Co-Application of Milk Tea Waste and NPK Fertilizers to Improve Sandy Soil Biochemical Properties and Wheat Growth
Abstract
:1. Introduction
2. Results
2.1. Effect of Milk Tea Waste (TW) Application on Plant Growth
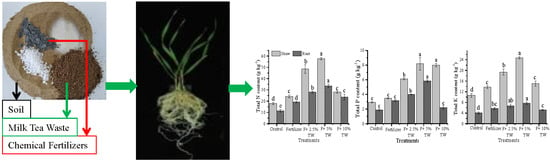
2.2. N, P, and K Contents in Shoot and Root
2.3. Root Traits
2.4. Soil Chemical Properties
2.5. Soil Enzyme Activities
2.6. Relationships among Root and Plant NPK, Soil Enzymes Activities and Soil Properties
3. Discussion
3.1. Effects of Milk Tea Waste (TW) on Wheat Growth Traits
3.2. Impact of TW on Wheat Root Traits
3.3. Effect of Amendments on N, P, and K Uptake in Wheat Shoots and Roots
3.4. Soil Enzymes Activities
3.5. Effect of Amendment on Soil Chemical Properties
4. Materials and Methods
4.1. Collection of Milk Tea Waste (TW) and Sandy Soil
4.2. Experimental Setup
4.3. Plant Growth Parameters
4.3.1. Chlorophyll Content
4.3.2. Root Traits
4.4. Chemical Analysis of Soil, Plant, and Milk Tea Waste
4.5. Soil Enzyme Assays
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laghari, M.; Mirjat, M.S.; Hu, Z.; Fazal, S.; Xiao, B.; Hu, M. Effects of biochar application rate on sandy desert soil properties and sorghum growth. Catena 2015, 135, 313–320. [Google Scholar] [CrossRef]
- Peng, Y.F.; Guo, D.L.; Yang, Y.H. Global patterns of root dynamics under nitrogen enrichment. Glob. Ecol. Biogeogr. 2017, 26, 102–114. [Google Scholar] [CrossRef]
- Asperen, H.L.; Bor, A.M.C.; Sonneveld, M.P.W.; Bruins, H.J.; Lazarovitch, N. Properties of anthropogenic soils in ancient run-off capturing agricultural terraces in the Central Negev desert (Israel) and related effects of biochar and ash on crop growth. Plant Soil 2013, 374, 779–792. [Google Scholar] [CrossRef]
- Li, Z.; Schneider, R.L.; Morreale, S.J.; Xie, Y.; Li, C.; Li, J. Woody organic amendments for retaining soil water, improving soil properties and enhancing plant growth in desertified soils of Ningxia, China. Geoderma 2018, 310, 143–152. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Children and the environment. J. Environ. Health Perspect. 1997, 105, 370–371. [Google Scholar]
- Dong, Z.B.; Chen, G.T.; He, X.D.; Han, Z.W.; Wang, X.M. Controlling blown sand along the highway crossing the Taklimakan desert. J. Arid Environ. 2004, 57, 329–344. [Google Scholar] [CrossRef]
- Zhang, Y.; Smil, V. An estimate of economic loss for desertificationin China. China Popul. Resour. Environ. 1996, 6, 45–49. [Google Scholar]
- Sadaf, J.; Shah, G.A.; Shahzad, K.; Ali, N.; Shahid, M.; Ali, S.; Hussain, R.A.; Ahmed, Z.I.; Traore, B.I.; Ismail, M.I.; et al. Improvements in wheat productivity and soil quality can accomplish by co-application of biochars and chemical fertilizers. Sci. Total Environ. 2017, 607–608, 715–724. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, B.; Olk, D.C.; Jia, Z.; Mao, J.; Cai, Y.; Zhang, J. Contributions of residue-C and -N to plant growth and soil organic matter pools under planted and unplanted conditions. Soil Biol. Biochem. 2018, 120, 91–104. [Google Scholar] [CrossRef]
- Pan, F.; Yu, W.; Ma, Q.; Zhou, H.; Jiang, C.; Xu, Y. Do organic amendments improve the synchronism between soil n supply and wheat demand? Appl. Soil Ecol. 2018, 125, 184–191. [Google Scholar] [CrossRef]
- Razaq, M.; Salahuddin; Shen, H.; Sher, H.; Zhang, P. Influence of biochar and nitrogen on fine root morphology, physiology, and chemistry of acer mono. Sci. Rep. 2017, 7, 5367. [Google Scholar] [CrossRef] [PubMed]
- Chocano, C.; García, C.; González, D.; Aguilar, J.M.D.; Hernández, T. Organic plum cultivation in the mediterranean region: The medium-term effect of five different organic soil management practices on crop production and microbiological soil quality. Agric. Ecosyst. Environ. 2016, 221, 60–70. [Google Scholar] [CrossRef]
- Neff, J.C.; Townsend, A.R.; Gleixner, G.; Lehman, S.J.; Turnbull, J.; Bowman, W.D. Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 2002, 419, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Mi, W.; Sun, Y.; Xia, S.; Zhao, H.; Mi, W.; Brookes, P.C.; Liu, Y.; Wu, L. Effect of inorganic fertilizers with organic amendments on soil chemical properties and rice yield in a low-productivity paddy soil. Geoderma 2018, 320, 23–29. [Google Scholar] [CrossRef]
- Wang, R.; Yao, Z.; Lei, Y. Modeling of soil available phosphorus surplus in an intensive wheat–maize rotation production area of the North China Plain. Agric. Ecosyst. Environ. 2019, 269, 22–29. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, W.; He, X.; Zhu, P.; Gao, H.; Sun, N.; Xu, M. Chemical fertilizers could be completely replaced by manure to maintain high maize yield and soil organic carbon (SOC) when SOC reaches a threshold in the northeast china plain. J. Integr. Agric. 2017, 16, 937–946. [Google Scholar] [CrossRef]
- Uga, Y.; Kitomi, Y.; Ishikawa, S.; Yano, M. Genetic improvement for root growth angle to enhance crop production. Breed. Sci. 2015, 65, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Ludewig, U.; Neuhauser, B.; Dynowski, M. Molecular mechanisms of ammonium transport and accumulation in plants. FEBS Lett. 2007, 581, 2301–2308. [Google Scholar] [CrossRef] [Green Version]
- Loque, D.; Von Wiren, N. Regulatory levels for the transport of ammonium in plant roots. J. Exp. Bot. 2004, 55, 1293–1305. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Yanai, R.D. The ecology of root lifespan. Adv. Ecol. Res. 1997, 27, 1–60. [Google Scholar]
- Xiang, Y.; Deng, Q.; Duan, H.; Guo, Y. Effects of biochar application on root traits: A meta- analysis. GCB Bioenergy 2017, 9, 1563–1572. [Google Scholar] [CrossRef]
- Ryser, P. Intra- and interspecific variation in root length, root turnover and the underlying parameters. In Inherent Variation in Plant Growth: Physiological Mechanisms and Ecological Consequences; Lambers, H., Poorter, H., Van Vuuren, M.M.I., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1998; pp. 441–465. [Google Scholar]
- Coleman, J.S.; Mc Connaughay, K.D.M.; Ackerly, D.D. Interpreting phenotypic variation in plants. Trends Ecol. Evol. 1994, 9, 187–191. [Google Scholar] [CrossRef]
- Herencia, J.F.; Garcia-Galavis, P.A.; Maqueda, C. Long-term effect of organic and mineral fertilization on soil physical properties under greenhouse and outdoor management practices. Pedosphere 2011, 21, 443–453. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, H.V.; Rao, L.J.M. Influence of milk and sugar on antioxidant potential of black tea. Food Res. Int. 2008, 41, 124–129. [Google Scholar] [CrossRef]
- Burn, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Bharali, A.; Baruaha, K.K.; Bhattacharyya, P.; Gorh, D. Integrated nutrient management in wheat grown in a northeast India soil: Impacts on soil organic carbon fractions in relation to grain yield. Soil Tillage Res. 2017, 168, 81–91. [Google Scholar] [CrossRef]
- Ali, A.; Guo, D.; Zhang, Y.; Sun, X.; Jiang, S.; Guo, Z.; Huang, H.; Liang, W.; Li, R.; Zhang, Z. Using bamboo biochar with compost for the stabilization and phytotoxicity reduction of heavy metals in mine-contaminated soils of China. Sci. Rep. 2017, 7, 2690. [Google Scholar] [CrossRef]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. Crop yield, plant nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on nitisols. Soil Tillage Res. 2016, 160, 1–13. [Google Scholar] [CrossRef]
- Cucina, M.; Tacconi, C.; Ricci, A.; Pezzolla, D.; Sordi, S.; Zadra, C.; Gigliotti, G. Evaluation of benefits and risks associated with the agricultural use of organic wastes of pharmaceutical origin. Sci. Total Environ. 2018, 613, 773–782. [Google Scholar] [CrossRef]
- Gaiotti, F.; Marcuzzo, P.; Belfiore, N.; Lovat, L.; Fornasier, F.; Tomasi, D. Influence of compost addition on soil properties, root growth and vine performances of Vitis vinifera cv Cabernet sauvignon. Sci. Hortic. 2017, 225, 88–95. [Google Scholar] [CrossRef]
- Barraclough, P.B.; Kuhlmann, H.; Weir, A.H. The effects of prolonged drought and nitrogen fertiliser on root and shoot growth and water uptake by winter wheat. J. Agron. Crop Sci. 1989, 163, 352–360. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Carbon-nutrient stoichiometry to increase soil carbon sequestration. Soil Biol. Biochem. 2013, 60, 77–86. [Google Scholar] [CrossRef]
- Siczek, A.; Lipiec, J. Soybean nodulation and symbiotic nitrogen fixation in response to soil compaction and mulching. Pet. Refin. Eng. 2009, 11, 447–453. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-ur-Rehman, M.; Qayyum, M.F.; Sik Ok, Y.; Murtaza, G. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ. Sci. Pollut. Res. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Khan, K.S.; Marschner, P.; Hassan, F. Microbial biomass, nutrient availability and nutrient uptake by wheat in two soils with organic amendments. J. Soil Sci. Plant Nutr. 2013, 13, 955–966. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Feng, Y.; Yang, G. Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Tillage Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Sial, T.A.; Khan, M.N.; Lan, Z.; Kumbhar, F.; Zhao, Y.; Zhang, J.; Sun, D.; Xiu, L. Contrasting effects of banana peels waste and its biochar on greenhouse gas emissions and soil biochemical properties. Process Saf. Environ. Prot. 2018, 122, 366–377. [Google Scholar] [CrossRef]
- Blagodatskaya, E.V.; Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol. Fertil. Soils 2008, 45, 115–131. [Google Scholar] [CrossRef]
- Mandal, A.; Patra, A.K.; Singh, D.; Swarup, A.; Masto, R.E. Effect of long-term application of manure and fertilizer on biological and biochemical activities in soil during crop development stages. Bioresource Technol. 2007, 98, 3585–3592. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.Y.; Dodla, S.K.; Wang, J.J. Fundamental and molecular composition characteristics of biochars produced from sugarcane and rice crop residues and by-products. Chemosphere 2016, 142, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.J.; Hansen, N.; Wallenstein, M.; Cotrufo, M.F. Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric. Ecosyst. Environ. 2016, 233, 404–414. [Google Scholar] [CrossRef]
- Arif, M.S.; Riaz, M.; Shahzad, S.M.; Yasmeen, T.; Ashraf, M.; Siddique, M.; Mubarik, M.S.; Bragazza, L.; Buttler, A. Fresh and composted industrial sludge restore soil functions in surface soil of degraded agricultural land. Sci. Total Environ. 2018, 619, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.A.; Zhou, L.M. Soil organic carbon sequestration and fertility response to newly-built terraces with organic manure and mineral fertilizer in a semi-arid environment. Soil Tillage Res. 2017, 172, 39–47. [Google Scholar] [CrossRef]
- Dwyer, L.M.; Stewart, D.W. Leaf area development in field grown maize. Agron. J. 1986, 78, 334–343. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls A, and B, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Sochan, A.; Bieganowski, A.; Ryżak, M.; Dobrowolski, R.; Bartmiński, P. Comparison of soil texture determined by two dispersion units of Mastersizer 2000. Int. Agrophys. 2012, 26, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Parkinson, J.A.; Allen, S.E. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Geochim. Cosmochim. Acta 1975, 52, 730–733. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Knudsen, D.; Peterson, G.A.; Pratt, P.F. Lithium, Sodium, and Potassium. In Method of Soil Analysis; Part 2; American Society of Agronomy: Madison, WI, USA, 1982; pp. 225–245. [Google Scholar]
- Shaheen, S.M.; Rinklebe, J.; Rupp, H.; Meissner, R. Lysimeter trials to assess the impact of different flood-dry-cycles the dynamics of pore water concentrations of As, Cr, Mo, and V in a contaminated floodplain soil. Geoderma 2014, 13, 228–229. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Method of Soil Analysis Part 2. Microbiological Properties; Weaver, R.W., Angle, S., Bottomely, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; Soil Science Society of America (SSSA): Madison, WI, USA, 1994; pp. 775–883. [Google Scholar]
Sample Availability: Not available. |








| Treatment | Plant Height (cm) | Shoot Dry Weight (g·pot−1) | Root Dry Weight (g·pot−1) | Leaf Area Index (cm2) |
|---|---|---|---|---|
| T1 | 18.0 ± 1.6 d | 0.14 ± 0.01 c | 0.16 ± 0.01 b | 0.30 ± 0.01 c |
| T2 | 20.5 ± 0.3 c | 0.23 ± 0.02 c | 0.19 ± 0.01 b | 0.41 ± 0.05 bc |
| T3 | 25.0 ± 0.6 b | 0.33 ± 0.01 b | 0.28 ± 0.03 a | 0.62 ± 0.06 b |
| T4 | 29.5 ± 0.6 a | 0.50 ± 0.07 a | 0.33± 0.03 a | 0.87 ± 0.06 a |
| T5 | 19.2 ± 1.0 c | 0.25 ± 0.03 bc | 0.21 ± 0.02 b | 0.57 ± 0.01 b |
| Treatment | Root Length Density (cm·cm−3) | Root Length Ratio (cm·g−1) | Root Mass Ratio (g·g−1) | Root Fineness (cm·cm−3) |
|---|---|---|---|---|
| T1 | 0.43 ± 0.06 b | 674 ± 41 c | 0.53 ± 0.01 a | 457 ± 62 c |
| T2 | 0.67 ± 0.05 b | 683 ± 63 c | 0.42 ± 0.04 bc | 525 ± 49 c |
| T3 | 1.23 ± 0.22 a | 928 ± 30 ab | 0.45 ± 0.05 abc | 848 ± 30 ab |
| T4 | 1.31 ± 0.30 a | 1145 ± 137 a | 0.41 ± 0.01 c | 956 ± 80 a |
| T5 | 0.75 ± 0.08 b | 801 ± 16 bc | 0.51 ± 0.03 ab | 789 ± 16 b |
| pH | SOC | NO3− | NH4+ | AP | AK | Urease | Glucosidase | Dehydrogenase | |
|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | −0.839 ** | −0.399 | −0.641 ** | −0.730 ** | −0.817 ** | −0.760 ** | −0.934 ** | −0.812 ** |
| SOC | 1 | 0.209 | 0.928 ** | 0.950 ** | 0.942 ** | 0.975 ** | 0.944 ** | 0.985 ** | |
| NO3− | 1 | 0.046 | 0.129 | 0.485 | 0.267 | 0.309 | 0.248 | ||
| NH4+ | 1 | 0.926 ** | 0.837 ** | 0.950 ** | 0.780 ** | 0.913 ** | |||
| AP | 1 | 0.905 ** | 0.969 ** | 0.877 ** | 0.950 ** | ||||
| AK | 1 | 0.955 ** | 0.915 ** | 0.954 ** | |||||
| Urease | 1 | 0.896 ** | 0.975 ** | ||||||
| Glucosidase | 1 | 0.921 ** | |||||||
| Dehydrogenase | 1 |
| Parameters | Soil | Milk Tea Waste (TW) |
|---|---|---|
| Clay (%) | 5.50 | |
| Silt (%) | 15.61 | |
| Sand (%) | 78.89 | |
| Water holding capacity (WHC) | 16% | |
| pH (1:2.5) | 9.21 ± 0.5 | 5.36 ± 0.3 (1:10) |
| EC (1:2.5) (μS·cm−1) | 1513 ± 40.7 | 1600 ± 48.5 (1:10) |
| Total carbon (%) | 1.97 ± 0.05 | 48.10 ± 4.5 |
| Total N (%) | 0.02 ± 0.0 | 3.76 ± 0.5 |
| C:N | 84.92 ± 4 | 12.79 ± 2 |
| Total P (%) | 0.42 ± 0.01 | |
| Total K (%) | 0.52 ± 0.01 | |
| Olsen P (mg·kg−1) | 12.15 ± 1 | |
| K exchangeable (mg·kg−1) | 125.56 ± 3 | |
| Total Zn (mg·kg−1) | 34.20 ± 2.1 | |
| Total Cu (mg·kg−1) | 6.05 ± 0.9 | |
| Total Fe (mg·kg−1) | 774.34 ± 18.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sial, T.A.; Liu, J.; Zhao, Y.; Khan, M.N.; Lan, Z.; Zhang, J.; Kumbhar, F.; Akhtar, K.; Rajpar, I. Co-Application of Milk Tea Waste and NPK Fertilizers to Improve Sandy Soil Biochemical Properties and Wheat Growth. Molecules 2019, 24, 423. https://doi.org/10.3390/molecules24030423
Sial TA, Liu J, Zhao Y, Khan MN, Lan Z, Zhang J, Kumbhar F, Akhtar K, Rajpar I. Co-Application of Milk Tea Waste and NPK Fertilizers to Improve Sandy Soil Biochemical Properties and Wheat Growth. Molecules. 2019; 24(3):423. https://doi.org/10.3390/molecules24030423
Chicago/Turabian StyleSial, Tanveer Ali, Jiao Liu, Ying Zhao, Muhammad Numan Khan, Zhilong Lan, Jianguo Zhang, Farhana Kumbhar, Kashif Akhtar, and Inayatullah Rajpar. 2019. "Co-Application of Milk Tea Waste and NPK Fertilizers to Improve Sandy Soil Biochemical Properties and Wheat Growth" Molecules 24, no. 3: 423. https://doi.org/10.3390/molecules24030423