Carminic Acid Stabilized with Aluminum-Magnesium Hydroxycarbonate as New Colorant Reducing Flammability of Polymer Composites
Abstract
:1. Introduction
2. Results
2.1. X-Ray Photoelectron Spectroscopy (XPS)
2.2. Secondary Ion Mass Spectrometry (TOF-SIMS)
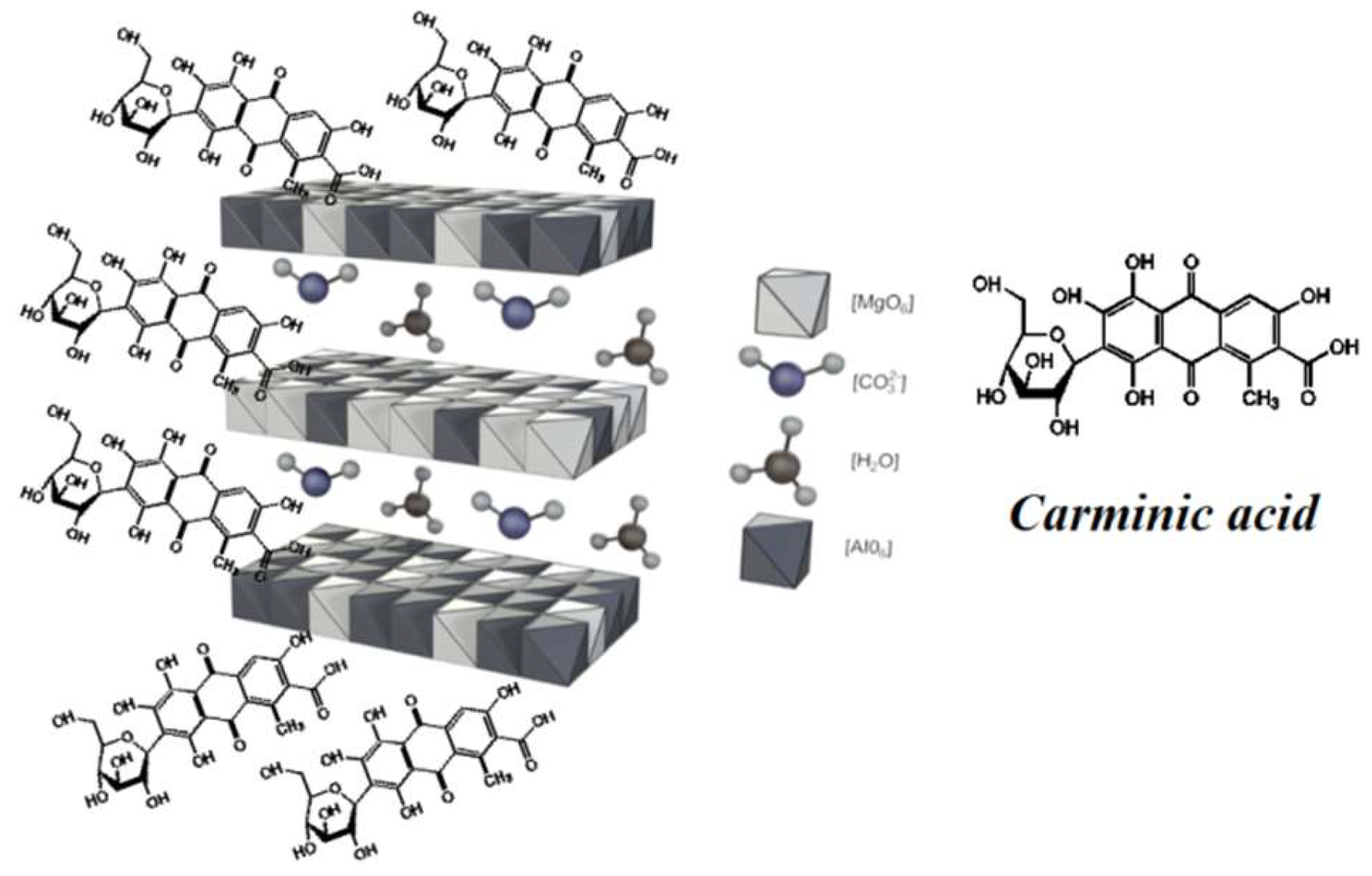
2.3. X-Ray Diffraction Analysis (XRD)
2.4. Thermogravimetric Analysis (TGA)
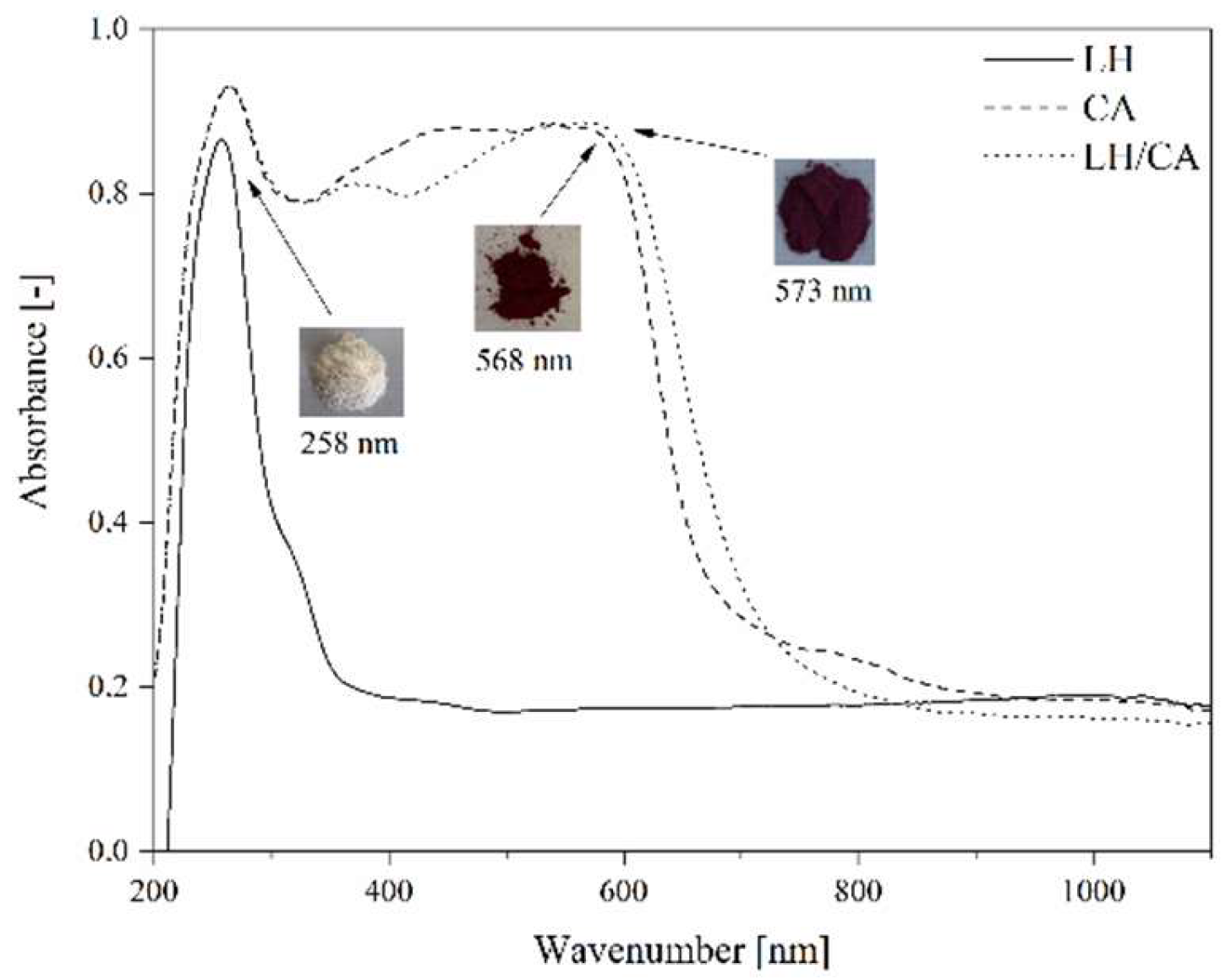
2.5. Color Stability of LH/CA Pigments
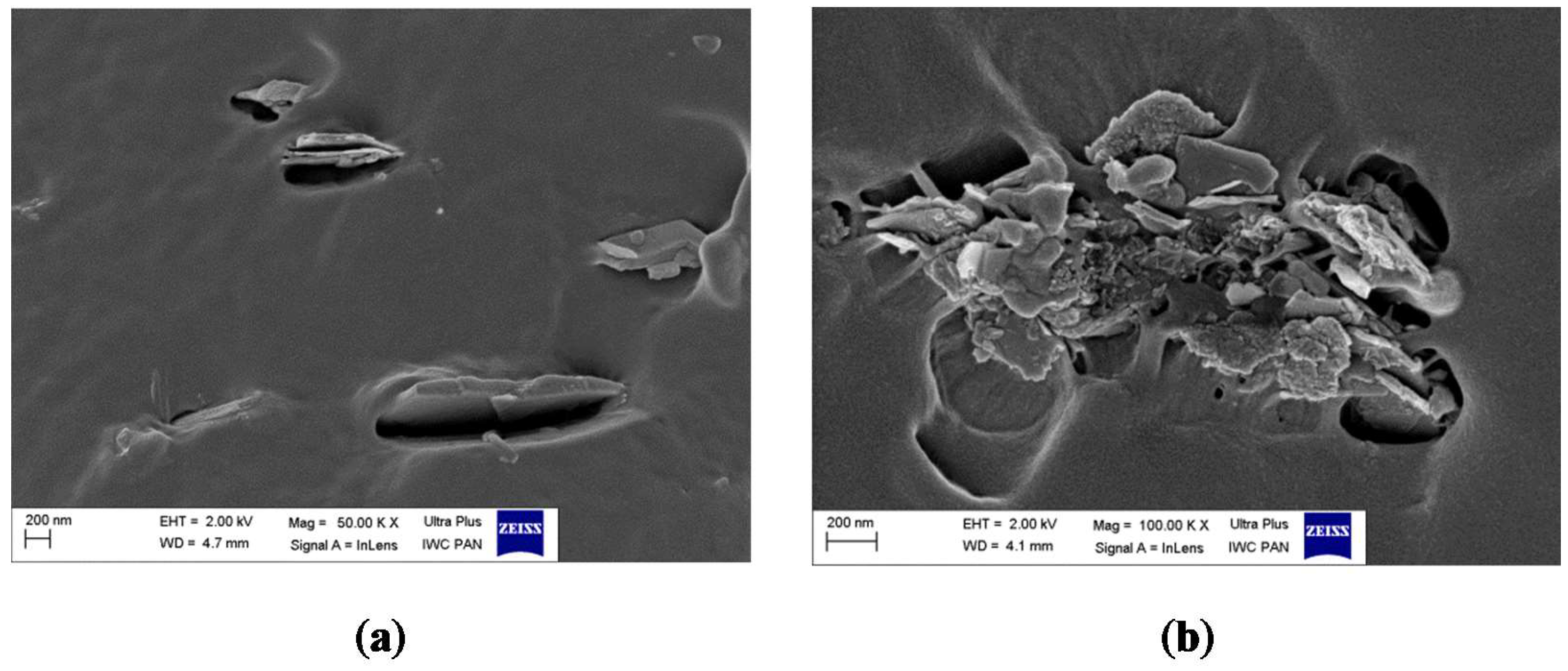
2.6. Scanning Electron Microscopy (SEM)
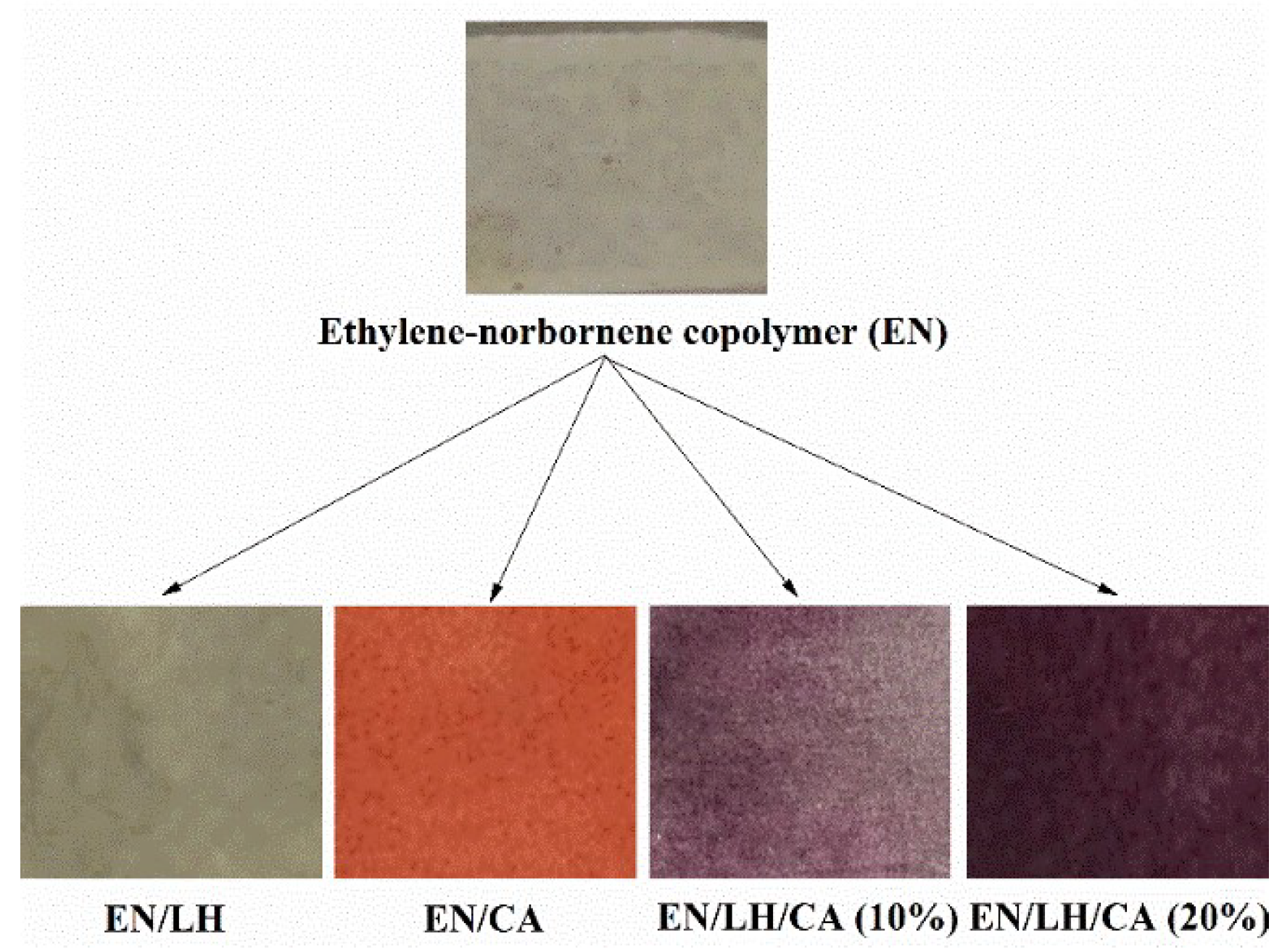
2.7. Colorimetric and Structural Analysis of EN/Hybrid Pigment Compounds
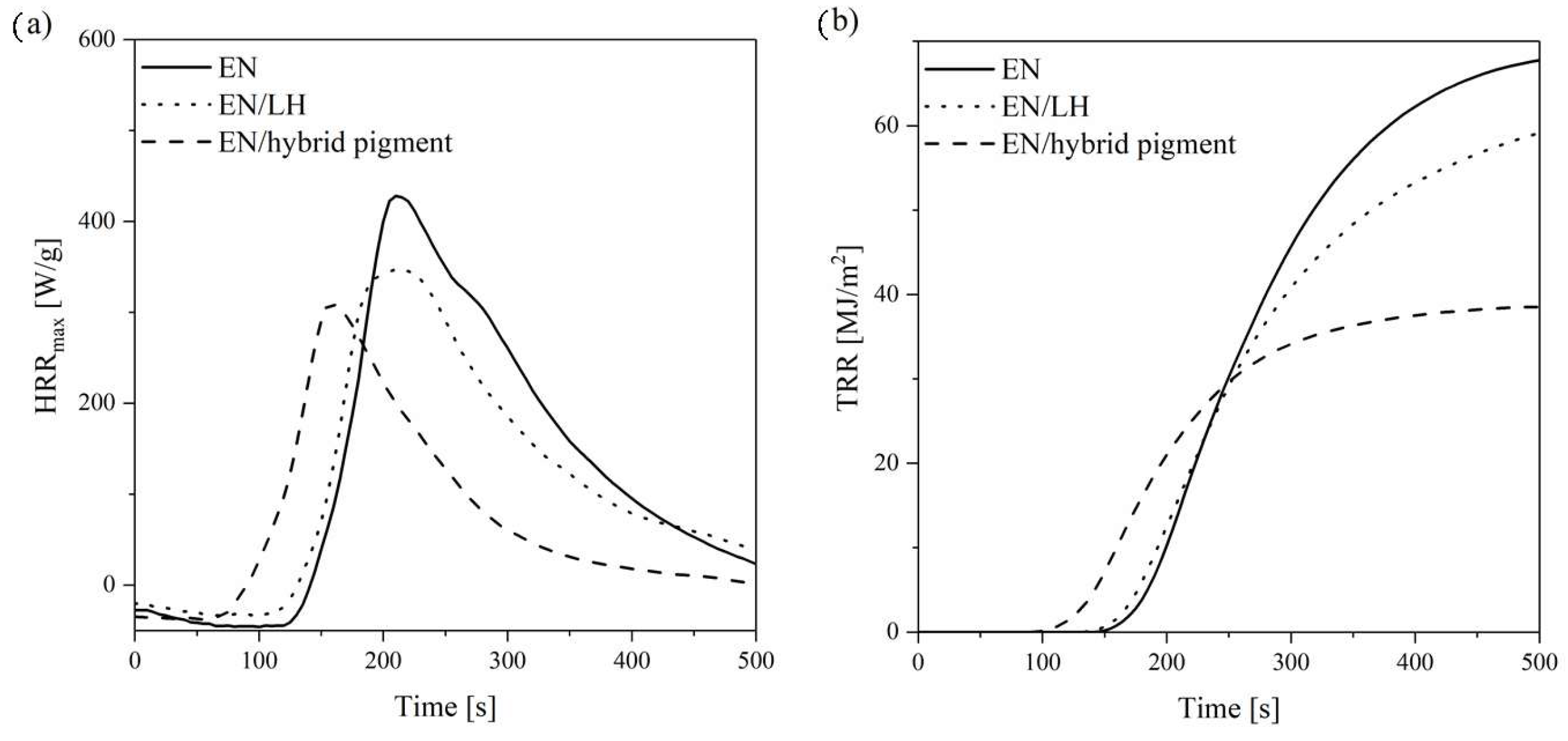
2.8. Flammability of EN/Hybrid Pigment Compounds
3. Materials and Methods
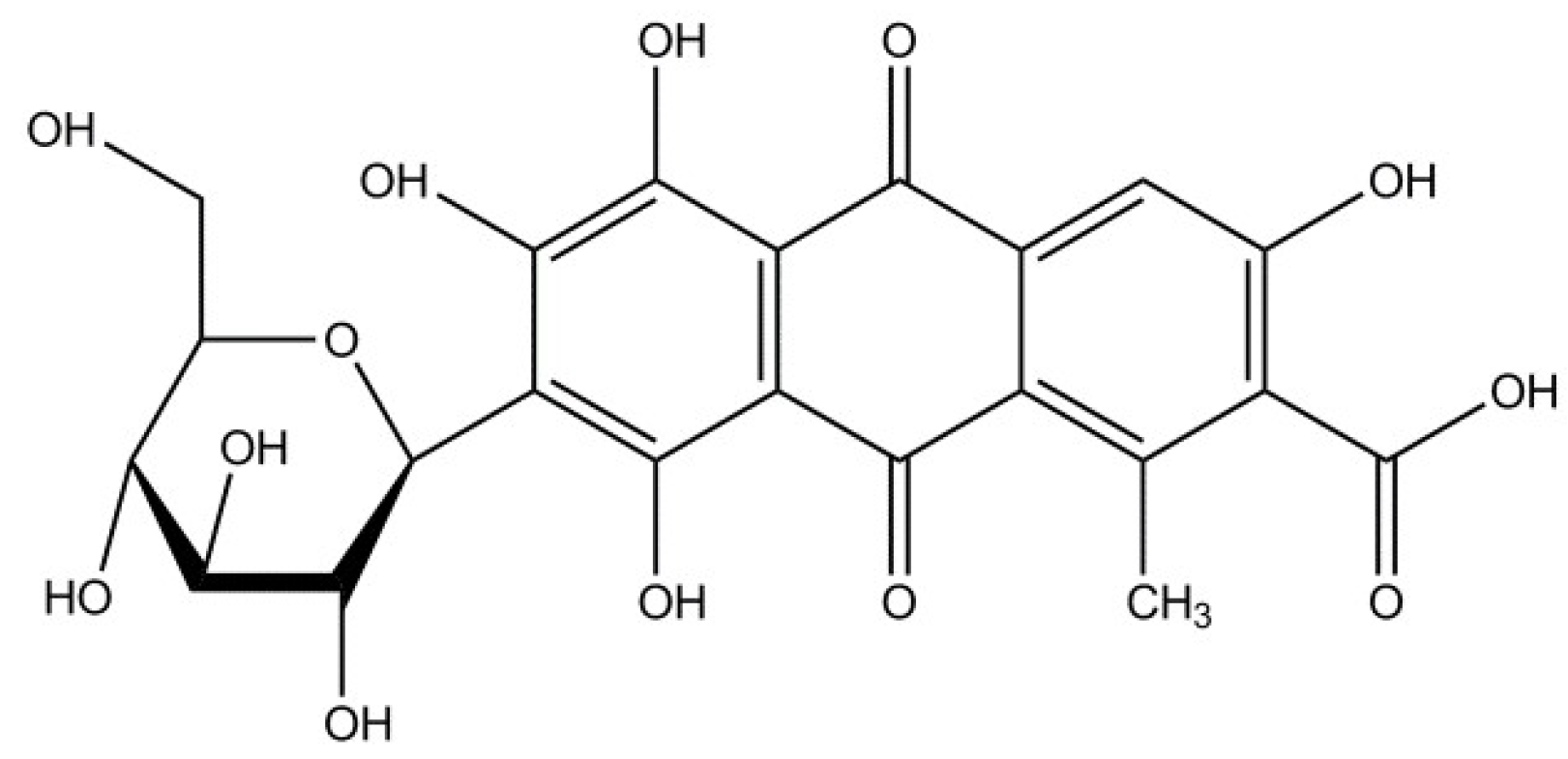
3.1. Raw Materials
3.2. Hybrid Pigment Preparation
3.3. Characterization of Powders
3.4. Preparation and Characterization of Polymer Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fleischmann, C.; Lievenbrück, M.; Ritter, H. Polymers and dyes: Developments and applications. Polymers 2015, 7, 717–746. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.D.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Mahmud-Ali, A.; Fitz-Binder, C.; Bechtold, T. Aluminium based dye lakes for plant extracts for textile coloration. Dyes Pigm. 2012, 94, 533–540. [Google Scholar] [CrossRef]
- Deveoglu, O.; Cakmakc, E.; Taskopru, T.; Torgan, E.; Karadag, R. Identyfication by RP-HPLC-DAD, FTIR, TGA and FESEM-EDAX of natural pigments prepared from Datissca cannabina L. Dyes Pigm. 2012, 94, 437–442. [Google Scholar] [CrossRef]
- Kohno, Y.; Totsuka, K.; Ikoma, S.; Yoda, K.; Shibata, M.; Matsushima, R.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Photostability enhancement of anionic natural dye by intercalation into hydrotalcite. J. Colloid Interface Sci. 2009, 337, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourhis, K.; Blanc, S.; Mathe, C.; Dupin, J.-C.; Vieillescazes, C. Spectroscopic and chromatographic analysis of yellow flavonoidic lakes: Quercetin chromophore. Appl. Clay Sci. 2011, 53, 598–607. [Google Scholar] [CrossRef]
- Itoh, T.; Yano, K.; Inada, Y.; Fukushima, Y. Photostabilized chlorophyll a in mesoporous silica: Adsorption properties and photoreduction activity of chlorophyll a. J. Am. Chem. Soc. 2002, 124, 13437–13441. [Google Scholar] [CrossRef]
- Lima, E.; Bosch, P.; Loera, S.; Ibarra, I.A.; Laguna, H.; Lara, V. Non-toxic hybrid pigments: Sequestering betanidin chromophores on inorganic matrices. Appl. Clay. Sci. 2009, 42, 478–482. [Google Scholar] [CrossRef]
- Kohno, Y.; Kinoshita, R.; Ikoma, S.; Yoda, K.; Shibata, M.; Matsushima, R.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Stabilization of natural anthocyanin by intercalation into montmorillonite. Appl. Clay. Sci. 2009, 42, 519–523. [Google Scholar] [CrossRef] [Green Version]
- Kohno, Y.; Senga, M.; Shibata, M.; Yoda, K.; Matsushima, R.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Stabilization of flavylium dye by incorporation into Fe-containing mesoporous silicate. Microporous Mesoporous Mater. 2011, 141, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, T.; Kohno, Y.; Shibata, M.; Tomita, Y.; Fukuhara, C.; Maeda, Y. An easy and effective method for the intercalation of hydrophobic natural dye into organo-montomorillonite for improved photostability. J. Phys. Chem. Solids 2018, 116, 168–173. [Google Scholar] [CrossRef]
- Kohno, Y.; Shibata, Y.; Oyaizu, N.; Yoda, K.; Shibata, M.; Matsushima, R. Stabilization of flavylium dye by incorporation into the pore of protonated zeolites. Microporous Mesoporous Mater. 2008, 114, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Kohno, Y.; Kato, Y.; Shibata, M.; Fukuhara, C.; Maeda, Y.; Tomita, Y.; Kobayashi, K. Fixation and stability enhancement of beta-carotene by organo-modified mesoporous silica. Microporous Mesoporous Mater. 2016, 220, 1–6. [Google Scholar] [CrossRef]
- Berlier, G.; Gastaidi, L.; Uqazio, E.; Mietto, I.; Iliade, P.; Sapino, S. Stabilization of quercetin flavonoid in MCM-41 mesoporous silica: Positive effect of surface functionalization. J. Colloid. Interface Sci. 2013, 393, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Christie, R.M.; Mackay, J.L. Metal salt azo pigments. Color. Technol. 2008, 124, 133–144. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Stewart, H.; Eremin, K.; Stenger, J. Lithol Red: A systematic structural study on salts of a sulfonated azo pigment. Chem. Eur. J. 2012, 18, 3064–3069. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.E.; Tejera, R.L.; Diaz, L.; Esparza, P.; Ibáñez, E. Natural dyes extraction from cochineal (Dactylopius coccus). New extraction methods. Food Chem. 2012, 132, 1855–1860. [Google Scholar] [CrossRef]
- Pozzi, F.; van den Berg, K.J.; Fielder, I.; Casadio, F.A. A systematic analysis of red lake pigments in French impressionist and post-impressionist paintings by surface-enhanced Raman spectroscopy (SERS). J. Raman Spectrosc. 2014, 45, 1119–1126. [Google Scholar] [CrossRef]
- Perez, E.; Ibarra, I.A.; Guzman, A.; Lima, E. Hybrid pigments resulting from several guest dyes onto γ-alumina host: A spectroscopic analysis. Spectrochim. Acta A 2017, 172, 174–181. [Google Scholar] [CrossRef]
- Fournier, F.; Viguerie, L.; Balme, S.; Janot, J.M.; Walter, P.; Jaber, M. Physico-chemical characterization of lake pigments based on montmorillonite and carminic acid. Appl. Clay Sci. 2016, 130, 12–17. [Google Scholar] [CrossRef]
- Guillermin, D.; Debroise, T.; Trigueiro, P.; de Viguerie, L.; Rigaud, B.; Morlet-Savary, F.; Balme, S.; Janot, J.M.; Tielens, F.; Michot, L.; et al. New pigments based on carminic acid and smectites: A molecular investigation. Dyes Pigm. 2019, 160, 971–982. [Google Scholar] [CrossRef]
- Velho, S.R.K.; Brum, L.F.W.; Petter, C.O.; dos Santos, J.H.Z.; Simunić, S.; Kappa, W.H. Development of structured natural dyes for use into plastics. Dyes Pigm. 2017, 136, 248–254. [Google Scholar] [CrossRef]
- Tang, P.; Xu, X.; Lin, Y.; Li, D. Enhancement of the thermo- and photo-stability of an anionic dye by intercalation in a zinc-aluminum layered double hydroxide host. End. Eng. Chem. Res. 2008, 47, 2478–2483. [Google Scholar] [CrossRef]
- Chakraborty, C.; Dana, K.; Malik, S. Intercalation of perylenediimde dye into LDH clays: Enhancement of photostability. J. Phys. Chem. C. 2010, 115, 1996–2004. [Google Scholar] [CrossRef]
- Sun, Z.; Jin, L.; Shi, W.; Wei, M.; Duan, X. Preparation of an anion dye intercalated into layered double hydroxides and its controllable luminescence properties. Chem. Eng. J. 2010, 161, 293–300. [Google Scholar] [CrossRef]
- Bauer, J.; Behrens, P.; Speckbacher, M.; Langhals, H. Composites of perylene chromophores and layered double hydroxides: Direct synthesis, characterization, and photo- and chemical stability. Adv. Funct. Mater. 2003, 13, 241–248. [Google Scholar] [CrossRef]
- Maruyama, S.A.; Tavares, S.R.; Leitao, A.A.; Wypych, F. Intercalation of indigo carmine anions into zinc hydroxide salt: A novel alternative blue pigment. Dyes Pigm. 2016, 128, 158–164. [Google Scholar] [CrossRef]
- Kameshima, Y.; Yasumori, A.; Okada, K. XPS and X-ray AES (XAES) study of various aluminate compounds. Hyomen Kagaku 2000, 21, 481–487. [Google Scholar] [CrossRef]
- Fournier, V.; Marcus, P.; Olefjord, I. Oxidation of magnesium. Surf. Interface Anal. 2002, 34, 494–497. [Google Scholar] [CrossRef]
- Fotea, C.; Callaway, J.; Alexander, M.R. Characterisation of the surface chemistry of magnesium exposed to the ambient atmosphere. Surf. Interface Anal. 2006, 38, 1363–1371. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Fadoni, M.; Vercelli, B. Magnesium salts and oxide: An XPS overview. Appl. Surf. Sci. 1997, 119, 253–259. [Google Scholar] [CrossRef]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Moszyński, D.; Kozanecki, M.; Zaborski, M. Characterization and properties of new color-tunable hybrid pigments based on layered double hydroxides (LDH) and 1,2-dihydroxyanthraquinone dye. J. Ind. Eng. Chem. 2018, 70, 427–438. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Kanezaki, E. Effect of atomic ratio Mg/Al in layers of Mg and Al layered double hydroxide on thermal stability of hydrotalcite-like layered structure by means of in situ high temperature powder X-Ray diffraction. Mater. Res. Bull. 1998, 33, 773–778. [Google Scholar] [CrossRef]
- Norman, N.; Bartczak, P.; Zdarta, J.; Tylus, W.; Szatkowski, T.; Stelling, A.L.; Ehrlich, H.; Jesionowski, T. Adsorption of C.I. Natural Red 4 onto Spongin Skeleton of Marine Demosponge. Materials 2015, 8, 96–116. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Rybiński, P.; Maniukiewicz, W.; Zaborski, M. Aluminum-magnesium hydroxycarbonate/azo dye hybrids as novel multifunctional colorants for elastomer composites. Polymers 2019, 11, 43. [Google Scholar] [CrossRef]
- Trigueiro, P.; Pereira, F.A.R.; Guillermin, D.; Rigaud, B.; Balme, S.; Janot, J.M.; Santos, I.M.G.; Fonseca, M.G.; Walter, P.; Jaber, M. When anthraquinone dyes meet pillared montmorillonite: Stability or fading upon exposure to light? Dyes Pigm. 2018, 159, 384–394. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
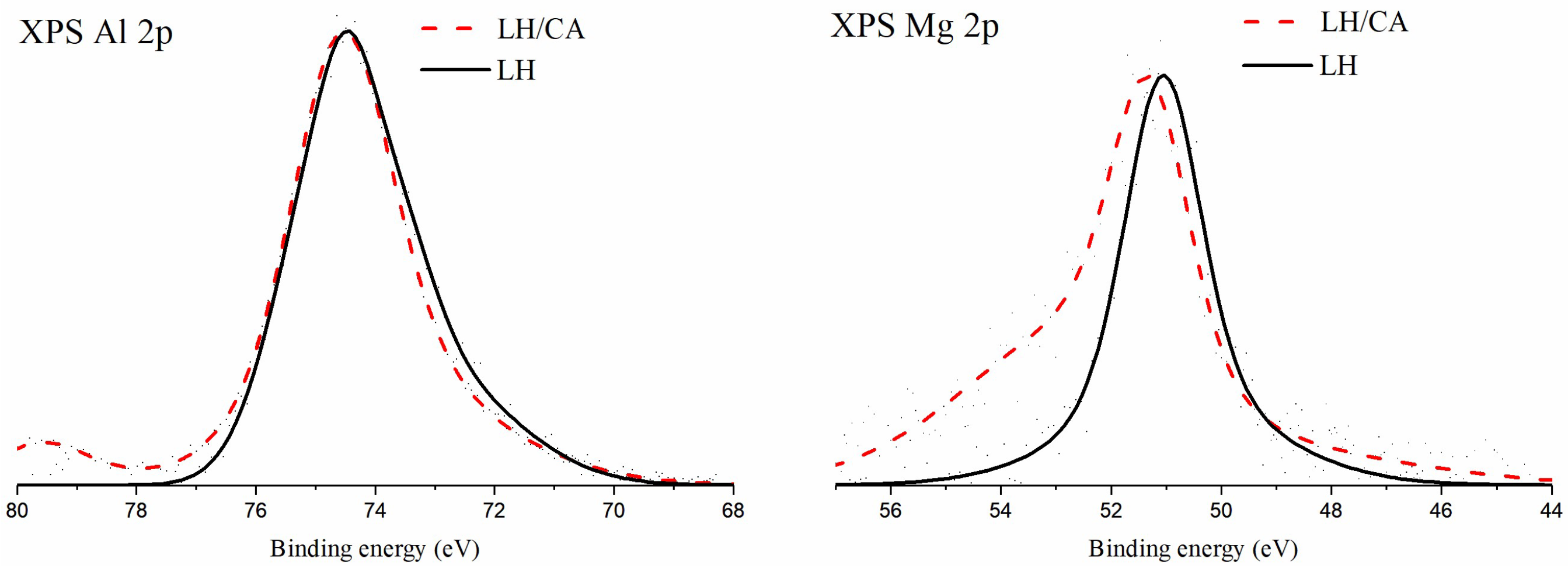
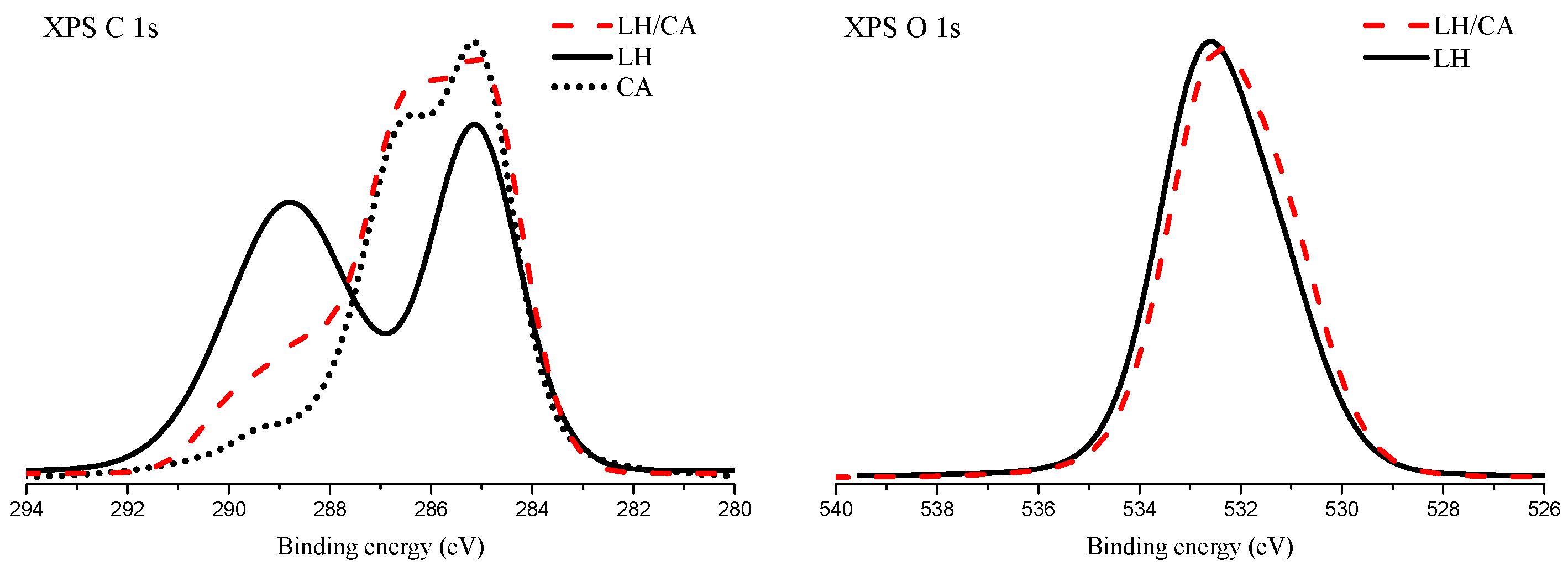
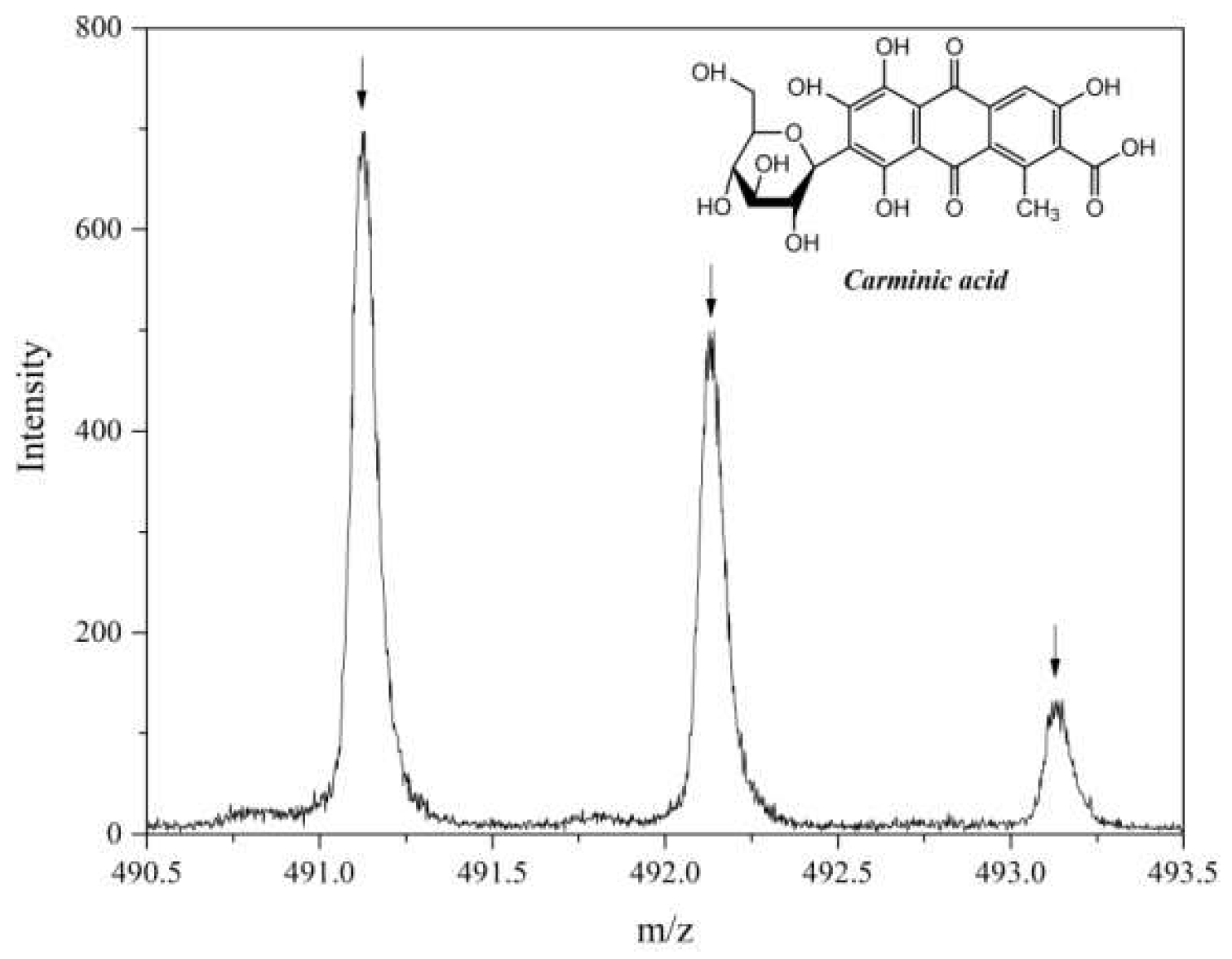

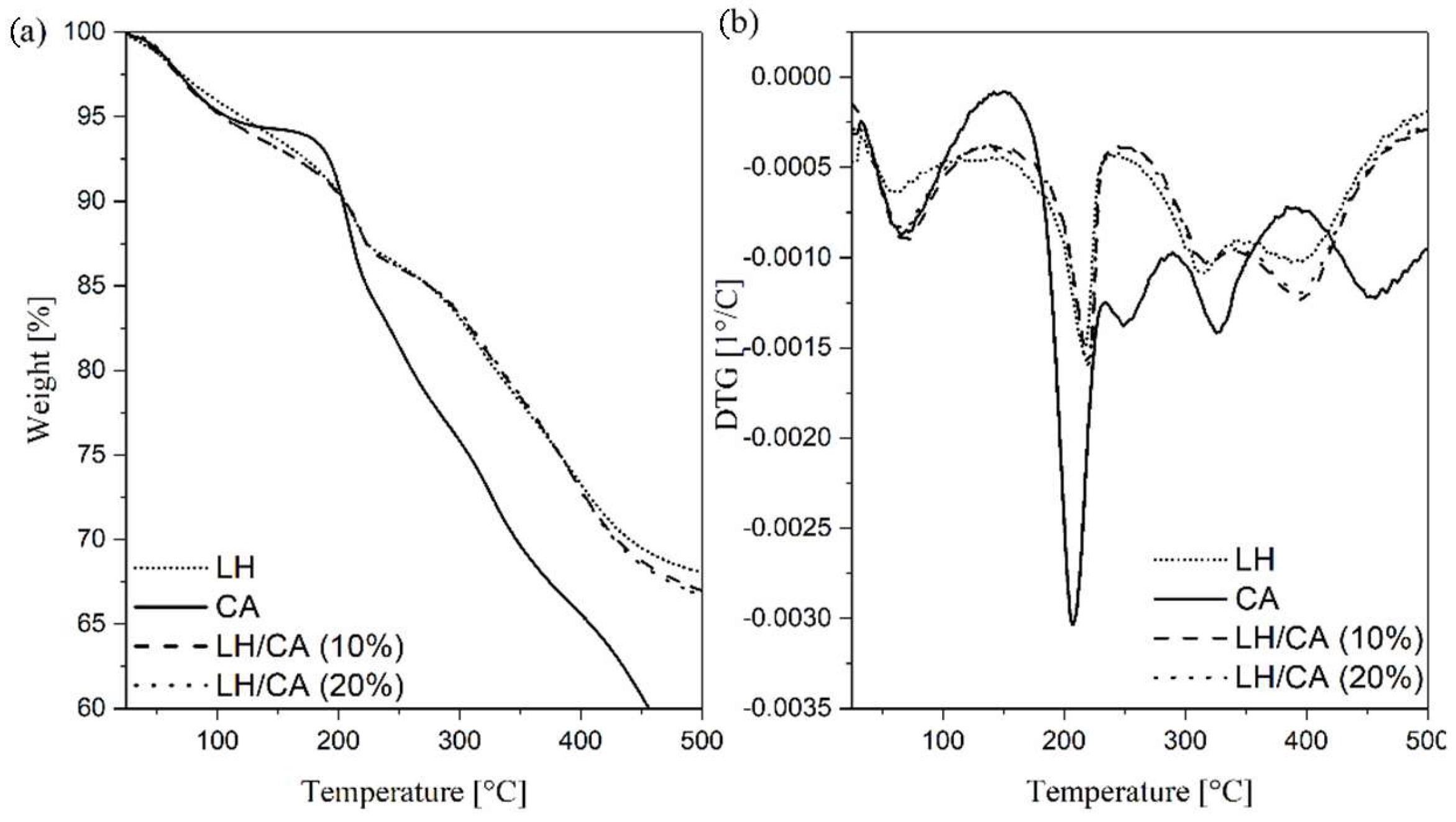




| Sample | Carbon | Oxygen | Magnesium | Aluminum |
|---|---|---|---|---|
| At% | ||||
| LH | 6 | 34 | 8 | 52 |
| CA | 68 | 32 | - | - |
| LH/CA | 12 | 25 | 9 | 54 |
| Sample | Thermal Stability | ||
|---|---|---|---|
| T5% 1 (°C) | T10% 1 (°C) | T20% 1 (°C) | |
| CA | 107 | 203 | 260 |
| LH | 119 | 205 | 329 |
| LH/CA (10%) | 105 | 205 | 334 |
| LH/CA (20%) | 105 | 206 | 335 |
| Sample | Colorimetric Parameters 1 | |||
|---|---|---|---|---|
| L* | a* | b* | ∆E | |
| EN | 85.19 | 0.83 | 4.78 | - |
| EN/LH | 77.33 | −1.11 | 5.99 | 8.19 |
| EN/CA | 56.77 | 34.58 | 20.58 | 46.87 |
| EN/LH/CA (10%) | 42.78 | 25.51 | −2.42 | 49.60 |
| EN/LH/CA (20%) | 37.09 | 22.67 | −2.27 | 53.30 |
| Sample | Flammability Parameters | ||||
|---|---|---|---|---|---|
| HRR 1 (kW/m2) | HRRMAX (kW/m2) | THR 2 (MJ/kg) | EHC 3 (MJ/kg) | MLR 4 (g/m2∙s) | |
| EN | 166.7 | 427.8 | 68.1 | 33.6 | 6.9 |
| EN/LH | 89.9 | 347.4 | 61.1 | 32.9 | 6.6 |
| EN/hybrid pigment | 75.3 | 308.7 | 38.5 | 23.5 | 6.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzec, A.; Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Moszyński, D.; Rybiński, P.; Zaborski, M. Carminic Acid Stabilized with Aluminum-Magnesium Hydroxycarbonate as New Colorant Reducing Flammability of Polymer Composites. Molecules 2019, 24, 560. https://doi.org/10.3390/molecules24030560
Marzec A, Szadkowski B, Rogowski J, Maniukiewicz W, Moszyński D, Rybiński P, Zaborski M. Carminic Acid Stabilized with Aluminum-Magnesium Hydroxycarbonate as New Colorant Reducing Flammability of Polymer Composites. Molecules. 2019; 24(3):560. https://doi.org/10.3390/molecules24030560
Chicago/Turabian StyleMarzec, Anna, Bolesław Szadkowski, Jacek Rogowski, Waldemar Maniukiewicz, Dariusz Moszyński, Przemysław Rybiński, and Marian Zaborski. 2019. "Carminic Acid Stabilized with Aluminum-Magnesium Hydroxycarbonate as New Colorant Reducing Flammability of Polymer Composites" Molecules 24, no. 3: 560. https://doi.org/10.3390/molecules24030560








