Effects of Substituents on the Blue Luminescence of Disilane-Linked Donor‒Acceptor‒Donor Triads
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
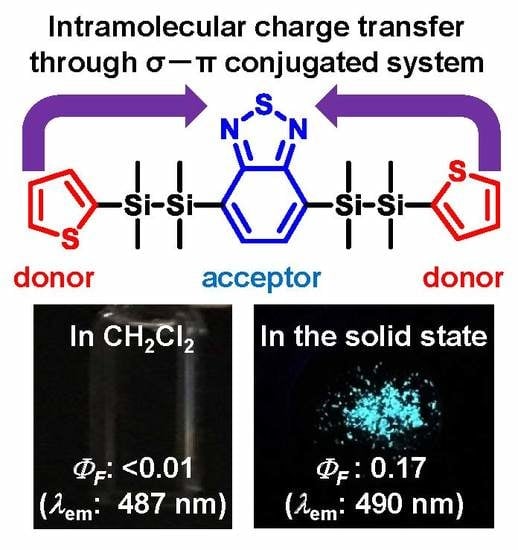
2.2. Optical Properties of the Disilane-Bridged Compounds in Solution and in the Solid States
2.3. XRD Analysis
2.4. Computational Investigation
3. Materials and Methods
3.1. General Information
3.2. X-ray Crystallographic Analysis
3.3. Powder XRD Analysis
3.4. Computational Details
3.5. Typical Experimental Procedure for the Synthesis of 1–5
3.6. Characterization Data for 1–5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Guo, Z.-H.; Jin, Z.-X.; Wang, J.-Y.; Pei, J. A donor–acceptor–donor conjugated molecule: Twist intramolecular charge transfer and piezochromic luminescent properties. Chem. Commun. 2014, 50, 6088–6090. [Google Scholar]
- Sasaki, S.; Drummen, G.P.C.; Konishi, G. Recent advances in twisted intramolecular charge transfer (TICT) fluorescence and related phenomena in materials chemistry. J. Mater. Chem. C 2016, 4, 2731–2743. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, Z. The Strong Light-Emission Materials in the Aggregated State: What Happens from a Single Molecule to the Collective Group. Adv. Sci. 2017, 4, 1600484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, M.; Hiyama, T. Organic Fluorophores Exhibiting Highly Efficient Photoluminescence in the Solid State. Chem. Asian J. 2010, 5, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Cornil, J.; Beljonne, D.; Calbert, J.-P.; Brédas, J.L. Interchain Interactions in Organic π-conjugated materials: Impact on electronic structure, optical response, and charge transport. Adv. Mater. 2001, 13, 1053–1067. [Google Scholar] [CrossRef]
- Karatsu, T. Photochemistry and photophysics of organomonosilane and oligosilanes: Updating their studies on conformation and intramolecular interactions. J. Photochem. Photobiol. C 2008, 9, 111–137. [Google Scholar] [CrossRef]
- Sakurai, H.; Tasaka, S.; Kira, M. Electronic Spectra of l,l,2,2-Tetramethyl-3,4-benzo-l,2-disilacyclopentene-3 and Related Compounds. Stereoelectronic Verification of σ-π Conjugation between Silicon-Silicon σ Bonds and Benzenoid π Systems. J. Am. Chem. Soc. 1972, 94, 9285–9286. [Google Scholar] [CrossRef]
- Tajima, Y.; Ishikawa, H.; Miyazawa, M.; Kira, M.; Mikami, N. First Observation of Intramolecular Charge-Transfer Emission from Jet-Cooled (p-Cyanophenyl)pentamethyldisilane in an Isolated Molecular Condition. J. Am. Chem. Soc. 1997, 119, 7400–7401. [Google Scholar] [CrossRef]
- Kira, M.; Miyazawa, T.; Mikami, N.; Sakurai, H. Conformational Analysis of Phenylpentamethyldlsllane and Related Compounds as Studied by Free-Jet Laser Spectroscopy. Organometallics 1991, 10, 3793–3795. [Google Scholar] [CrossRef]
- Shimizu, M.; Oda, K.; Bando, T.; Hiyama, T. Preparation, Structure, and Properties of Tris(trimethylsilyl)silyl-substituted Anthracenes: Realization of Ideal Conformation for σ‒π Conjugation Involving Eclipse of Si–Si σ-Bond with π-Orbital of Aromatic Ring. Chem. Lett. 2006, 35, 1022–1023. [Google Scholar] [CrossRef]
- Yabusaki, Y.; Ohshima, N.; Kondo, H.; Kusamoto, T.; Yamanoi, Y.; Nishihara, H. Versatile Synthesis of Blue Luminescent Siloles and Germoles and Hydrogen-Bond-Assisted Color Alteration. Chem. Eur. J. 2010, 16, 5581–5585. [Google Scholar] [CrossRef] [PubMed]
- Inubushi, H.; Hattori, Y.; Yamanoi, Y.; Nishihara, H. Structures and optical properties of tris(trimethylsilyl)silylated oligothiophene derivatives. J. Org. Chem. 2014, 79, 2974–2979. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Yamanoi, Y.; Matsushita, T.; Kondo, T.; Nishibori, E.; Hatakeyama, A.; Sugimoto, K.; Nishihara, H. Optical Properties of Disilane-Bridged Donor-Acceptor Architectures: Strong Effect of Substituents on Fluorescence and Nonlinear Optical Properties. J. Am. Chem. Soc. 2015, 137, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Yamanoi, Y.; Ohto, T.; Pham, S.-T.; Yamada, R.; Tada, H.; Omoto, K.; Tashiro, S.; Shionoya, M.; Hattori, M.; et al. Multifunctional Octamethyltetrasila[2.2]cyclophanes: Conformational Variations, Circularly Polarized Luminescence, and Organic Electroluminescence. J. Am. Chem. Soc. 2017, 139, 11214–11221. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Tsuchiya, M.; Sakamoto, R.; Yamanoi, Y.; Nishibori, E.; Sugimoto, K.; Nishihara, H. Bright Solid-State Emission of Disilane-Bridged Donor‒Acceptor‒Donor and Acceptor‒Donor‒Acceptor Chromophores. Angew. Chem. Int. Ed. 2016, 55, 3022–3026. [Google Scholar] [CrossRef]
- Usuki, T.; Shimada, M.; Yamanoi, Y.; Ohto, T.; Tada, H.; Kasai, H.; Nishibori, E.; Nishihara, H. Aggregation-induced Enhanced Emission from Disilane bridged Donor‒Acceptor‒Donor Luminogens Based on Triarylamine Functionality. ACS Appl. Mater. Interfaces 2018, 10, 12164–12172. [Google Scholar] [CrossRef]
- Shimada, M.; Yamanoi, Y.; Nishihara, H. Unusual reactivity of group 14 hydrides toward organic halides: Synthetic studies and application to functional materials. J. Synth. Org. Chem. Jpn. 2016, 74, 1098–1107. [Google Scholar] [CrossRef]
- Yamanoi, Y.; Nishihara, H. Efficient synthesis of arylsilanes by cross-coupling of aromatic compound with hydrosilanes as silicon sources. J. Synth. Org. Chem. Jpn. 2009, 67, 778–786. [Google Scholar] [CrossRef]
- Lesbani, A.; Kondo, H.; Sato, J.-I.; Yamanoi, Y.; Nishihara, H. Facile synthesis of hypersilylated aromatic compounds by palladium-mediated arylation reaction. Chem. Commun. 2010, 46, 7784–7786. [Google Scholar] [CrossRef]
- Skorotetcky, M.S.; Krivtsova, E.D.; Borshchev, O.V.; Surin, N.M.; Svidchenko, E.A.; Fedorov, Y.V.; Pisarev, S.A.; Ponomarenko, S.A. Influence of the structure of electron-donating aromatic units in organosilicon luminophores based on 2,1,3-benzothiadiazole electron-withdrawing core on their absorption-luminescent properties. Dyes Pigment. 2018, 155, 284–291. [Google Scholar] [CrossRef]
- Fluorescence of related disilane compounds as dispersion in polymer matrix has been investigated and no dependence on emission properties was observed at all in comparison with pure solid compound. In addition, there was no concentration dependence in solution in the previous related reports. Please see references [15,16].
- CCDC 1887619 (1) and 1887703 (2) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the Cambridge Crystallographic Data Center.
- Beagley, B.; Monaghan, J.J.; Hewitt, T.G. Electron-diffraction studies of tetramethylsilane and hexamethyldisilane, and discussion of the lengths of Si-C bonds. J. Mol. Struct. 1971, 8, 401–411. [Google Scholar] [CrossRef]
- Baxter, S.G.; Mislow, K.; Blount, J.F. Conformational analysis of 1,1,2,2-tetramethyldisilane. Tetrahedron 1980, 36, 605–616. [Google Scholar] [CrossRef]
- Söldner, M.; Šander, M.; Schier, M.; Schmidbaur, H. Synthesis, structure and photoluminescence of 1,2-disila-acenaphthene Si2C10H10 and 1,2-diaryldisilane reference compounds. Chem. Ber. 1997, 130, 1671–1676. [Google Scholar] [CrossRef]
- Maus, M.; Rettig, W.; Bonafoux, D.; Lapouyade, R. Photoinduced Intramolecular Charge Transfer in a Series of Differently Twisted Donor-Acceptor Biphenyls as Revealed by Fluorescence. J. Phys. Chem. A 1999, 103, 3388. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bijleveld, J.C.; Shahid, M.; Gilot, J.; Wienk, M.M.; Janssen, R.A.J. Copolymers of Cyclopentadithiophene and Electron-Deficient Aromatic Units Designed for Photovoltaic Applications. Adv. Funct. Mater. 2009, 19, 3262–3270. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97, Program for the Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Nishibori, E.; Takata, M.; Kato, K.; Sakata, M.; Kubota, Y.; Aoyagi, S.; Kuroiwa, Y.; Yamakata, M.; Ikeda, N. The large Debye-Scherrer camera installed at SPring-8 BL02B2 for charge density studies. Nucl. Instrum. Methods Phys. Res. A 2001, 467, 1045–1048. [Google Scholar] [CrossRef]
- Boultif, A.; Louër, D. Powder pattern indexing with the dichotomy method. J. Appl. Crystallogr. 2004, 37, 724–731. [Google Scholar] [CrossRef]
- Nishibori, E.; Ogura, T.; Aoyagi, S.; Sakata, M. Ab initio structure determination of a pharmaceutical compound, prednisolone succinate, from synchrotron powder data by combination of a genetic algorithm and the maximum entropy method. J. Appl. Crystallogr. 2008, 41, 292–301. [Google Scholar] [CrossRef]
- Nishibori, E.; Sunaoshi, E.; Yoshida, A.; Aoyagi, S.; Kato, K.; Takata, M.; Sakata, M. Accurate structure factors and experimental charge densities from synchrotron X-ray powder diffraction data at SPring-8. Acta Crystallogr. 2007, A63, 43–52. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds 1–5 are available from the authors. |




| Compound | In CH2Cl2 a | In the Solid State | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λabs (nm) b | ε (M−1 cm−1) c | λem (nm) d | ΦFe | τ (ns) f | kf (ns−1) g | knr (ns−1) h | λem (nm) i | ΦFe | τ (ns) f | kf (ns−1) g | knr (ns−1) h | |
| 1 | 360 | 7960 | 487 | 0.004 | − j | − j | − j | 536 | 0.006 | 0.47 | 0.013 | 2.1 |
| 2 | 353 | 7670 | 497 | 0.002 | − j | − j | − j | 490 | 0.167 | 2.8 | 0.060 | 0.30 |
| 3 | 385 | 4280 | 503 | 0.097 | 2.8 | 0.035 | 0.32 | 456 | 0.019 | 0.91 | 0.021 | 1.1 |
| 4 | 380 | 5280 | 495 | 0.021 | 0.58 | 0.034 | 1.7 | − k | − k | − k | − k | − k |
| 5 | 388 | 9960 | 460 | 0.023 | 0.69 | 0.033 | 1.4 | − k | − k | − k | − k | − k |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usuki, T.; Omoto, K.; Shimada, M.; Yamanoi, Y.; Kasai, H.; Nishibori, E.; Nishihara, H. Effects of Substituents on the Blue Luminescence of Disilane-Linked Donor‒Acceptor‒Donor Triads. Molecules 2019, 24, 521. https://doi.org/10.3390/molecules24030521
Usuki T, Omoto K, Shimada M, Yamanoi Y, Kasai H, Nishibori E, Nishihara H. Effects of Substituents on the Blue Luminescence of Disilane-Linked Donor‒Acceptor‒Donor Triads. Molecules. 2019; 24(3):521. https://doi.org/10.3390/molecules24030521
Chicago/Turabian StyleUsuki, Tsukasa, Kenichiro Omoto, Masaki Shimada, Yoshinori Yamanoi, Hidetaka Kasai, Eiji Nishibori, and Hiroshi Nishihara. 2019. "Effects of Substituents on the Blue Luminescence of Disilane-Linked Donor‒Acceptor‒Donor Triads" Molecules 24, no. 3: 521. https://doi.org/10.3390/molecules24030521