Novel O-alkyl Derivatives of Naringenin and Their Oximes with Antimicrobial and Anticancer Activity
Abstract
:1. Introduction
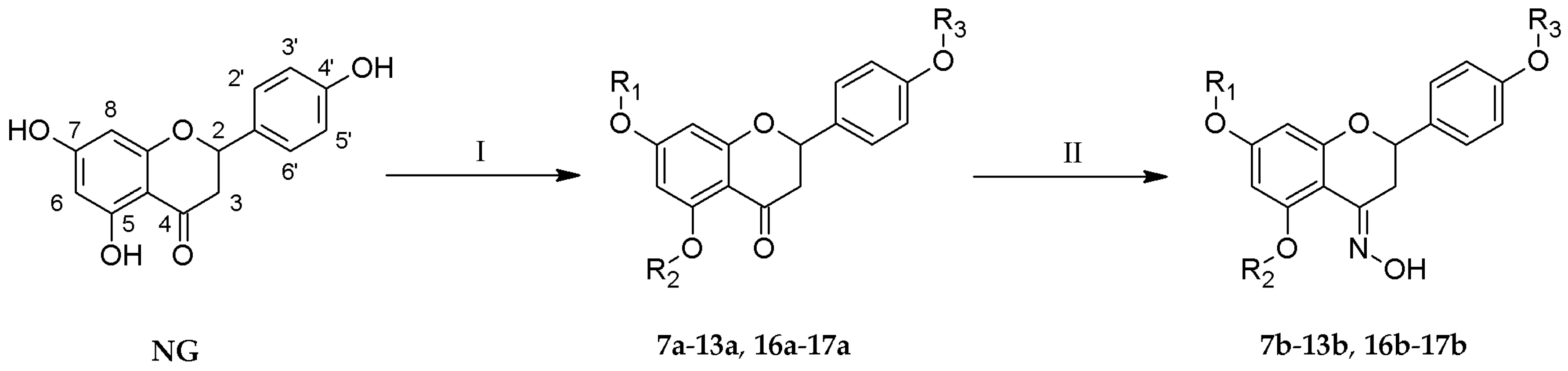
2. Results and Discussion
2.1. Chemistry
2.2. Antibacterial Activity
2.3. Anticancer Activity
3. Materials and Methods
3.1. Chemicals
3.2. Analysis
3.3. Synthesis of Mono- (7a, 10a, 12a, 16a) and Di-O-alkyl Derivatives of Naringenin (8a, 11a, 13a, 17a)
3.4. Synthesis of 5,7,4′-Tri-O-propylnaringenin (9a)
3.5. Synthesis of Oximes (7b–13b, 16b–17b)
3.6. Minimal Inhibitory Concentration (MIC) Evaluation
3.7. Sulforhodamine B (SRB) Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vuorela, P.; Leinonen, M.; Saikku, P.; Tammela, P.; Rauha, J.-P.; Wennberg, T.; Vuorela, H. Natural products in the Process of Finding New Drug Candidates. Curr. Med. Chem. 2004, 11, 1375–1389. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Céliz, G.; Daz, M.; Audisio, M.C. Antibacterial activity of naringin derivatives against pathogenic strains. J. Appl. Microbiol. 2011, 111, 731–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Leonardi, T.; Vanamala, J.; Taddeo, S.S.; Davidson, L.A.; Murphy, M.E.; Patil, B.S.; Wang, N.; Carroll, R.J.; Chapkin, R.S.; Lupton, J.R.; et al. Apigenin and naringenin suppress colon carcinogenesis through the aberrant crypt stage in azoxymethane-treated rats. Exp. Biol. Med. 2010, 235, 710–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocyigit, A.; Koyuncu, I.; Dikilitas, M.; Bahadori, F.; Turkkan, B. Cytotoxic, genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines. Asian Pac. J. Trop. Biomed. 2016, 6, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Khan, M.K.; Zill-E-Huma; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Zou, W.; Luo, Y.; Liu, M.; Chen, S.; Wang, S.; Nie, Y.; Cheng, G.; Su, W.; Zhang, K. Human intestinal microbial metabolism of naringin. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 363–367. [Google Scholar] [CrossRef]
- Fuhr, U.; Klittich, K.; Staib, A. Inhibitory effect of grapefruit juice and its bitter principal, naringenin, on CYP1A2 dependent metabolism of caffeine in man. Br. J. Clin. Pharmacol. 1993, 35, 431–436. [Google Scholar] [CrossRef]
- Shimizu, T.; Lin, F.; Hasegawa, M.; Okada, K.; Nojiri, H.; Yamane, H. Purification and identification of naringenin 7-O-methyltransferase, a key enzyme in biosynthesis of flavonoid phytoalexin sakuranetin in rice. J. Biol. Chem. 2012, 287, 19315–19325. [Google Scholar] [CrossRef] [PubMed]
- Nobakht, M.; Grkovic, T.; Trueman, S.J.; Wallace, H.M.; Katouli, M.; Quinn, R.J.; Brooks, P.R. Chemical constituents of kino extract from Corymbia torelliana. Molecules 2014, 19, 17862–17871. [Google Scholar] [CrossRef] [PubMed]
- Sakoda, C.P.P.; de Toledo, A.C.; Perini, A.; Pinheiro, N.M.; Hiyane, M.I.; dos Santos Grecco, S.; de Fátima Lopes Calvo Tibério, I.; Câmara, N.O.S.; de Arruda Martins, M.; Lago, J.H.G.; et al. Sakuranetin reverses vascular peribronchial and lung parenchyma remodeling in a murine model of chronic allergic pulmonary inflammation. Acta Histochem. 2016, 118, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.A.S.; Agra, M.D.F.; Tavares, J.F.; Da-Cunha, E.V.L.; Barbosa-Filho, J.M.; Silva, M.S. Flavanones from aerial parts of Cordia globosa (Jacq.) Kunth, Boraginaceae. Braz. J. Pharmacogn. 2010, 20, 675–681. [Google Scholar] [CrossRef]
- Copmans, D.; Orellana-Paucar, A.M.; Steurs, G.; Zhang, Y.; Ny, A.; Foubert, K.; Exarchou, V.; Siekierska, A.; Kim, Y.; De Borggraeve, W.; et al. Methylated flavonoids as anti-seizure agents: Naringenin 4′,7-dimethyl ether attenuates epileptic seizures in zebrafish and mouse models. Neurochem. Int. 2018, 112, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Moon, S.-H.; Lee, J.-Y.; Kim, K.-T.; Park, Y.-S.; Paik, H.-D. Antibacterial activity of a novel flavonoid, 7-O-butyl naringenin, against methicillin-resistant Staphylococcus aureus (MRSA). Food Sci. Biotechnol. 2013, 22, 1725–1728. [Google Scholar] [CrossRef]
- Moon, S.H.; Lee, J.H.; Kim, K.T.; Park, Y.S.; Nah, S.Y.; Ahn, D.U.; Paik, H.D. Antimicrobial effect of 7-O-butylnaringenin, a novel flavonoid, and various natural flavonoids against Helicobacter pylori strains. Int. J. Environ. Res. Public Health 2013, 10, 5459–5469. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, J.-W.; Paik, H.-D.; Cho, S.G.; Nah, S.-Y.; Park, Y.-S.; Han, Y.S. Cytotoxic effects of 7-O-butyl naringenin on human breast cancer MCF-7 cells. Food Sci. Biotechnol. 2010, 19, 717–724. [Google Scholar] [CrossRef]
- Kozłowska, J.; Potaniec, B.; Żarowska, B.; Anioł, M. Synthesis and biological activity of novel O-alkyl derivatives of naringenin and their oximes. Molecules 2017, 22, 1485. [Google Scholar] [CrossRef]
- Gaur, R.; Gupta, V.K.; Pal, A.; Darokar, M.P.; Bhakuni, R.S.; Kumar, B. In vitro and in vivo synergistic interaction of substituted chalcone derivatives with norfloxacin against methicillin resistant Staphylococcus aureus. RSC Adv. 2015, 5, 5830–5845. [Google Scholar] [CrossRef]
- Yenjai, C.; Wanich, S. Cytotoxicity against KB and NCI-H187 cell lines of modified flavonoids from Kaempferia parviflora. Bioorg. Med. Chem. Lett. 2010, 20, 2821–2823. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Meng, W.; Xu, Y.; Cao, J.; Qing, F. Synthesis and Anticancer Effect of Chrysin Derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 881–884. [Google Scholar] [CrossRef]
- Fonseca, S.F.; Lima, D.B.; Alves, D.; Jacob, R.G.; Perin, G.; Lenardao, E.J.; Savegnago, L. Synthesis, characterization and antioxidant activity of organoselenium and organotellurium compound derivatives of chrysin. New J. Chem. 2015, 39, 3043–3050. [Google Scholar] [CrossRef]
- Fonseca, S.F.; Padilha, N.B.; Thurow, S.; Roehrs, J.A.; Savegnago, L.; De Souza, M.N.; Fronza, M.G.; Collares, T.; Buss, J. Ultrasound-promoted copper-catalyzed synthesis of bis-arylselanyl chrysin derivatives with boosted antioxidant and anticancer activities. Ultrason. Sonochem. 2017, 39, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.K.D.; Huynh, T.K.C.; Nguyen, T.D. Synthesis, characterization, anti-inflammatory and anti-proliferative activity against MCF-7 cells of O-alkyl and O-acyl flavonoid derivatives. Bioorg. Chem. 2015, 63, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, K.S.; Lee, C.; Chong, Y. Synthesis of a complete series of O-methyl analogues of naringenin and apigenin. Bull. Korean Chem. Soc. 2007, 28, 2527–2530. [Google Scholar] [CrossRef]
- Deka, N.; Mariotte, A.-M.; Boumendjel, A. Microwave mediated solvent-free acetylation of deactivated and hindered phenols. Green Chem. 2001, 3, 263–264. [Google Scholar] [CrossRef]
- Grela, E.; Dziełak, A.; Szydłowska, K.; Mucha, A.; Kafarski, P.; Grabowiecka, A.M. Whole-cell Proteus mirabilis urease inhibition by aminophosphinates for the control of struvite formation. J. Med. Microbiol. 2016, 65, 1123–1129. [Google Scholar] [CrossRef]
- Kozioł, A.; Stryjewska, A.; Librowski, T.; Sałat, K.; Gaweł, M.; Moniczewski, A.; Lochyński, S. An Overview of the Pharmacological Properties and Potential Applications of Natural Monoterpenes. Mini-Rev. Med. Chem. 2014, 14, 1156–1168. [Google Scholar]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Wayne, P. CLSI document. In Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard—9th ed.; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32, ISBN 1562387839. [Google Scholar]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef]
- Yenjai, C.; Wanich, S.; Pitchuanchom, S.; Sripanidkulchai, B. Structural modification of 5,7-dimethoxyflavone from Kaempferia parviflora and biological activities. Arch. Pharm. Res. 2009, 32, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rasamiravaka, T.; Stévigny, C.; Duez, P.; Rajaonson, S.; Diallo, B.; Mol, A.; Baucher, M.; El Jaziri, M. The flavanone naringenin reduces the production of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Microbiology 2011, 157, 2120–2132. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Kim, T.W.; Shin, S.Y.; Park, M.J.; Yong, Y.; Kim, D.W.; Islam, T.; Lee, Y.H.; Jung, K.Y.; Lim, Y. Design, synthesis and inhibitory activities of naringenin derivatives on human colon cancer cells. Bioorg. Med. Chem. Lett. 2013, 23, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xie, Y.; Chen, X. Type 2 diabetes diminishes the benefits of dietary antioxidants: Evidence from the different free radical scavenging potential. Food Chem. 2015, 186, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Xie, Y.; Cao, H.; Yang, H.; Chen, X.; Xiao, J. Fetal bovine serum influences the stability and bioactivity of resveratrol analogues: A polyphenol-protein interaction approach. Food Chem. 2017, 219, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.P.; Nguyen, K.P.P.; Kamounah, F.S.; Zhang, W.; Hansen, P.E. NMR of a series of novel hydroxyflavothiones. Magn. Reson. Chem. 2009, 47, 1043–1054. [Google Scholar] [CrossRef]
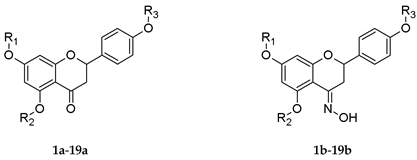
| No. | R1 | R2 | R3 |
|---|---|---|---|
| 1a, 1b | Me | H | H |
| 2a, 2b | Me | H | Me |
| 3a, 3b | Me | Me | Me |
| 4a, 4b | Et | H | H |
| 5a, 5b | Et | H | Et |
| 6a, 6b | Et | Et | Et |
| 7a★, 7b★ | n-Pr | H | H |
| 8a★, 8b★ | n-Pr | H | n-Pr |
| 9a★, 9b★ | n-Pr | n-Pr | n-Pr |
| 10a★, 10b★ | i-Pr | H | H |
| 11a★, 11b★ | i-Pr | H | i-Pr |
| 12a, 12b★ | Bu | H | H |
| 13a★, 13b★ | Bu | H | Bu |
| 14a, 14b | Pe | H | H |
| 15a, 15b | Pe | H | Pe |
| 16a, 16b★ | De | H | H |
| 17a★, 17b★ | De | H | De |
| 18a, 18b | Dod | H | H |
| 19a, 19b | Dod | H | Dod |
| No. | Minimal Inhibitory Concentration (µg/mL) | No. | Minimal Inhibitory Concentration (µg/mL) | ||||
|---|---|---|---|---|---|---|---|
| Escherichia coli ATCC25922 | Bacillus subtilis ATCC19659 | Staphylococcus aureus ATCC11632 | Escherichia coli ATCC25922 | Bacillus subtilis ATCC19659 | Staphylococcus aureus ATCC11632 | ||
| NG | >400 | 200 | 200 | NG-OX | >400 | 100 | 100 |
| 1a | 400 | >400 | >400 | 1b | 400 | 50 | 50 |
| 2a | 200 | 50 | 50 | 2b | 400 | 100 | 200 |
| 3a | 400 | >400 | 200 | 3b | >400 | >400 | 400 |
| 4a | >400 | 25 | 25 | 4b | 400 | 25 | 25 |
| 5a | 400 | 100 | 100 | 5b | 400 | >400 | 100 |
| 6a | 400 | 50 | 100 | 6b | 400 | >400 | 100 |
| 7a★ | 400 | 200 | 100 | 7b★ | >400 | >400 | 100 |
| 8a★ | 400 | >400 | >400 | 8b★ | 400 | 400 | 400 |
| 9a★ | >400 | >400 | >400 | 9b★ | >400 | >400 | >400 |
| 10a★ | >400 | 12.5 | 12.5 | 10b★ | >400 | 12.5 | 12.5 |
| 11a★ | 400 | >400 | >400 | 11b★ | 400 | 6.25 | 12.5 |
| 12a | 400 | 6.25 | 6.25 | 12b★ | 200 | 50 | 50 |
| 13a★ | >400 | >400 | >400 | 13b★ | 200 | 100 | >400 |
| 14a | 200 | 50 | 25 | 14b | 200 | 50 | 25 |
| 15a | 400 | >400 | 200 | 15b | 200 | >400 | >400 |
| 16a | >400 | 100 | 100 | 16b★ | >400 | 400 | 200 |
| 17a★ | >400 | >400 | >400 | 17b★ | >400 | 400 | >400 |
| 18a | 400 | >400 | >400 | 18b | 400 | >400 | >400 |
| 19a | >400 | >400 | >400 | 19b | 400 | 200 | 200 |
| Gentamicin | 1.5 | 1.0 | 1.5 | ||||
| Nalidixic acid | 6.25 | 25 | 50 | ||||
| Novobiocin | 400 | 1.0 | 0.5 | ||||
| No. | HT-29 Cell Line IC50 (μg/mL) | No. | HT-29 Cell Line IC50 (μg/mL) |
|---|---|---|---|
| NG | 38.93 ± 13.51 | NG-OX | 29.44 ± 3.16 |
| 1a | 24.98 ± 3.95 | 1b | 13.13 ± 1.02 |
| 2a | >100 | 2b | 11.45 ± 0.34 |
| 3a | 20.84 ± 2.05 | 3b | >100 |
| 4a | 14.82 ± 1.25 | 4b | 13.75 ± 2.09 |
| 5a | >100 | 5b | 7.65 ± 1.23 |
| 6a | >100 | 6b | >100 |
| 7a★ | 11.99 ± 0.58 | 7b★ | 9.11 ± 1.34 |
| 8a★ | >100 | 8b★ | 4.59 ± 0.56 |
| 9a★ | 31.77 ± 6.00 | 9b★ | >100 |
| 10a★ | 10.41 ± 2.14 | 10b★ | 7.26 ± 0.31 |
| 11a★ | 9.81 ± 0.72 | 11b★ | 4.89 ± 0.56 |
| 12a | 9.71 ± 1.28 | 12b★ | 6.22 ± 0.30 |
| 13a★ | >100 | 13b★ | 3.32 ± 0.29 |
| 14a | 13.23 ± 0.61 | 14b | 7.00 ± 0.48 |
| 15a | >100 | 15b | 5.89 ± 1.29 |
| 16a | 8.35 ± 0.45 | 16b★ | 3.63 ± 0.47 |
| 17a★ | >100 | 17b★ | >100 |
| 18a | 22.16 ± 4.33 | 18b | 7.46 ± 1.21 |
| 19a | >100 | 19b | >100 |
| Cisplatin | 16.73 ± 0.58 | ||
| Doxorubicin | 0.33 ± 0.02 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowska, J.; Grela, E.; Baczyńska, D.; Grabowiecka, A.; Anioł, M. Novel O-alkyl Derivatives of Naringenin and Their Oximes with Antimicrobial and Anticancer Activity. Molecules 2019, 24, 679. https://doi.org/10.3390/molecules24040679
Kozłowska J, Grela E, Baczyńska D, Grabowiecka A, Anioł M. Novel O-alkyl Derivatives of Naringenin and Their Oximes with Antimicrobial and Anticancer Activity. Molecules. 2019; 24(4):679. https://doi.org/10.3390/molecules24040679
Chicago/Turabian StyleKozłowska, Joanna, Ewa Grela, Dagmara Baczyńska, Agnieszka Grabowiecka, and Mirosław Anioł. 2019. "Novel O-alkyl Derivatives of Naringenin and Their Oximes with Antimicrobial and Anticancer Activity" Molecules 24, no. 4: 679. https://doi.org/10.3390/molecules24040679





