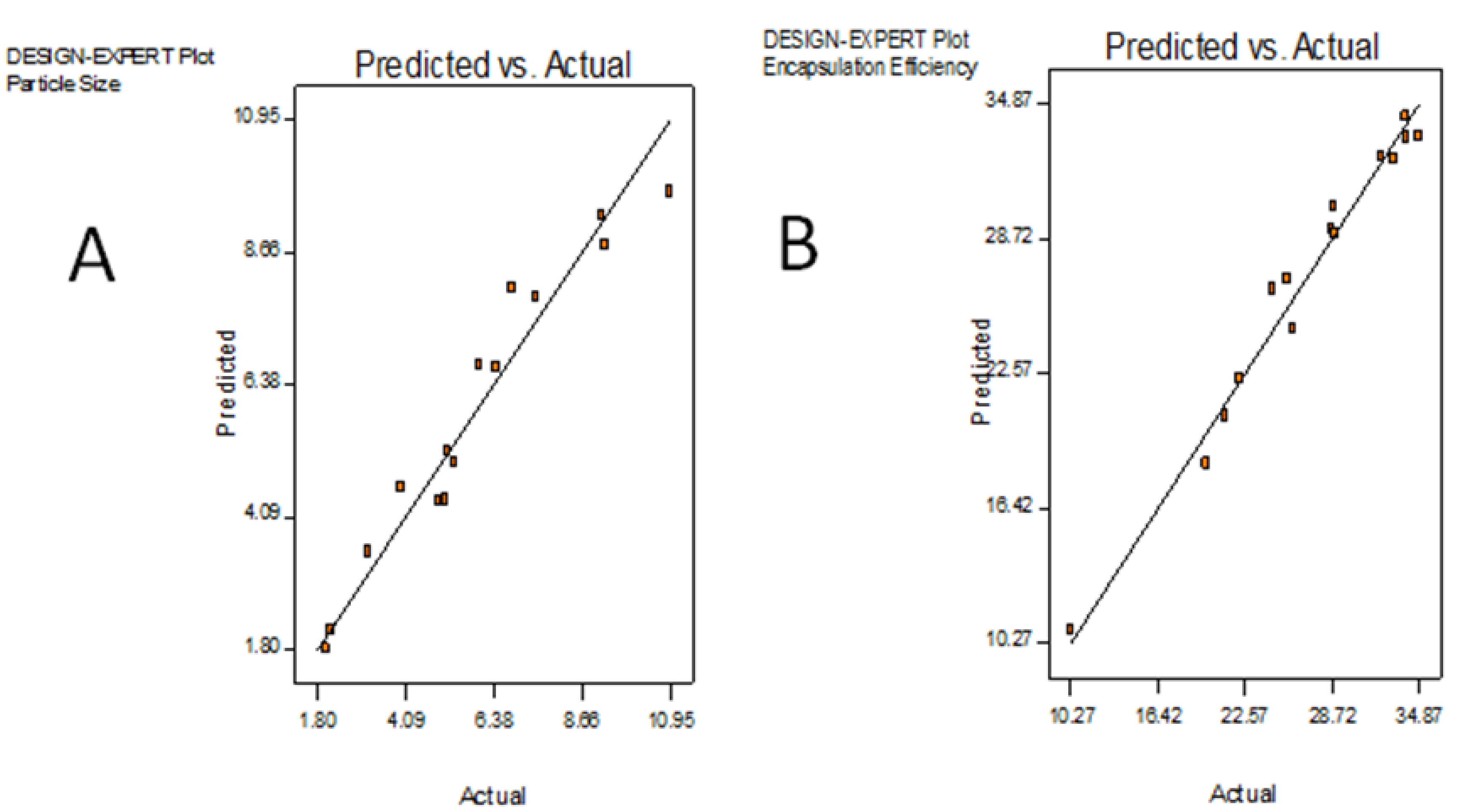
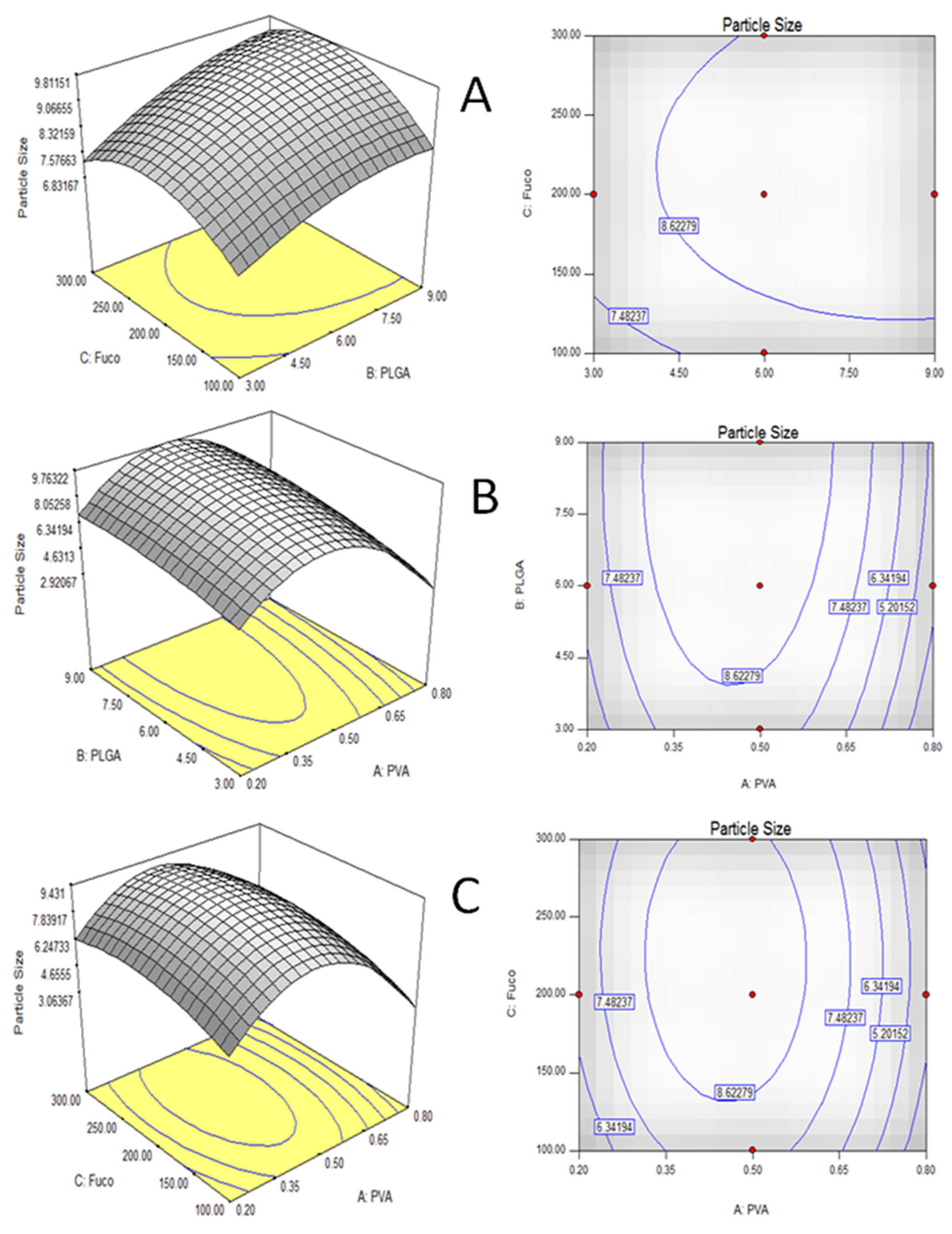
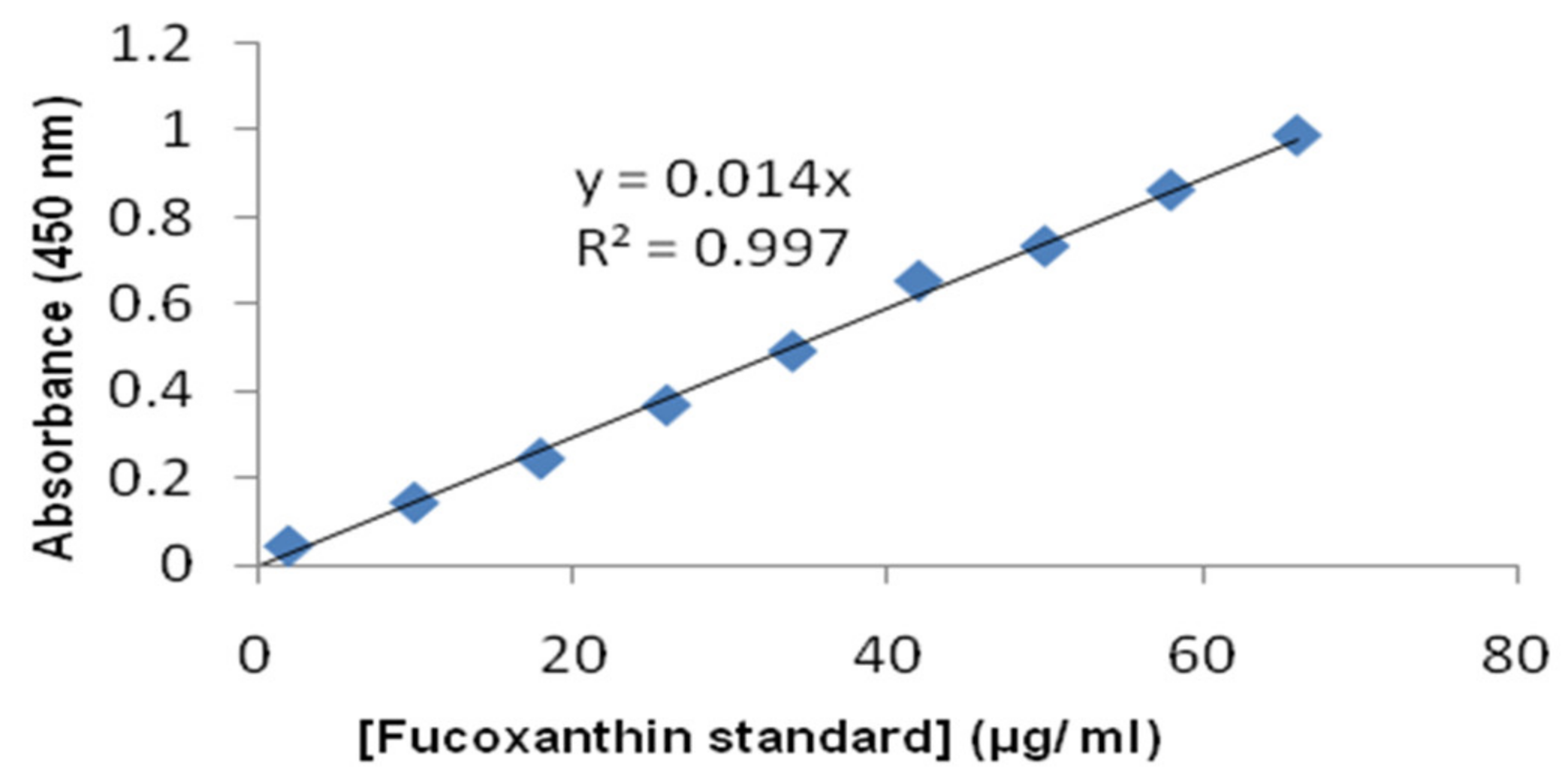

| Run | PVA (% w/v) | PLGA (% w/v) | Fuco (µg/mL) | PS (µm) | EE (%) | ||
|---|---|---|---|---|---|---|---|
| Ex. | Pr.* | Ex. | Pr.** | ||||
| 1 | 0.80 (+1) | 6.00 (0) | 200.00 (0) | 5.12 | 4.38 | 28.91 | 28.93 |
| 2 | 0.50 (0) | 6.00 (0) | 100.00 (−1) | 7.48 | 7.90 | 22.25 | 22.35 |
| 3 | 0.20 (−1) | 9.00 (+1) | 300.00 (+1) | 6.45 | 6.67 | 33.97 | 33.33 |
| 4 | 0.20 (−1) | 3.00 (−1) | 100.00 (−1) | 4.95 | 4.34 | 19.85 | 18.43 |
| 5 | 0.20 (−1) | 9.00 (+1) | 100.00 (−1) | 5.18 | 5.22 | 25.57 | 26.85 |
| 6 | 0.80 (+1) | 9.00 (+1) | 100.00 (−1) | 3.12 | 3.48 | 21.15 | 20.64 |
| 7 | 0.50 (0) | 3.00 (−1) | 200.00 (0) | 6.85 | 8.04 | 24.56 | 26.40 |
| 8 | 0.20 (−1) | 6.00 (0) | 200.00 (0) | 6.01 | 6.69 | 33.94 | 34.30 |
| 9 | 0.20 (−1) | 3.00 (−1) | 300.00 (+1) | 5.36 | 5.02 | 28.72 | 29.13 |
| 10 | 0.80 (+1) | 9.00 (+1) | 300.00 (+1) | 3.97 | 4.59 | 28.87 | 30.19 |
| 11 | 0.50 (0) | 9.00 (+1) | 200.00 (0) | 10.95 | 9.71 | 34.87 | 33.41 |
| 12 | 0.50 (0) | 6.00 (0) | 200.00 (0) | 9.18 | 9.30 | 33.09 | 32.34 |
| 13 | 0.80 (+1) | 3.00 (−1) | 300.00 (+1) | 2.16 | 2.13 | 25.97 | 24.59 |
| 14 | 0.50 (0) | 6.00 (0) | 300.00 (+) | 9.26 | 8.79 | 32.19 | 32.47 |
| 15 | 0.80 (+1) | 3.00 (−1) | 100.00 (−1) | 2.01 | 1.80 | 10.27 | 10.82 |
| Source | Sum of Square | F-Value | p-Value |
|---|---|---|---|
| Model | 88.67 | 8.99 | 0.0131 |
| PVA, A | 13.39 | 12.22 | 0.0174 |
| PLGA, B | 6.96 | 6.35 | 0.0532 |
| Fuco, C | 1.99 | 1.82 | 0.2357 |
| A2 | 36.34 | 33.17 | 0.0022 |
| B2 | 0.46 | 0.42 | 0.5442 |
| C2 | 2.34 | 2.14 | 0.2036 |
| AB | 0.32 | 0.29 | 0.6121 |
| AC | 0.058 | 0.054 | 0.8274 |
| BC | 0.30 | 0.28 | 0.6208 |
| Source | Sum of Square | F-value | p-value |
|---|---|---|---|
| Model | 614.88 | 23.17 | 0.0015 |
| PVA, A | 72.25 | 24.51 | 0.0043 |
| PLGA, B | 122.92 | 41.69 | 0.0013 |
| Fuco, C | 256.34 | 86.95 | 0.0002 |
| A2 | 1.35 | 0.46 | 0.5287 |
| B2 | 15.24 | 5.71 | 0.0721 |
| C2 | 62.48 | 21.19 | 0.0058 |
| AB | 0.99 | 0.33 | 0.5879 |
| AC | 4.73 | 1.60 | 0.2612 |
| BC | 8.93 | 3.03 | 0.1424 |
| Equation | Zero Order | First Order | Higuchi |
|---|---|---|---|
| R2 | 0.719 | 0.792 | 0.841 |
| Model | Plot | Equation |
|---|---|---|
| Zero order | Qt vs. t | Qt = K0t |
| First order | ln (Q0 − Qt) vs. t | ln Qt = ln Q0 − K1t |
| Higuchi | Qt vs. t1/2 | Qt = Kht1/2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaswir, I.; Noviendri, D.; Taher, M.; Mohamed, F.; Octavianti, F.; Lestari, W.; Mukti, A.G.; Nirwandar, S.; Hamad Almansori, B.B. Optimization and Formulation of Fucoxanthin-Loaded Microsphere (F-LM) Using Response Surface Methodology (RSM) and Analysis of Its Fucoxanthin Release Profile. Molecules 2019, 24, 947. https://doi.org/10.3390/molecules24050947
Jaswir I, Noviendri D, Taher M, Mohamed F, Octavianti F, Lestari W, Mukti AG, Nirwandar S, Hamad Almansori BB. Optimization and Formulation of Fucoxanthin-Loaded Microsphere (F-LM) Using Response Surface Methodology (RSM) and Analysis of Its Fucoxanthin Release Profile. Molecules. 2019; 24(5):947. https://doi.org/10.3390/molecules24050947
Chicago/Turabian StyleJaswir, Irwandi, Dedi Noviendri, Muhammad Taher, Farahidah Mohamed, Fitri Octavianti, Widya Lestari, Ali Ghufron Mukti, Sapta Nirwandar, and Bubaker B. Hamad Almansori. 2019. "Optimization and Formulation of Fucoxanthin-Loaded Microsphere (F-LM) Using Response Surface Methodology (RSM) and Analysis of Its Fucoxanthin Release Profile" Molecules 24, no. 5: 947. https://doi.org/10.3390/molecules24050947






