Study on Antidepressant Activity of Pseudo-Ginsenoside HQ on Depression-Like Behavior in Mice
Abstract
:1. Introduction
2. Results
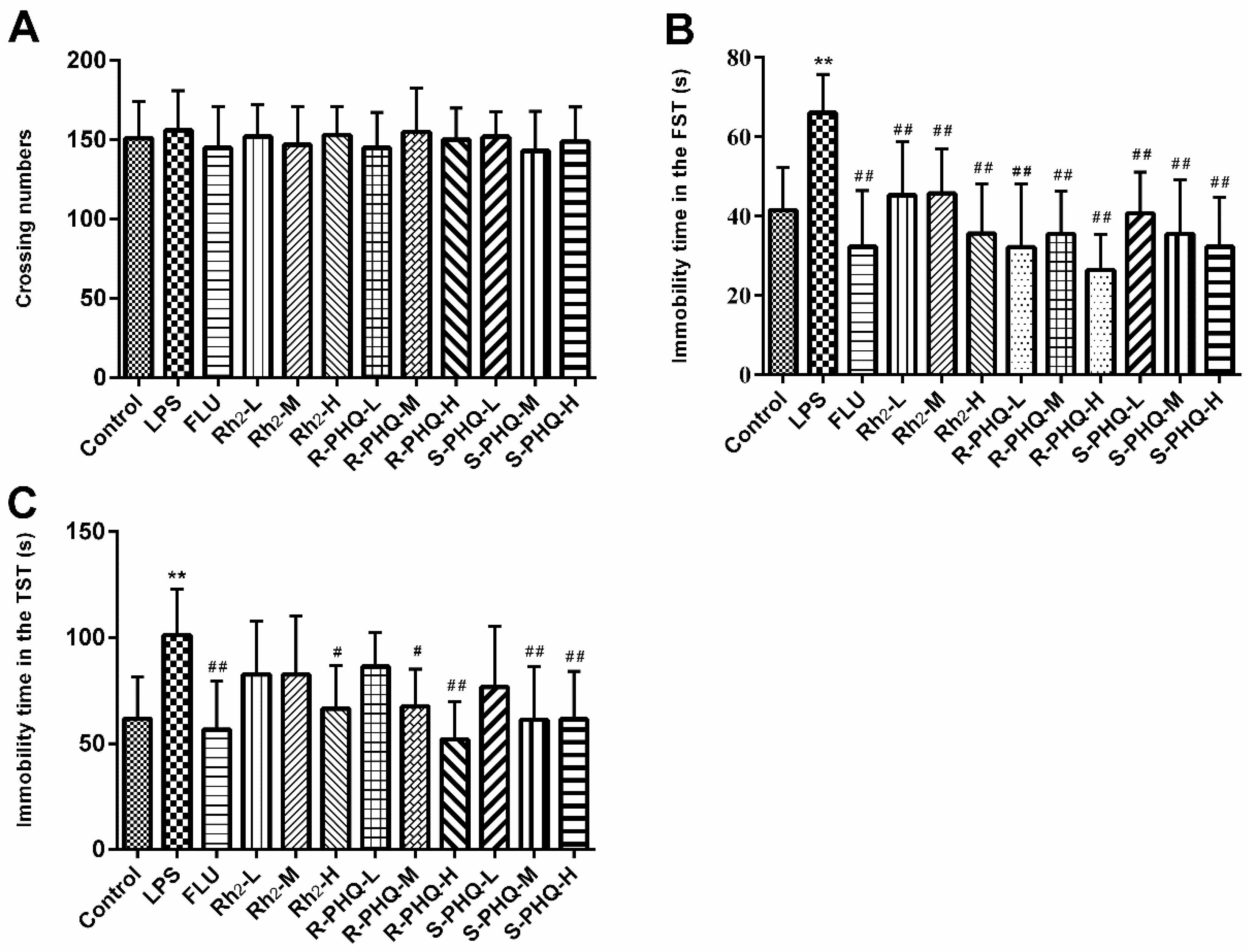
2.1. Open-Field Test
2.2. Effects of Rh2, R-PHQ, and S-PHQ on LPS-Induced Depressive-Like Behavior
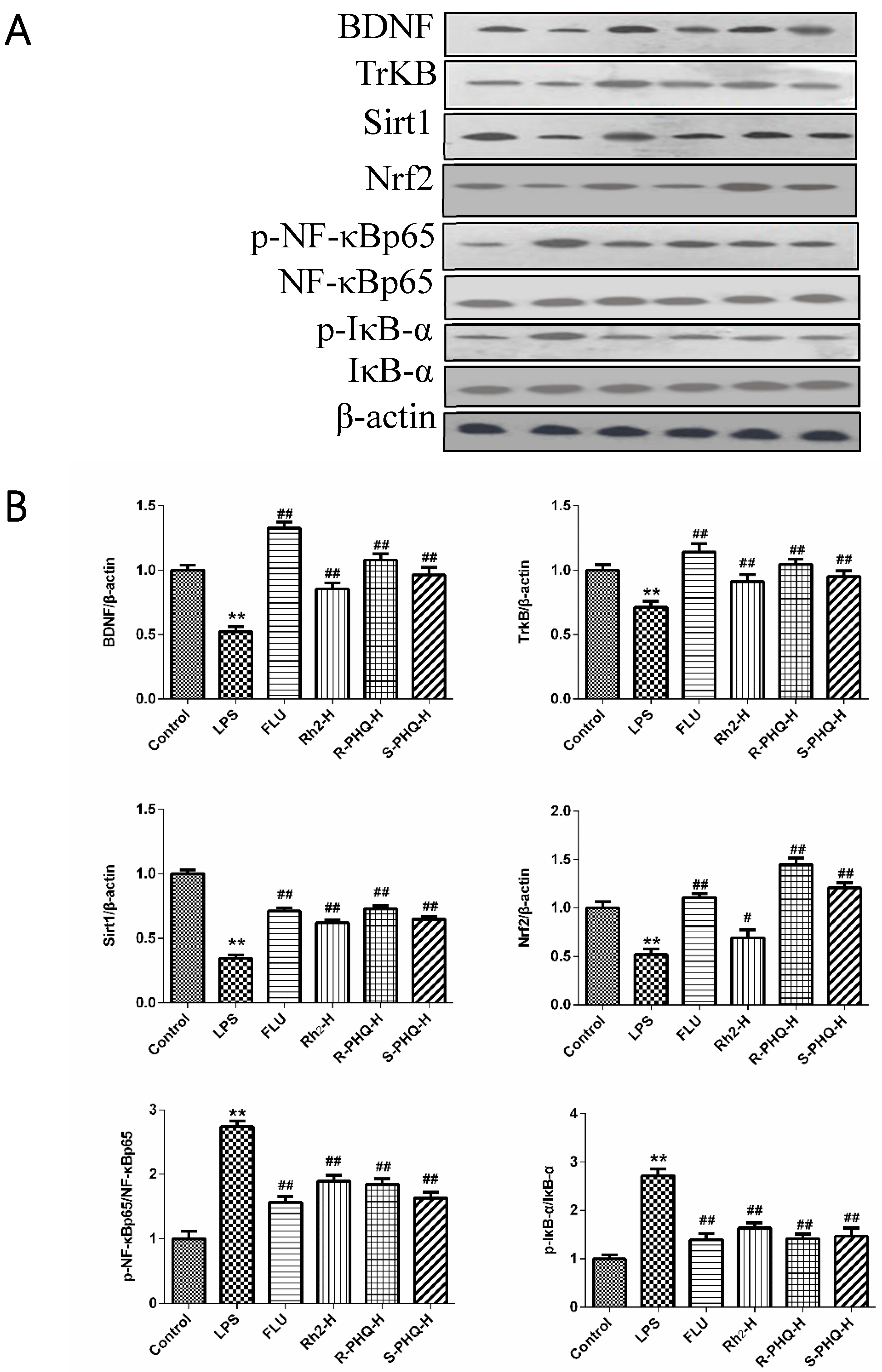
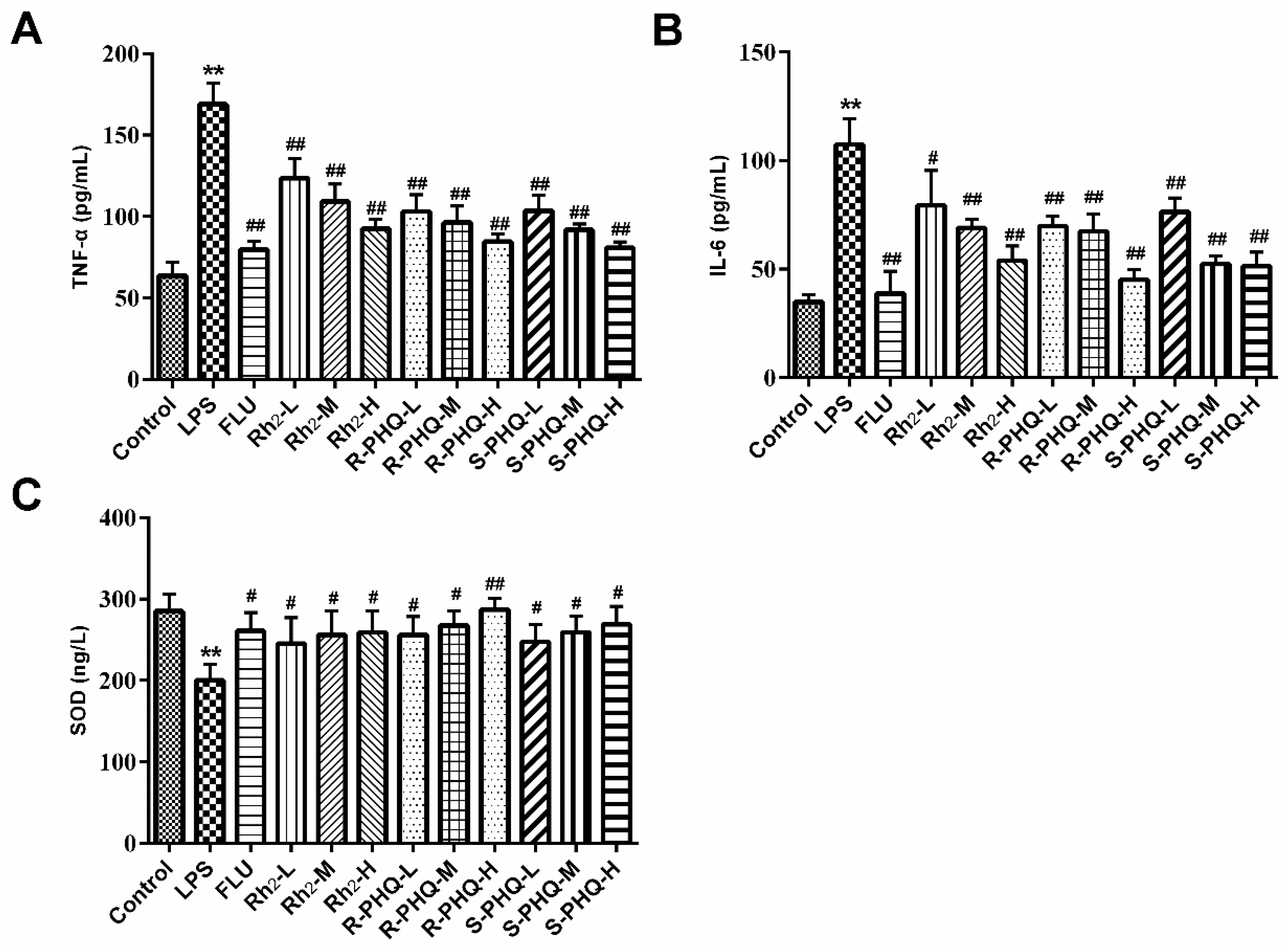
2.3. Rh2, R-PHQ, and S-PHQ Mediate LPS-Induced Inflammatory Reaction
2.4. Effects of Rh2, R-PHQ, and S-PHQ on LPS-Induced Oxidative Stress in the Hippocampus
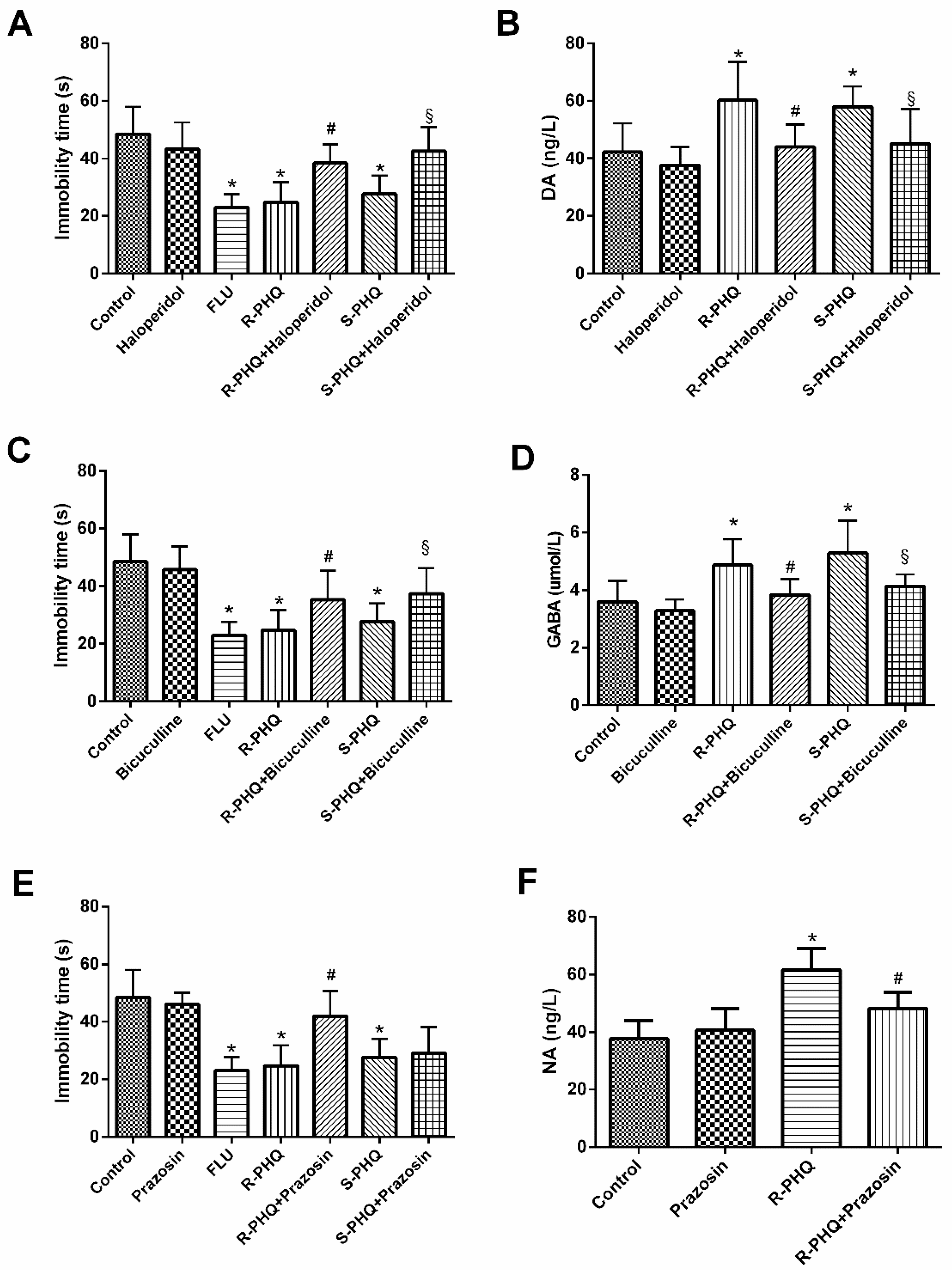
2.5. Role of the Different Facets of the Central Nervous System including Dopaminergic (DA), γ-Aminobutyric Acid (GABA)ergic, and Noradrenergic (NA) Systems in the Antidepressant-Like Effect of R-PHQ and S-PHQ in the FST
2.5.1. Involvement of the Dopaminergic System in the Antidepressant-Like Effect of R-PHQ and S-PHQ in the FST
2.5.2. Involvement of the GABAergic System in the Antidepressant-Like Effect of R-PHQ and S-PHQ in the FST
2.5.3. Involvement of the Noradrenergic System in the Antidepressant-Like Effect of R-PHQ and S-PHQ in the FST
3. Discussion
4. Materials and Methods
4.1. Preparation of Experimental Drugs
4.1.1. Plant Material
4.1.2. Alkaline Hydrolysis of the Total Ginsenosides
4.1.3. Isolation of Rh2
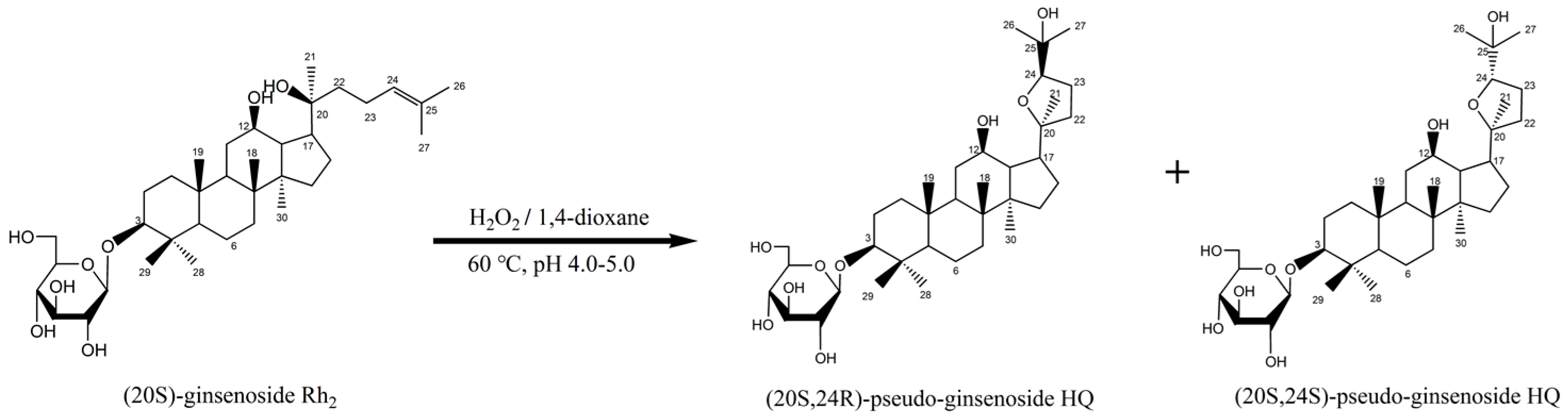
4.1.4. Synthesis of R-PHQ and S-PHQ
4.1.5. Structure Elucidation of Rh2, R-PHQ, and S-PHQ
4.2. Experimental Animal Model and Drug Treatment
4.3. Enzyme-Linked Immunosorbent Assay
4.4. Assessment of Biochemical Parameter
4.5. Western Blot
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BDNF | brain-derived neurotrophic factor |
| CNS | central nervous system |
| FST | forced swimming test |
| IκB-α | inhibitor of κB-α |
| IL-6 | interleukin-6 |
| LPS | lipopolysaccharide |
| MDD | major depressive disorder |
| NF-κB | nuclear factor-κB |
| Nrf2 | nuclear-related factor 2 |
| Sirt1 | sirtuin type 1 |
| SOD | superoxide dismutase |
| TNF-α | tumor necrosis factor-α |
| TrkB | tropomyosin-related kinase B |
| TST | tail suspension test |
References
- Duman, R.S.; Aghajanian, G.K. Synaptic Dysfunction in Depression: Potential Therapeutic Targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Koretz, D.; Merikangas, K.R.; John Rush, A.; Walters, E.E.; Wang, P.S. The epidemiology of major depressive disorder: Results from the national comorbidity survey replication (ncs-r). JAMA 2003, 289, 3095–3105. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Detraux, J.; De Lepeleire, J.; De Hert, M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015, 14, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, X.; Liu, X.; Liu, J.; Zhao, Y.; Li, H.; Cai, E.; Li, P.; Gao, Y. Study on antidepressant activity of chiisanoside in mice. Int. Immunopharmacol. 2018, 57, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Sekio, M.; Seki, K. Lipopolysaccharide-Induced Depressive-Like Behavior is Associated with α1-Adrenoceptor Dependent Downregulation of the Membrane GluR1 Subunit in the Mouse Medial Prefrontal Cortex and Ventral Tegmental Area. Int. J. Neuropsychoph. 2015, 18. [Google Scholar] [CrossRef] [PubMed]
- Kang, A.; Xie, T.; Zhu, D.; Shan, J.; Di, L.; Zheng, X. Suppressive Effect of Ginsenoside Rg3 against Lipopolysaccharide-Induced Depression-Like Behavior and Neuroinflammation in Mice. J. Agric. Food Chem. 2017, 65, 6861–6869. [Google Scholar] [CrossRef] [PubMed]
- Seimon, T.A.; Amrom, O.; Moore, K.J.; Golenbock, D.T.; Ira, T. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc. Natl. Acad. Sci. USA 2006, 103, 19794–19799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Liu, J.; Cai, E.; Zhao, Y.; Li, H.; Zhang, L.; Li, P.; Gao, Y. Sesquiterpenoids from the Root of Panax ginseng Attenuates Lipopolysaccharide- Induced Depressive-Like Behavior through the Brain-Derived Neurotrophic Factor/Tropomyosin-Related Kinase B and Sirtuin Type 1/Nuclear Factor-κB Signaling Pathways. J. Agric. Food Chem. 2018, 66, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Meng, X.; Zhai, Y.; Zhou, P.; Ye, T.; Wang, Z.; Sun, G.; Sun, X. Panax Notoginseng Saponins: A Review of Its Mechanisms of Antidepressant or Anxiolytic Effects and Network Analysis on Phytochemistry and Pharmacology. Molecules 2018, 23, 940. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Yao, Q.; Shen, J.; Gu, Z.; Xu, H.; Wu, Z.; Chen, C.; Li, L. Antidepressant-like effects of ginsenoside Rg3 in mice via activation of the hippocampal BDNF signaling cascade. J. Nat. Med. 2017, 71, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Lee, J.; Cho, I.H.; Kim, H.J.; Lee, S.W.; Kim, Y.O.; Park, C.G.; Lee, S. Ginsenoside Rg12, a new dammarane-type triterpene saponin from Panax ginseng root. J. Gin. Res. 2017, 41, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Wang, Z.; Zhou, B.; Fu, S.; Hong, T.; Li, P.; Liu, J. A new ocotillol-type ginsenoside from stems and leaves of Panax quinquefolium L. and its anti-oxidative effect on hydrogen peroxide exposed A549 cells. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Boonlert, W.; Benya-aphikul, H.; Umka Welbat, J.; Rodsiri, R. Ginseng Extract G115 Attenuates Ethanol-Induced Depression in Mice by Increasing Brain BDNF Levels. Nutrients 2017, 9, 931. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Li, W.; Tan, J.; Wang, C.; Lin, H.; Zhou, B.; Liu, J.; Li, P. Effect of ginsenoside Rh2 on renal apoptosis in cisplatin-induced nephrotoxicity in vivo. Phytomedicine 2019. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, L.; Li, Z.; Shao, Z.; Qi, Y.; Gao, K.; Liu, S.; Sun, Y.; Li, P.; Liu, J. Immunomodulatory Effects of (24R)-Pseudo-Ginsenoside HQ and (24S)-Pseudo-Ginsenoside HQ on Cyclophosphamide-Induced Immunosuppression and Their Anti14 Tumor Effects Study. Int. J. Mol. Sci. 2019, 20, 836. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, C.; Zhao, W.; Gao, S.; Cui, Z. Antidepressant-like effects of ginsenoside Rg5 in mice: Involving of hippocampus BDNF signaling pathway. Neurosci. Lett. 2017, 645, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Jung, I.H.; Van Le, T.K.; Jeong, J.J.; Kim, N.J.; Kim, D.H. Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. J. Ethnopharmacol. 2013, 146, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Tae Woo, K.; Hyuck-Jai, C.; Nam-Jae, K.; Dong-Hyun, K. Anxiolytic-like effects of ginsenosides Rg3 and Rh2 from red ginseng in the elevated plus-maze model. Planta Med. 2009, 75, 836–839. [Google Scholar]
- Willner, P. Animal models as simulations of depression. Trends Pharmacol. Sci. 1991, 12, 131–136. [Google Scholar] [CrossRef]
- Borsini, F.; Volterra, G.; Meli, A. Does the behavioral “despair” test measure “despair”? Physiol. Behav. 1986, 38, 385–386. [Google Scholar] [CrossRef]
- Sulakhiya, K.; Kumar, P.; Jangra, A.; Dwivedi, S.; Hazarika, N.K.; Baruah, C.C.; Lahkar, M. Honokiol abrogates lipopolysaccharide-induced depressive like behavior by impeding neuroinflammation and oxido-nitrosative stress in mice. Eur. J. Pharmacolo. 2014, 744, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, Y.; Cai, E.; Li, B.; Zhao, Y.; Zhu, H.; Zhang, L.; Gao, Y. l-menthol exhibits antidepressive-like effects mediated by the modification of 5-HTergic, GABAergic and DAergic systems. Cogn. Neurodynami. 2018. [Google Scholar] [CrossRef]
- Ueno, H.; Shimada, A.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Matsumoto, Y.; Okamoto, M.; Ishihara, T. Antidepressive-like effect of 2-phenylethanol inhalation in mice. Biomed. Pharmacot. 2019, 111, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Björkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, H.Y.; Bian, X.B.; Zhao, Y.; Zang, P.; Gao, Y.G.; Zhang, L.X. The antidepressant effect of 4-hydroxybenzyl alcohol 2-naphthoate through monoaminergic, GABAergic system and BDNF signaling pathway. Nat. Prod. Res. 2018, 436, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bortolotto, V.; Cuccurazzu, B.; Canonico, P.L.; Grilli, M. NF-B Mediated Regulation of Adult Hippocampal Neurogenesis: Relevance to Mood Disorders and Antidepressant Activity. Biomed. Res. Int. 2014, 2014, 612798. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Li, Z.; Li, W.; Liu, Y.; Wang, C.; Lin, H.; Liu, J.; Li, P. Pseudoginsengenin DQ Exhibits Therapeutic Effects in Cisplatin-Induced Acute Kidney Injury via Sirt1/NF-κB and Caspase Signaling Pathway without Compromising Its Antitumor Activity in Mice. Molecules 2018, 23, 3038. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sang, W.; Yang, X.S.; Zhai, M.J.; Wang, L.l.; Qin, P.Y.; Wu, L.; Zhou, X.R.; Wang, L.J.; Li, J.Y.; et al. Antidepressant Effects of Ginsenosides from Panax notoginseng. J. Integr. Agr. 2012, 11, 483–488. [Google Scholar] [CrossRef]
- Wang, G.L.; He, Z.M.; Zhu, H.Y.; Gao, Y.G.; Zhao, Y.; Yang, H.; Zhang, L.X. Involvement of serotonergic, noradrenergic and dopaminergic systems in the antidepressant-like effect of ginsenoside Rb1, a major active ingredient of Panax ginseng C.A. Meyer. J. Ethnopharmacol. 2017, 204, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Sun, T.; Gao, Y.; Chen, Y.; Jin, Y.; Li, Y. Semisynthesis and bioactive evaluation of oxidized products from 20(S)-ginsenoside Rg3, Rh2, protopanaxadiol (PPD) and their 20(R)-epimers as cytotoxic agents. Steroids 2016, 106, 26–34. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds ginsenoside Rh2, (24R)-pseudo-ginsenoside HQ, and (24S)-pseudo-ginsenoside HQ are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-x.; Qi, Z.; Shao, Z.-j.; Li, S.-s.; Qi, Y.-l.; Gao, K.; Liu, S.-x.; Li, Z.; Sun, Y.-s.; Li, P.-y. Study on Antidepressant Activity of Pseudo-Ginsenoside HQ on Depression-Like Behavior in Mice. Molecules 2019, 24, 870. https://doi.org/10.3390/molecules24050870
Chen L-x, Qi Z, Shao Z-j, Li S-s, Qi Y-l, Gao K, Liu S-x, Li Z, Sun Y-s, Li P-y. Study on Antidepressant Activity of Pseudo-Ginsenoside HQ on Depression-Like Behavior in Mice. Molecules. 2019; 24(5):870. https://doi.org/10.3390/molecules24050870
Chicago/Turabian StyleChen, Li-xue, Zeng Qi, Zi-jun Shao, Shan-shan Li, Yu-li Qi, Kun Gao, Song-xin Liu, Zhuo Li, Yin-shi Sun, and Ping-ya Li. 2019. "Study on Antidepressant Activity of Pseudo-Ginsenoside HQ on Depression-Like Behavior in Mice" Molecules 24, no. 5: 870. https://doi.org/10.3390/molecules24050870





