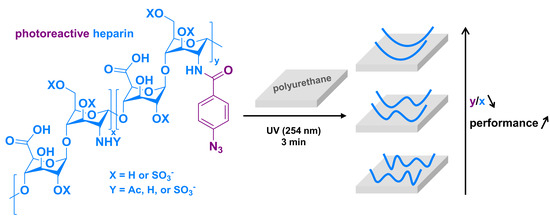
Heparinized Polyurethane Surface Via a One-Step Photografting Method
Abstract
:1. Introduction
2. Results
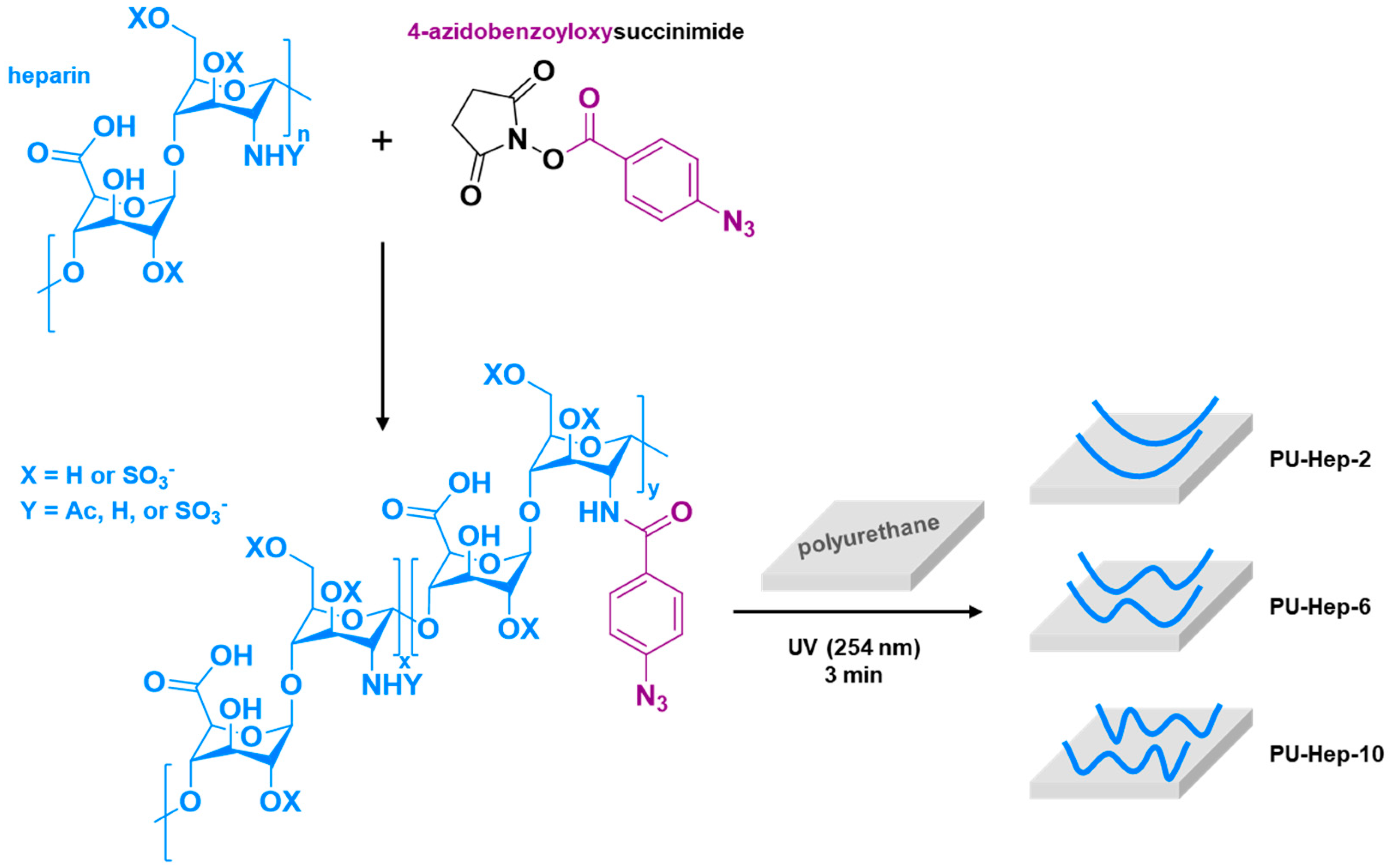
2.1. Heparin Grafting onto PU Surface
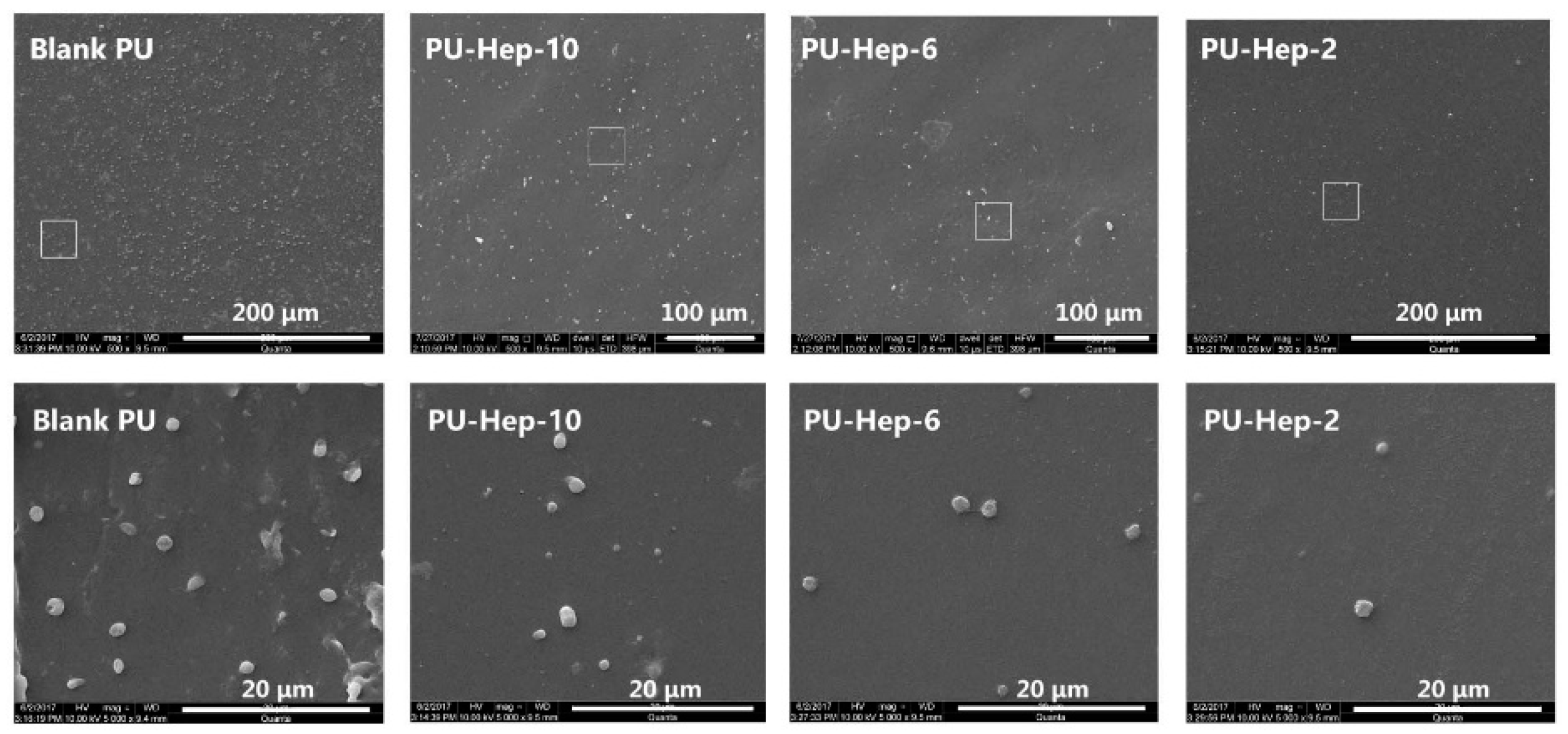
2.2. Characterization of Heparin on Surface
2.3. Hemocompatibility Evaluation
2.4. Cell Assays
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Heparin Surface Grafting
4.3. Characterization
4.4. Blood Compatibility Tests
4.5. Cell Viability
4.6. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGee, D.C.; Gould, M.K. Preventing Complications of Central Venous Catheterization. N. Engl. J. Med. 2003, 348, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Akl, E.A.; Vasireddi, S.R.; Gunukula, S.; Yosuico, V.E.; Barba, M.; Sperati, F.; Cook, D.; Schünemann, H. Anticoagulation for patients with cancer and central venous catheters. Cancer 2010, 112, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Walenga, J.M.; Frenkel, E.P.; Bick, R.L. Heparin-induced thrombocytopenia, paradoxical thromboembolism, and other adverse effects of heparin-type therapy. Med. Clin. N. Am. 2003, 17, 259–282. [Google Scholar] [CrossRef]
- Minet, V.; Dogné, J.M.; Mullier, F. Functional assays in the diagnosis of heparin-induced thrombocytopenia: A review. Molecules 2017, 22, 617. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wei, J.; Shi, Q.; Zhao, J.; Yin, J.; Stagnaro, P. Fabrication of PP-g-PEGMA-g-heparin and its hemocompatibility: From protein adsorption to anticoagulant tendency. Appl. Surf. Sci. 2012, 258, 5841–5849. [Google Scholar] [CrossRef]
- Murugesan, S.; Xie, J.; Linhardt, R.J. Immobilization of heparin: Approaches and applications. Curr. Top. Med. Chem. 2008, 8, 80–100. [Google Scholar] [PubMed]
- Leiro, V.; Parreira, P.; Freitas, S.C.; Martins, M.C.L.; Pêgo, A.P. Conjugation Chemistry Principles and Surface Functionalization of Nanomaterials. In Biomedical Applications of Functionalized Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 35–66. [Google Scholar]
- Kang, I.K.; Kwon, O.H.; Kim, M.K.; Lee, Y.M.; Sung, Y.K. In vitro blood compatibility of functional group grafted and heparin-immobilized polyurethanes prepared by plasma glow discharge. Biomaterials 1997, 18, 1099–1107. [Google Scholar] [CrossRef]
- Sask, K.N.; Berry, L.R.; Chan, A.K.; Brash, J.L. Modification of polyurethane surface with an antithrombin-heparin complex for blood contact: Influence of molecular weight of polyethylene oxide used as a linker/spacer. Langmuir 2012, 28, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Feng, Y.; Wang, H.; Zhang, L.; Khan, M.; Guo, J.; Chen, Q.; Liu, J. Immobilized bioactive agents onto polyurethane surface with heparin and phosphorylcholine group. Macromol. Res. 2013, 21, 541–549. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Liu, T.; Wang, X.; Liu, Y.; Wang, Y.; Chen, J.; Huang, N. Covalent co-immobilization of heparin/laminin complex that with different concentration ratio on titanium surface for selectively direction of platelets and vascular cells behavior. Appl. Surf. Sci. 2014, 317, 776–786. [Google Scholar] [CrossRef]
- Font Tellado, S.; Chiera, S.; Bonani, W.; Poh, P.S.P.; Migliaresi, C.; Motta, A.; Balmayor, E.R.; van Griensven, M. Heparin functionalization increases retention of TGF-β2 and GDF5 on biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Acta Biomater. 2018, 72, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Rider, C.C.; Mulloy, B. Heparin, heparan sulphate and the TGF-Cytokine superfamily. Molecules 2017, 22, 713. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; Rudd, T.; Yates, E. New applications of heparin and other glycosaminoglycans. Molecules 2017, 22, 749. [Google Scholar] [CrossRef] [PubMed]
- Chiodelli, P.; Bugatti, A.; Urbinati, C.; Rusnati, M. Heparin/heparan sulfate proteoglycans glycomic interactome in angiogenesis: Biological implications and therapeutical use. Molecules 2015, 20, 6342–6388. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Rånby, B. Bulk surface photografting process and its applications. II. Principal factors affecting surface photografting. J. Appl. Polym. Sci. 1996, 62, 545–555. [Google Scholar] [CrossRef]
- Delaittre, G.; Goldmann, A.S.; Mueller, J.O.; Barner-Kowollik, C. Efficient Photochemical Approaches for Spatially Resolved Surface Functionalization. Angew. Chem. Int. Ed. 2015, 54, 11388–11403. [Google Scholar] [CrossRef] [PubMed]
- Turgut, H.; Schmidt, A.C.; Wadhwani, P.; Welle, A.; Müller, R.; Delaittre, G. The para-fluoro-thiol ligation in water. Polym. Chem. 2017, 8, 1288–1293. [Google Scholar] [CrossRef]
- Giol, E.D.; Van Vlierberghe, S.; Unger, R.E.; Schaubroeck, D.; Ottevaere, H.; Thienpont, H.; Kirkpatrick, C.J.; Dubruel, P. Endothelialization and Anticoagulation Potential of Surface-Modified PET Intended for Vascular Applications. Macromol. Biosci. 2018, 18, e1800125. [Google Scholar] [CrossRef]
- Siegmann, K.; Inauen, J.; Villamaina, D.; Winkler, M. Photografting of perfluoroalkanes onto polyethylene surfaces via azide/nitrene chemistry. Appl. Surf. Sci. 2017, 396, 672–680. [Google Scholar] [CrossRef]
- Guowei, Z.; Yashao, C.; Tao, D.; Xiaoli, W. Surface Modification of Polyethylene by Heparin for Improvement of Antithrombogenicity. Plasma Sci. Technol. 2007, 9, 202–205. [Google Scholar] [CrossRef]
- Osborne, T.; Hoffa, A.; Gingles, B.; Urbanski, J. Hydrophilic Coated Medical Device. U.S. Patent US20050100580A1, 12 May 2005. [Google Scholar]
- Liu, L.-H.; Yan, M. Perfluorophenyl Azides: New Applications in Surface Functionalization and Nanomaterial Synthesis. Acc. Chem. Res. 2010, 43, 1434–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.I.; Sheffield, W.P.; Blajchman, M.A. Defining the heparin-binding domain of antithrombin. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 1994, 5, 83–95. [Google Scholar] [CrossRef]
- Hussain, M.; Rupp, F.; Wendel, H.P.; Gehring, F.K. Bioapplications of acoustic crystals, a review. TrAC Trends Anal. Chem. 2018, 102, 194–209. [Google Scholar] [CrossRef]
- Olsson, P.; Sanchez, J.; Mollnes, T.E.; Riesenfeld, J. On the blood compatibility of end-point immobilized heparin. J. Biomater. Sci. Polym. Ed. 2000, 11, 1261. [Google Scholar] [CrossRef] [PubMed]
- Mourier, P.A.J.; Guichard, O.Y.; Herman, F.; Sizun, P.; Viskov, C. New insights in thrombin inhibition structure-activity relationships by characterization of octadecasaccharides from low molecular weight heparin. Molecules 2017, 22, 428. [Google Scholar] [CrossRef]
- Linhardt, R.J. Heparin: Structure and Activity. J. Med. Chem. 2003, 46, 2551–2564. [Google Scholar] [CrossRef]
- Park, K.D.; Okano, T.; Nojiri, C.; Kim, S.W. Heparin immobilization onto segmented polyurethaneurea surfaces—Effect of hydrophilic spacers. J. Biomed. Mater. Res. Part A 1988, 22, 977–992. [Google Scholar] [CrossRef]
- Ran, F.; Nie, S.; Li, J.; Su, B.; Sun, S.; Zhao, C. Heparin-like macromolecules for the modification of anticoagulant biomaterials. Macromol. Biosci. 2012, 12, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cheng, C.; Xiang, T.; Tang, M.; Zhao, W.; Sun, S.; Zhao, C. Modification of polyethersulfone hemodialysis membrane by blending citric acid grafted polyurethane and its anticoagulant activity. J. Membr. Sci. 2012, 405–406, 261–274. [Google Scholar] [CrossRef]
- Park, K.D.; Piao, A.Z.; Jacobs, H.; Okano, T.; Kim, S.W. Synthesis and characterization of SPUU–PEO–heparin graft copolymers. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 1725–1737. [Google Scholar] [CrossRef]
- Tagaya, M.; Ikoma, T.; Takeguchi, M.; Hanagata, N.; Tanaka, J. Interfacial Serum Protein Effect on Biological Apatite Growth. J. Phys. Chem. C 2011, 115, 22523–22533. [Google Scholar] [CrossRef]
- Fang, B.; Ling, Q.; Zhao, W.; Ma, Y.; Bai, P.; Wei, Q.; Li, H.; Zhao, C. Modification of polyethersulfone membrane by grafting bovine serum albumin on the surface of polyethersulfone/poly(acrylonitrile-co-acrylic acid) blended membrane. J. Membr. Sci. 2009, 329, 46–55. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Luo, R.; Maitz, M.F.; Jing, F.; Sun, H.; Huang, N. The covalent immobilization of heparin to pulsed-plasma polymeric allylamine films on 316L stainless steel and the resulting effects on hemocompatibility. Biomaterials 2010, 31, 2072–2083. [Google Scholar] [CrossRef] [PubMed]
- Han, D.K.; Lee, N.Y.; Park, K.D.; Kim, Y.H.; Han, I.C.; Min, B.G. Heparin-like anticoagulant activity of sulphonated poly(ethylene oxide) and sulphonated poly(ethylene oxide)-grafted polyurethane. Biomaterials 1995, 16, 467. [Google Scholar] [CrossRef]
Sample Availability: Samples of heparin derivatives containing pendant azidobenzoic photosensitive groups are available from the authors. |




| Samples | PT (s) | TT (s) | APTT (s) | Bioactivity (%) |
|---|---|---|---|---|
| Control | 12.4 ± 0.4 | 18.5 ± 0.5 | 35.7 ± 0.6 | |
| Blank PU | 12.9 ± 0.4 * | 20.7 ± 2.3 * | 40.8 ± 0.6 * | |
| PU-Hep-10 | 23.4 ± 1.1 %, & | 89 ± 2.9 %, & | 84.3 ± 1.3 %, & | 22.9 %, & |
| PU-Hep-6 | 29.1 ± 0.9 % | 104.7 ± 3.2 % | 102.8 ± 2.2 % | 28.8 % |
| PU-Hep-2 | 37.3 ± 1.5 & | 124.3 ± 3.5 & | 117.5 ± 4.4 & | 36.9 & |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Fang, L.; Delaittre, G.; Ke, Y.; Wu, G. Heparinized Polyurethane Surface Via a One-Step Photografting Method. Molecules 2019, 24, 758. https://doi.org/10.3390/molecules24040758
Liu Z, Fang L, Delaittre G, Ke Y, Wu G. Heparinized Polyurethane Surface Via a One-Step Photografting Method. Molecules. 2019; 24(4):758. https://doi.org/10.3390/molecules24040758
Chicago/Turabian StyleLiu, Zhangshuan, Liming Fang, Guillaume Delaittre, Yu Ke, and Gang Wu. 2019. "Heparinized Polyurethane Surface Via a One-Step Photografting Method" Molecules 24, no. 4: 758. https://doi.org/10.3390/molecules24040758