Bioactivity Determination of a Therapeutic Recombinant Human Keratinocyte Growth Factor by a Validated Cell-based Bioassay
Abstract
:1. Introduction
2. Results
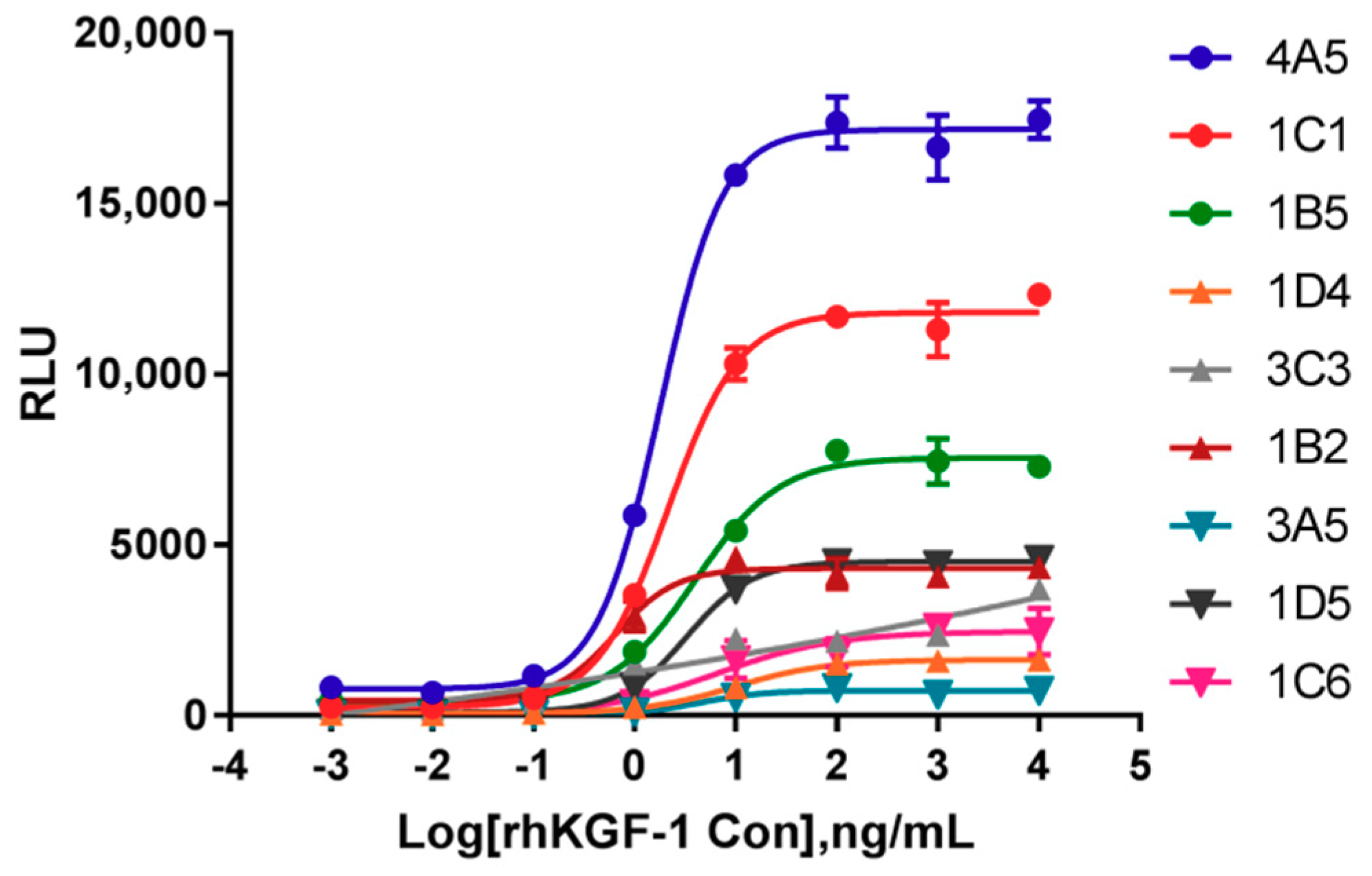
2.1. Identification of Cells Responsive to rhKGF-1
2.2. Optimization Procedure
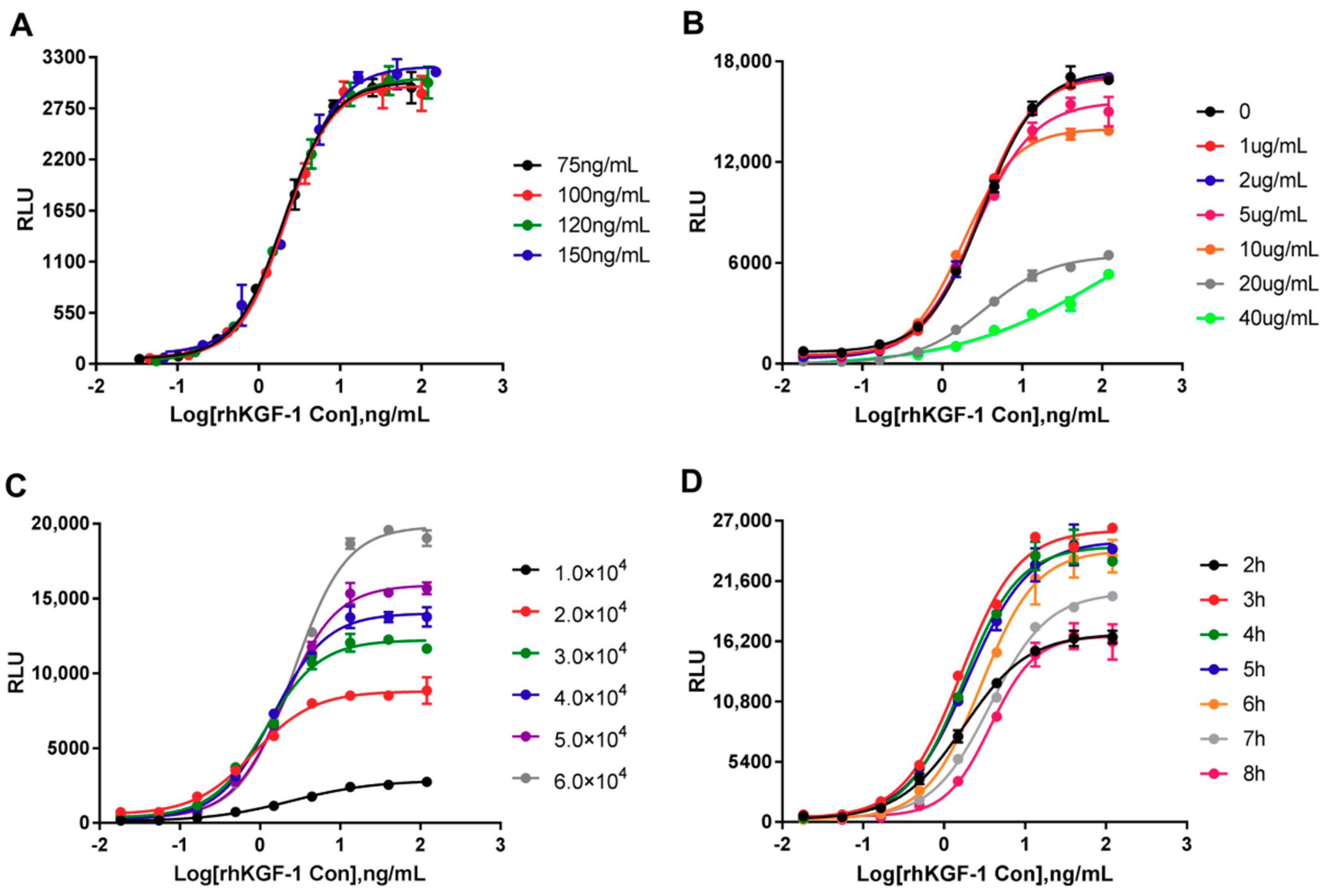
2.2.1. Optimal initial rhKGF-1 concentration
2.2.2. Optimization of Heparin Concentration in Assay Media
2.2.3. Optimization of Cell Numbers and Incubation Time
2.3. Validation of Bioactivity Procedures
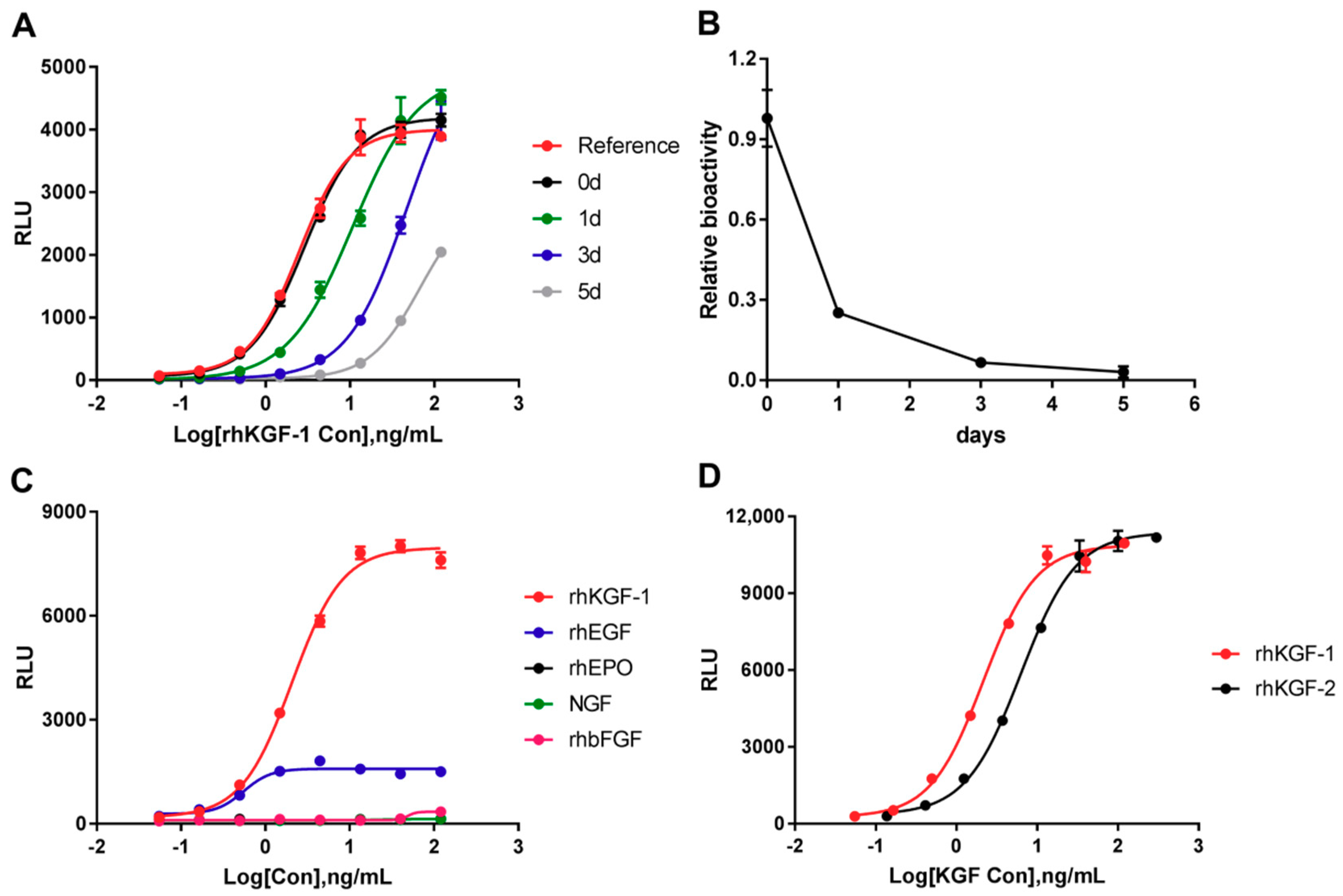
2.3.1. Specificity
2.3.2. Linearity
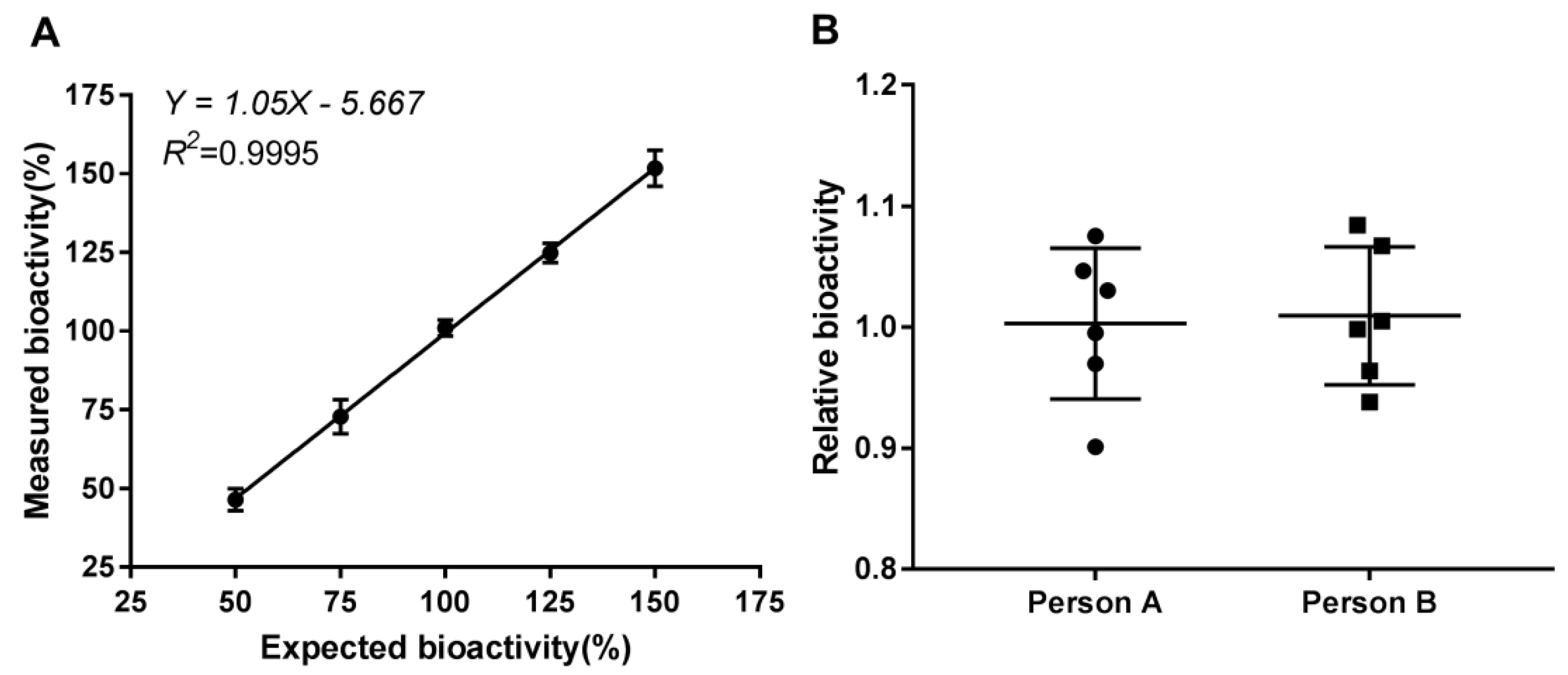
2.3.3. Accuracy
2.3.4. Precision
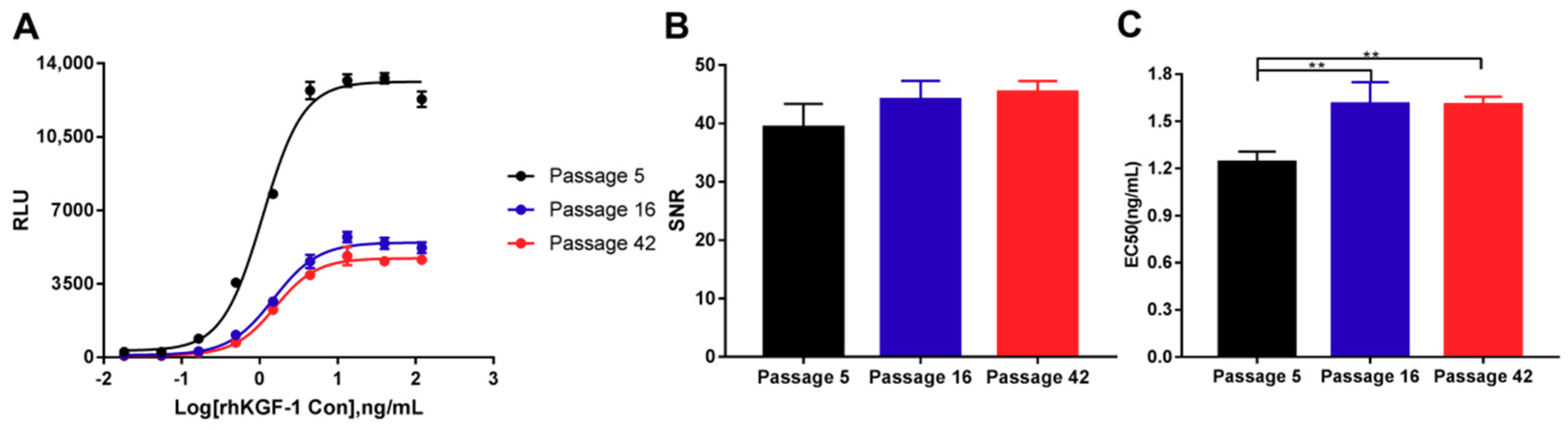
2.3.5. Stability of the HEK293-Luc Cell Line
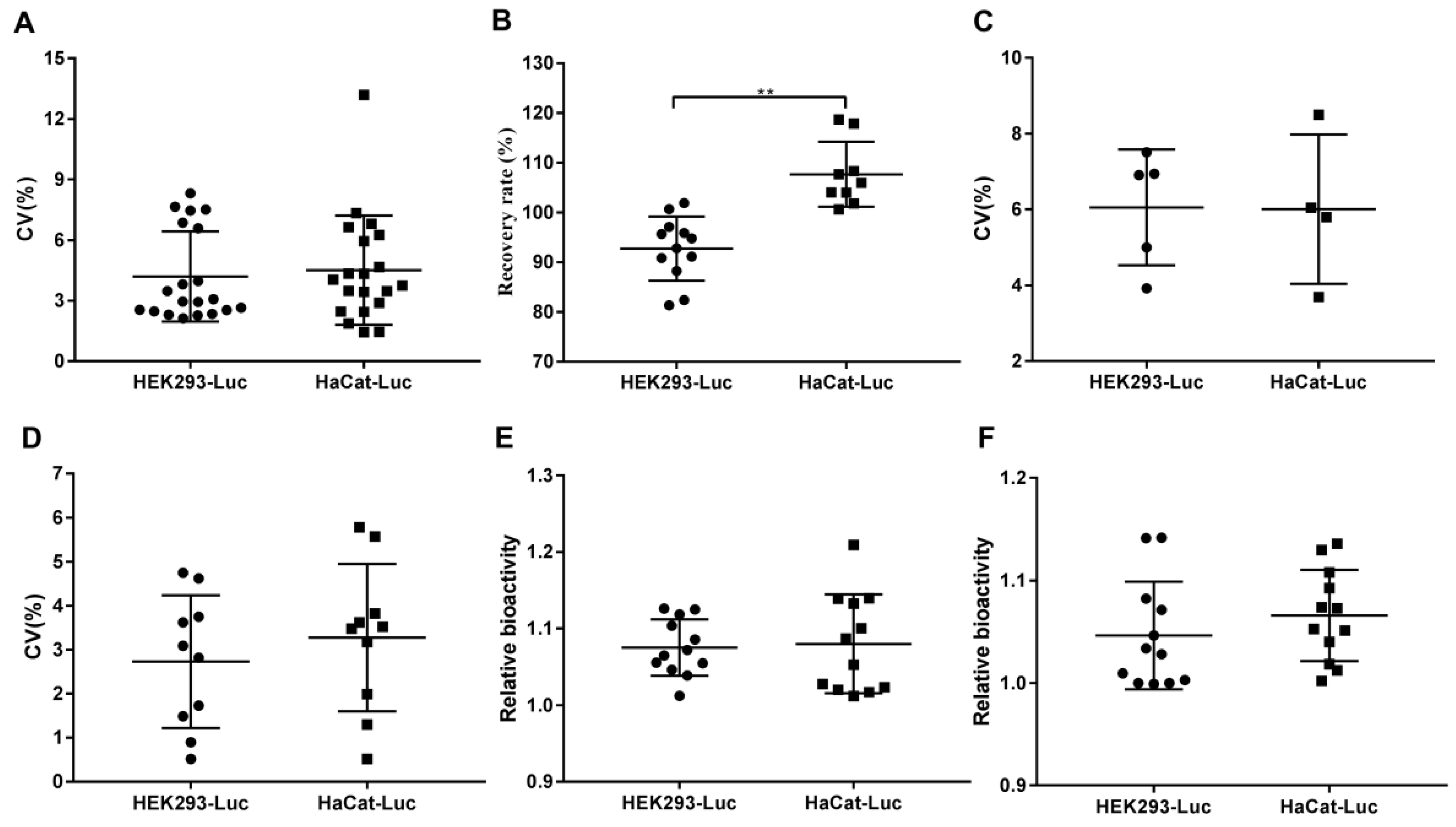
2.3.6. Comparison of Responsiveness to rhKGF-1 between HEK293-Luc and HaCat-Luc Cell Lines
3. Discussion
4. Materials and Methods
4.1. Cells and Materials
4.2. Preparation of Desired Responsive Cells to rhKGF-1
4.3. Bioactivity Assay
4.4. Preparation of Forced Degradation from rhKGF-1
4.5. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rubin, J.S.; Osada, H.; Finch, P.W.; Taylor, W.G.; Rudikoff, S.; Aaronson, S.A. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc. Natl. Acad. Sci. USA 1989, 86, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.L.; Scully, S.; Danilenko, D.M. Keratinocyte Growth Factor; Elservier Science Ltd.: Amsterdam, The Netherlands, 2002; pp. 1–16. [Google Scholar] [CrossRef]
- Nakao, Y.; Mitsuyasu, T.; Kawano, S.; Nakamura, N.; Kanda, S.; Nakamura, S. Fibroblast growth factors 7 and 10 are involved in ameloblastoma proliferation via the mitogen-activated protein kinase pathway. Int. J. Oncol. 2013, 43, 1377–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamasaki, M.; Miyake, A.; Tagashira, S.; Itoh, N. Structure and expression of the rat mRNA encoding a novel member of the fibroblast growth factor family. J. Biol. Chem. 1996, 271, 15918–15921. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, S.A.; Bottaro, D.P.; Miki, T.; Ron, D.; Finch, P.W.; Fleming, T.P.; Ahn, J.; Taylor, W.G.; Rubin, J.S. Keratinocyte growth factor. A fibroblast growth factor family member with unusual target cell specificity. Ann. N. Y. Acad. Sci. 1991, 638, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Finch, P.W.; Rubin, J.S.; Miki, T.; Ron, D.; Aaronson, S.A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science 1989, 245, 752–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emoto, H.; Tagashira, S.; Mattei, M.G.; Yamasaki, M.; Hashimoto, G.; Katsumata, T.; Negoro, T.; Nakatsuka, M.; Birnbaum, D.; Coulier, F.; et al. Structure and expression of human fibroblast growth factor-10. J. Biol. Chem. 1997, 272, 23191–23194. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Finch, P.W.; Aaronson, S.A. Characterization of recombinant human fibroblast growth factor (FGF)-10 reveals functional similarities with keratinocyte growth factor (FGF-7). J. Biol. Chem. 1998, 273, 13230–13235. [Google Scholar] [CrossRef]
- Hui, Q.; Jin, Z.; Li, X.; Liu, C.; Wang, X. FGF Family: From Drug Development to Clinical Application. Int. J. Mol. Sci. 2018, 19, 1875. [Google Scholar] [CrossRef]
- Radtke, M.L.; Kolesar, J.M. Palifermin (Kepivance) for the treatment of oral mucositis in patients with hematologic malignancies requiring hematopoietic stem cell support. J. Oncol. Pharm. Pract. 2005, 11, 121–125. [Google Scholar] [CrossRef]
- Moghadasi, M.; Ilghari, D.; Sirati-Sabet, M.; Amini, A.; Asghari, H.; Gheibi, N. Structural characterization of recombinant human fibroblast growth factor receptor 2b kinase domain upon interaction with omega fatty acids. Chem. Phys. Lipids 2017, 202, 21–27. [Google Scholar] [CrossRef]
- Dell, K.R.; Williams, L.T. A novel form of fibroblast growth factor receptor 2. Alternative splicing of the third immunoglobulin-like domain confers ligand binding specificity. J. Biol. Chem. 1992, 267, 21225–21229. [Google Scholar] [PubMed]
- Belleudi, F.; Purpura, V.; Torrisi, M.R. The receptor tyrosine kinase FGFR2b/KGFR controls early differentiation of human keratinocytes. PLoS ONE 2011, 6, e24194. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Ibrahimi, O.A.; Olsen, S.K.; Umemori, H.; Mohammadi, M.; Ornitz, D.M. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 2006, 281, 15694–15700. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: from development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Adjei, A.A. FGFR Signaling as a Target for Lung Cancer Therapy. J. Thorac. Oncol. 2016, 11, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portnoy, J.; Curran-Everett, D.; Mason, R.J. Keratinocyte growth factor stimulates alveolar type II cell proliferation through the extracellular signal-regulated kinase and phosphatidylinositol 3-OH kinase pathways. Am. J. Respir. Cell Mol. Biol. 2004, 30, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Geer, D.J.; Swartz, D.D.; Andreadis, S.T. Biomimetic Delivery of Keratinocyte Growth Factor upon Cellular Demand for Accelerated Wound Healing in Vitro and in Vivo. Am. J. Pathol. 2005, 167, 1575–1586. [Google Scholar] [CrossRef] [Green Version]
- Terakawa, J.; Rocchi, A.; Serna, V.A.; Bottinger, E.P.; Graff, J.M.; Kurita, T. FGFR2IIIb-MAPK Activity Is Required for Epithelial Cell Fate Decision in the Lower Mullerian Duct. Mol. Endocrinol. 2016, 30, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Osslund, T.D.; Syed, R.; Singer, E.; Hsu, E.W.; Nybo, R.; Chen, B.L.; Harvey, T.; Arakawa, T.; Narhi, L.O.; Chirino, A.; et al. Correlation between the 1.6 A crystal structure and mutational analysis of keratinocyte growth factor. Protein Sci. 1998, 7, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Sonis, S. Emerging therapies for the prevention and treatment of oral mucositis. Expert Opin. Emerg. Drugs 2014, 19, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Goyal, S.; Kim, M.M.; Cabrales, P.; Lybeck, M.; Caroen, S.; Oronsky, N.; Burbano, E.; Carter, C.; Oronsky, A. A Review of Clinical Radioprotection and Chemoprotection for Oral Mucositis. Transl. Oncol. 2018, 11, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. Efficacy of palifermin (keratinocyte growth factor-1) in the amelioration of oral mucositis. Core Evid. 2010, 4, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Athar, U.; Gentile, T.C. Keratinocyte growth factor. Expert Opin. Biol. Ther. 2009, 9, 779–787. [Google Scholar] [CrossRef]
- Bahadori, Z.; Kalhor, H.R.; Mowla, S.J. Producing functional recombinant human keratinocyte growth factor in Pichia pastoris and investigating its protective role against irradiation. Enzyme Microb. Technol. 2018, 111, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Beale, K.K.; Ma, J.D. Evaluation of current and upcoming therapies in oral mucositis prevention. Future Oncol. 2010, 6, 1751–1770. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Xu, J.; Colvin, J.S.; McEwen, D.G.; MacArthur, C.A.; Coulier, F.; Gao, G.; Goldfarb, M. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 1996, 271, 15292–15297. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Y.; Yu, L.; Li, X.; Shi, X.; Qin, X.; Rao, C.; Wang, J. A novel reporter gene assay for Recombinant Human Erythropoietin (rHuEPO) pharmaceutical products. J. Pharm. Biomed. Anal. 2014, 100, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Larocque, L.; Bliu, A.; Xu, R.; Diress, A.; Wang, J.; Lin, R.; He, R.; Girard, M.; Li, X. Bioactivity Determination of Native and Variant Forms of Therapeutic Interferons. J. Biomed. Biotechnol. 2011, 2011, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Igne, B.; Drennen, J.K., 3rd; Anderson, C.A. Method development and validation for pharmaceutical tablets analysis using transmission Raman spectroscopy. Int. J. Pharm. 2016, 498, 318–325. [Google Scholar] [CrossRef]
- Bates, C.M. Role of fibroblast growth factor receptor signaling in kidney development. Am. J. Physiol. Renal Physiol. 2011, 301, F245–F251. [Google Scholar] [CrossRef] [Green Version]
- Capone, A.; Visco, V.; Belleudi, F.; Marchese, C.; Cardinali, G.; Bellocci, M.; Picardo, M.; Frati, L.; Torrisi, M.R. Up-modulation of the expression of functional keratinocyte growth factor receptors induced by high cell density in the human keratinocyte HaCaT cell line. Cell Growth Differ. 2000, 11, 607–614. [Google Scholar]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.M.; Zhang, Z.Q.; Liu, Y.Y.; Zhou, X.; Shi, X.H.; Jiang, Q.; Fan, D.L.; Cao, C. Requirement of Galphai1/3-Gab1 signaling complex for keratinocyte growth factor-induced PI3K-AKT-mTORC1 activation. J. Invest. Dermatol. 2015, 135, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Price, C.A. Mechanisms of fibroblast growth factor signaling in the ovarian follicle. J. Endocrinol. 2016, 228, R31–R43. [Google Scholar] [CrossRef]
- Xu, R.; Rudd, T.R.; Hughes, A.J.; Siligardi, G.; Fernig, D.G.; Yates, E.A. Analysis of the fibroblast growth factor receptor (FGFR) signalling network with heparin as coreceptor: Evidence for the expansion of the core FGFR signalling network. FEBS J. 2013, 280, 2260–2270. [Google Scholar] [CrossRef] [PubMed]
- ICH. International Conference on Harmonization (ICH) Guidelines ICH Q2(R1), Validation of Analytical Procedures: Text and Methodology; ICH: Geneva, Switzerland, 2005. [Google Scholar]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia, 4th ed.; People’s Medical Publishing House: Beijing, China, 2015. [Google Scholar]
- Viswanathan, C.T.; Bansal, S.; Booth, B.; DeStefano, A.J.; Rose, M.J.; Sailstad, J.; Shah, V.P.; Skelly, J.P.; Swann, P.G.; Weiner, R. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm. Res. 2007, 24, 1962–1973. [Google Scholar] [CrossRef]
- Booth, B.; Arnold, M.E.; DeSilva, B.; Amaravadi, L.; Dudal, S.; Fluhler, E.; Gorovits, B.; Haidar, S.H.; Kadavil, J.; Lowes, S.; et al. Workshop report: Crystal City V--quantitative bioanalytical method validation and implementation: the 2013 revised FDA guidance. AAPS J. 2015, 17, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Hawe, A.; Wiggenhorn, M.; van de Weert, M.; Garbe, J.H.O.; Mahler, H.-C.; Jiskoot, W. Forced Degradation of Therapeutic Proteins. J. Pharm. Sci. 2012, 101, 895–913. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds rhKGF1 and rhKGF2 are available from the authors. |
| Recovery Rate (%) | Intra-day CV (%) | Mean | SD | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| 1 | 97.13 | 91.17 | 100.70 | 5.00 | 96.33 | 4.82 |
| 2 | 94.80 | 95.90 | 101.90 | 3.92 | 97.53 | 3.82 |
| 3 | 82.40 | 88.27 | 95.70 | 7.51 | 88.79 | 6.67 |
| 4 | 92.82 | 90.86 | 81.35 | 6.94 | 88.34 | 6.13 |
| Inter-day CV (%) | 6.91 | |||||
| Mean | 92.75 | |||||
| SD | 6.41 | |||||
| Sample | Intra-day CV (%) | Inter-day CV (%) | 95% CI of Relative Bioactivity | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| rhKGF-1 | 1.49 | 3.09 | 1.73 | 3.75 | 2.82 | 1.05–1.10 |
| rhKGF-1 bulk | 4.62 | 0.90 | 3.62 | 0.52 | 4.75 | 1.01–1.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, W.; Guo, Y.; Qin, X.; Yu, L.; Shi, X.; Liu, L.; Zhou, Y.; Hu, J.; Rao, C.; Wang, J. Bioactivity Determination of a Therapeutic Recombinant Human Keratinocyte Growth Factor by a Validated Cell-based Bioassay. Molecules 2019, 24, 699. https://doi.org/10.3390/molecules24040699
Yao W, Guo Y, Qin X, Yu L, Shi X, Liu L, Zhou Y, Hu J, Rao C, Wang J. Bioactivity Determination of a Therapeutic Recombinant Human Keratinocyte Growth Factor by a Validated Cell-based Bioassay. Molecules. 2019; 24(4):699. https://doi.org/10.3390/molecules24040699
Chicago/Turabian StyleYao, Wenrong, Ying Guo, Xi Qin, Lei Yu, Xinchang Shi, Lan Liu, Yong Zhou, Jinpan Hu, Chunming Rao, and Junzhi Wang. 2019. "Bioactivity Determination of a Therapeutic Recombinant Human Keratinocyte Growth Factor by a Validated Cell-based Bioassay" Molecules 24, no. 4: 699. https://doi.org/10.3390/molecules24040699






