A Green-emitting Fluorescent Probe Based on a Benzothiazole Derivative for Imaging Biothiols in Living Cells
Abstract
:1. Introduction
2. Results and Discussion
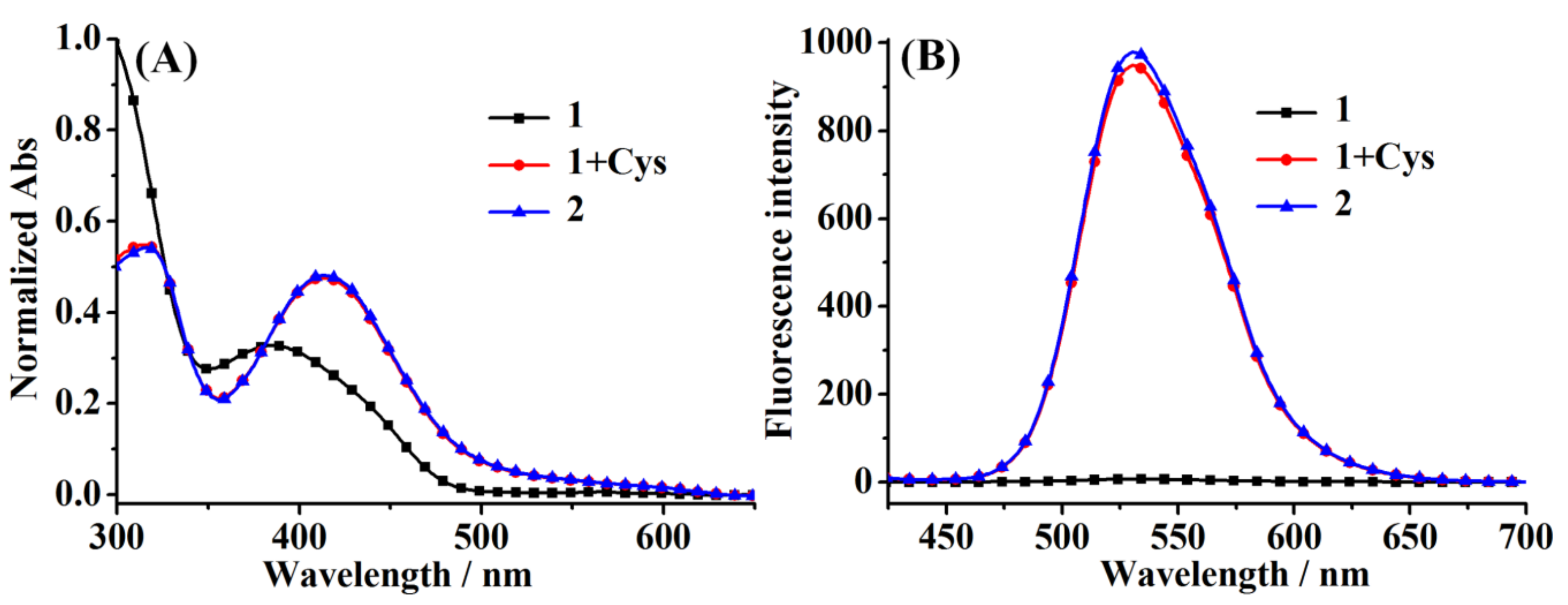
2.1. Spectroscopic Studies
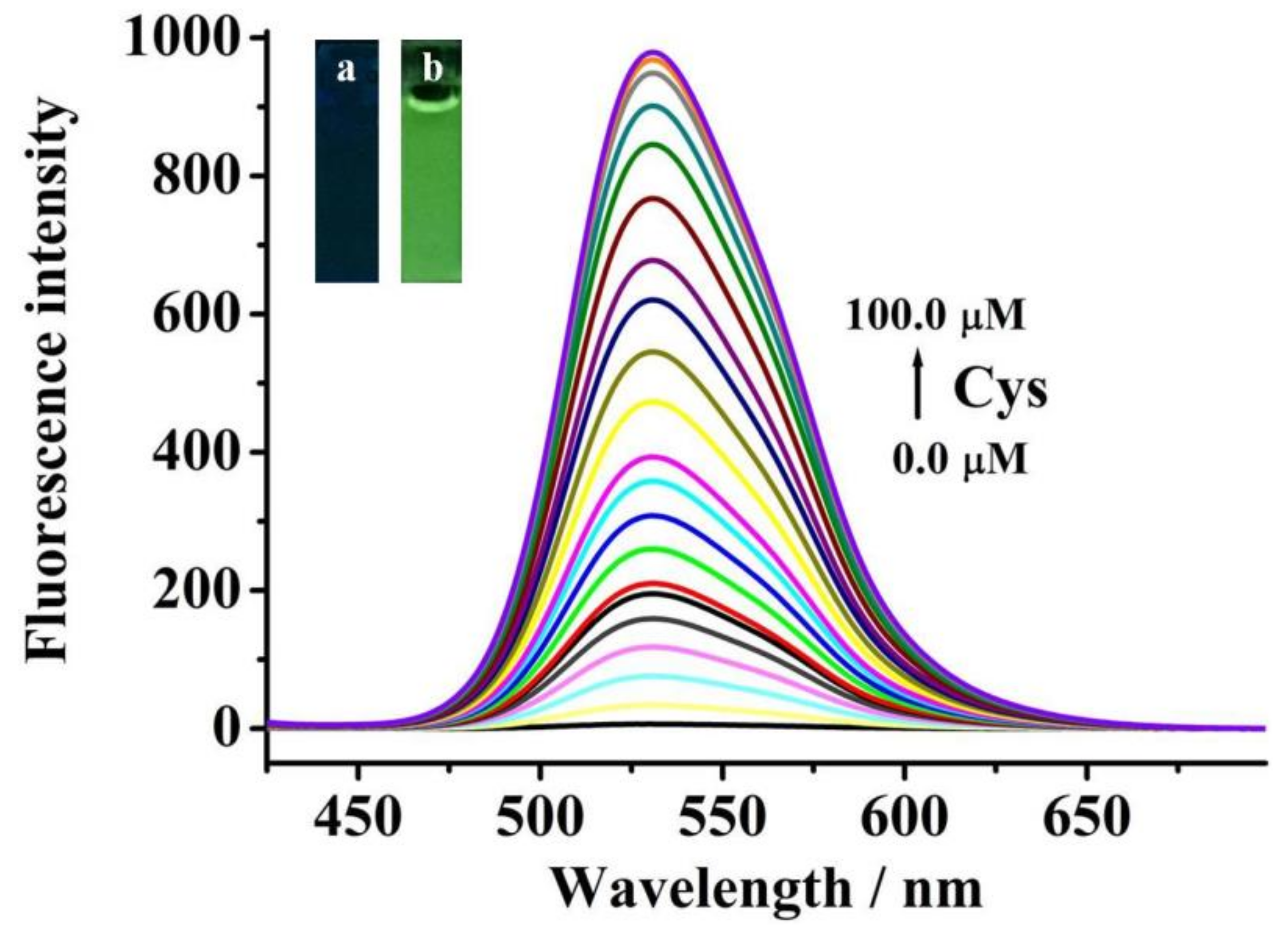
2.2. Sensitivity Studies
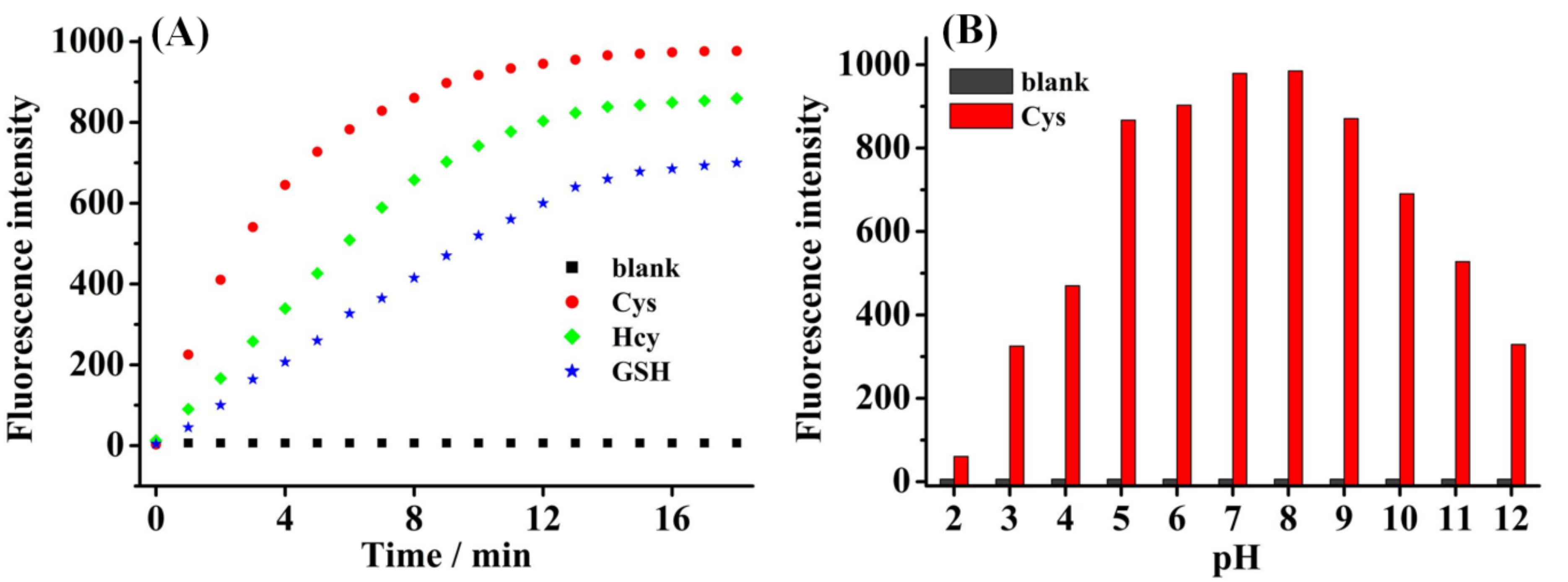
2.3. Selective and Interference Fluorescence Response to Thiols
2.4. Kinetics and the Effects of pH
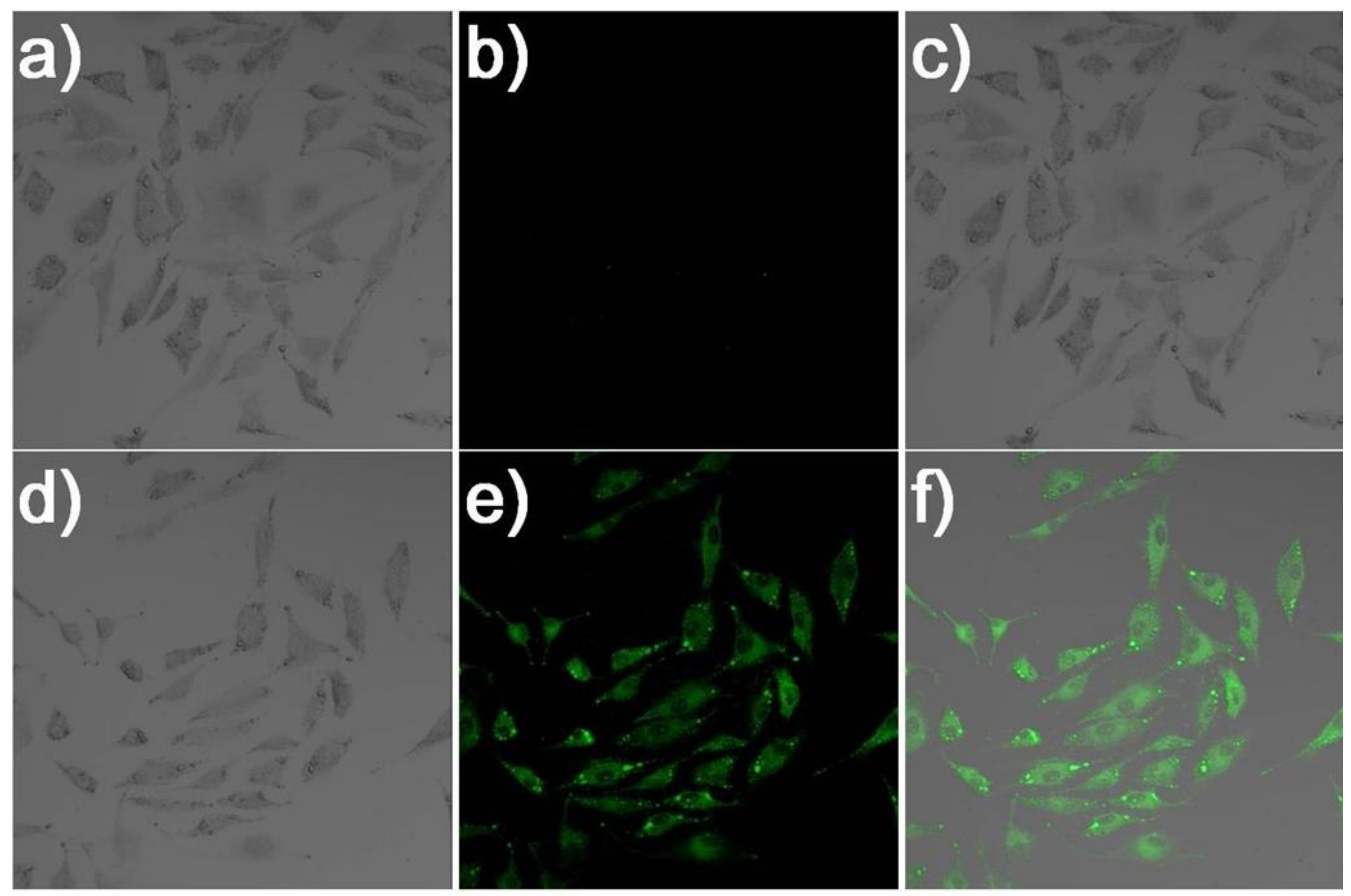
2.5. Bioimaging of Probe 1
3. Materials and Methods
3.1. Instruments and Chemicals
3.2. Preparation of Spectra Measurements
3.3. Cell Assay
3.4. Synthesis of Dye 2
3.5. Synthesis of Probe 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Ding, H.; Xu, H.J.; Lv, Y.L.; Liu, H.; Wang, H.D.; Tian, Z.Y. Dual-functional probes for sequential thiol and redox homeostasis sensing in live cells. Analyst 2015, 140, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Yu, Y.W.; Wang, B.X.; Jiang, Y.L. A sensitive fluorescent probe based on coumarin for detection of cysteine in living cells. J. Photochem. Photobiol. A 2017, 338, 178–182. [Google Scholar] [CrossRef]
- Gao, J.H.; Tao, Y.F.; Wang, N.N.; He, J.L.; Zhang, J.; Zhao, W.L. BODIPY-based turn-on fluorescent probes for cysteine and homocysteine. Spectrochim. Acta Part A 2018, 203, 77–84. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Zhang, S.; Ong, C.N.; Shen, H.M. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004, 208, 143–153. [Google Scholar] [CrossRef]
- Weerapana, E.; Wang, C.; Simon, G.M.; Richter, F.; Khare, S.; Dillon, M.B.D.; Bachovchin, D.A.; Mowen, K.; Baker, D.; Cravatt, B.F. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 2010, 468, 790–795. [Google Scholar] [CrossRef] [Green Version]
- Chu, P.Y.; Liu, M.Y. Amino acid cysteine induces senescence and decelerates cell growth in melanoma. J. Funct. Foods 2015, 18, 455–462. [Google Scholar] [CrossRef]
- Dorszewska, J.; Prendecki, M.; Oczkowska, A.; Dezor, M.; Kozubski, W. Molecular basis of familial and sporadic Alzheimer’s disease. Curr. Alzheimer. Res. 2016, 13, 952–963. [Google Scholar] [CrossRef]
- Kong, F.P.; Liu, R.P.; Chu, R.R.; Wang, X.; Xu, K.H.; Tang, B. A highly sensitive near-infrared fluorescent probe for cysteine and homocysteine in living cells. Chem. Commun. 2013, 49, 9176–9178. [Google Scholar] [CrossRef]
- Nekrassova, O.; Lawrence, N.S.; Compton, R.G. Analytical determination of homocysteine: A review. Talanta 2003, 60, 1085–1095. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.F.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Yin, C.X.; Huo, F.J.; Zhang, J.J.; Martinez-Manez, R.; Yang, Y.T.; Lv, H.G.; Li, S.D. Thiol-addition reactions and their applications in thiol recognition. Chem. Soc. Rev. 2013, 42, 6032–6059. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.Q.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A.; Herzenberg, L.A. N-Acetylcysteine—A safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007, 7, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.W.; Yu, J.J.; Xu, X.H.; Jiang, Y.L.; Wang, B.X. A novel fluorescent probe for highly sensitive and selective detection of cysteine and its application in cell imaging. Sensor. Actuat B Chem. 2017, 251, 902–908. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, H.R.; Sun, H.B.; Liu, S.J.; Wang, J.X.; Zhao, Q.; Liu, X.M.; Xu, W.J.; Li, S.B.; Huang, W. Rational design of an “off-on” phosphorescent chemodosimeter based on an Iridium (III) complex and its application for time-resolved luminescent detection and bioimaging of cysteine and homocysteine. Chem. Eur. J. 2013, 19, 1311–1319. [Google Scholar] [CrossRef]
- Wei, M.J.; Yin, P.; Shen, Y.M.; Zhang, L.L.; Deng, J.H.; Xue, S.Y.; Li, H.T.; Guo, B.; Zhang, Y.Y.; Yao, S.Z. A new turn-on fluorescent probe for selective detection of glutathione and cysteine in living cells. Chem. Common. 2013, 49, 4640–4642. [Google Scholar] [CrossRef]
- Cheng, D.; Xu, W.; Yuan, L.; Zhang, X. Investigation of drug-induced hepatotoxicity and its remediation pathway with reaction-based fluorescent probes. Anal. Chem. 2017, 89, 7693–7700. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.M.; Hou, P. A novel cyanobiphenyl benzothiazole-based fluorescent probe for detection of biothiols with a large Stokes shift and its application in cell imaging. Tetrahedron 2017, 73, 589–593. [Google Scholar] [CrossRef]
- Cheng, D.; Pan, Y.; Yin, B.C.; Yuan, L.; Zhang, X.B. A new fluorescent probe with ultralow background fluorescence for imaging of endogenous cellular selenol under oxidative stress. Chin. Chem. Lett. 2017, 28, 1987–1990. [Google Scholar] [CrossRef]
- Ren, T.B.; Xu, W.; Jin, F.P.; Cheng, D.; Zhang, L.L.; Yuan, L.; Zhang, X.B. rational engineering of bioinspired anthocyanidin fluorophores with excellent two-photon properties for sensing and imaging. Anal. Chem. 2017, 89, 11427–11434. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.M.; Hou, P. Imidazo[1,5-α]pyridine-derived fluorescent turn-on probe for cellular thiols imaging with a large Stokes shift. Tetrahedron Lett. 2017, 58, 2654–2657. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Zhao, W.; Zhang, X.; Luo, X.; Corkins, M.E.; Cole, S.L.; Wang, C.; Xiao, Y.; Bi, X.; et al. Two-photon near infrared fluorescent turn-on probe toward cysteine and its imaging applications. ACS Sensors 2016, 1, 882–887. [Google Scholar] [CrossRef]
- Liu, X.J.; Gao, L.; Yang, L.; Zou, L.F.; Chen, W.Q.; Song, X.Z. A phthalimide-based fluorescent probe for thiol detection with a large Stokes shift. RSC Adv. 2015, 5, 18177–18182. [Google Scholar] [CrossRef]
- Dai, X.; Wu, Q.H.; Wang, P.C.; Tian, J.; Xu, Y.; Wang, S.Q.; Miao, J.Y.; Zhao, B.X. A simple and effective coumarin-based fluorescent probe for cysteine. Biosens. Bioelectron. 2014, 59, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, X.; Zhang, Y.; Zhang, C.; Jin, J.; Li, H.; Yao, S. A novel colorimetric/fluorescence dual-channel sensor based on NBD for the rapid and highly sensitive detection of cysteine and homocysteine in living cells. Anal. Methods 2016, 8, 2420–2426. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, X.; Sun, G.; Wu, X. A Sensitive and selective fluorescent probe for cysteine based on a new response-assisted electrostatic attraction strategy: The role of spatial charge configuration. Chem. Eur. J. 2013, 19, 7817–7824. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Lv, X.; Liu, Y.; Zhao, Y.; Guo, W. A naphthalimide-based glyoxal hydrazone for selective fluorescence turn-on sensing of Cys and Hcy. Org. Lett. 2012, 14, 520–523. [Google Scholar] [CrossRef]
- Jiang, W.N.; Yang, S.L.; Lu, W.; Gao, B.H.; Xu, L.; Sun, X.; Jiang, D.; Xu, H.J.; Ma, M.T.; Cao, F.L. A novel fluorescence “turn off-on” nano-sensor for detecting Cu2+ and cysteine in living cells. J. Photochem. Photobiol. A 2018, 362, 14–20. [Google Scholar] [CrossRef]
- Chen, C.Y.; Liu, W.; Xu, C.; Liu, W.S. A colorimetric and fluorescent probe for detecting intracellular biothiols. Biosens. Bioelectron. 2016, 85, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hou, P.; Wang, J.; Fu, S.; Liu, L. A highly sensitive fluorescent probe based on the Michael addition mechanism with a large Stokes shift for cellular thiols imaging. Anal. Bioanal. Chem. 2018, 410, 4323–4330. [Google Scholar] [CrossRef]
- Chen, S.; Hou, P.; Wang, J.; Fu, S.; Liu, L. A simple but effective fluorescent probe with large stokes shift for specific detection of cysteine in living cells. J. Photochem. Photobiol A 2018, 363, 7–12. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, N.; Fang, Z. A novel dicyanoisophorone-based ratiometric fluorescent probe for selective detection of cysteine and its bioimaging application in living cells. Molecules 2018, 23, 475. [Google Scholar] [CrossRef]
- Zeng, R.-F.; Lan, J.-S.; Li, X.-D.; Liang, H.-F.; Liao, Y.; Lu, Y.-J.; Zhang, T.; Ding, Y. A fluorescent coumarin-based probe for the fast detection of cysteine with live cell application. Molecules 2017, 22, 1618. [Google Scholar] [CrossRef]
- Jiang, G.Y.; Liu, X.; Chen, Q.Q.; Zeng, G.J.; Wu, Y.Q.; Dong, X.B.; Zhang, G.X.; Li, Y.D.; Fan, X.L.; Wang, J.G. A new tetraphenylethylene based AIE probe for light-up and disxriminatory detection of Cys over Hcy and GSH. Sensor. Actuat B Chem. 2017, 252, 712–716. [Google Scholar] [CrossRef]
- Ding, S.Y.; Liu, M.J.; Hong, Y.N. Biothiol-specific fluorescent probes with aggregation-induced emission characteristics. Sci. China Chem. 2018, 61, 882–891. [Google Scholar] [CrossRef]
- Tang, Y.H.; Tang, B.Z. Principles and Applications of Aggregation-Induced Emission; Springer: Gewerbestrasse, Switzerland, 2019; pp. 391–407. [Google Scholar]
- Chen, S.; Li, H.M.; Hou, P. A novel imidazo[1,5-α]pyridine-based fluorescent probe with a large Stokes shift for imaging hydrogen sulfide. Sensor. Actuat B-Chem. 2018, 256, 1086–1092. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.M.; Hou, P. A large Stokes shift fluorescent probe for sensing of thiophenols based on imidazo[1,5-α]pyridine in both aqueous medium and living cells. Anal. Chim. Acta 2017, 993, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Hao, Y.Q.; Zeng, K.; Fan, S.N.; Li, F.; Yuan, S.K.; Ding, X.J.; Xu, M.T.; Liu, Y.N. A benzothiazole-based fluorescent probe for hypochlorous acid detection and imaging in living cells. Spectrochim. Acta Part A 2018, 199, 189–193. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, X.; Xu, K.; Kong, X.; Lin, W. A multi-signal fluorescent probe for simultaneously distinguishing and sequentially sensing cysteine/homocysteine, glutathione, and hydrogen sulfide in living cells. Chem. Sci. 2017, 8, 6257–6265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Hou, P.; Wang, J.X.; Song, X.Z. A highly sulfite-selective ratiometric fluorescent probe based on ESIPT. RSC Adv. 2012, 2, 10869–10873. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, T.; Miao, J.Y.; Zhao, B.X. A ratiometric fluorescent probe with DNBS group for biothiols in aqueous solution. Sensor. Actuat B Chem. 2016, 223, 274–279. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Hao, Y.; Liu, J.; Wu, G.; Liu, L. A Green-emitting Fluorescent Probe Based on a Benzothiazole Derivative for Imaging Biothiols in Living Cells. Molecules 2019, 24, 411. https://doi.org/10.3390/molecules24030411
Ma X, Hao Y, Liu J, Wu G, Liu L. A Green-emitting Fluorescent Probe Based on a Benzothiazole Derivative for Imaging Biothiols in Living Cells. Molecules. 2019; 24(3):411. https://doi.org/10.3390/molecules24030411
Chicago/Turabian StyleMa, Xiaohua, Yuanqiang Hao, Jiaxiang Liu, Guoguang Wu, and Lin Liu. 2019. "A Green-emitting Fluorescent Probe Based on a Benzothiazole Derivative for Imaging Biothiols in Living Cells" Molecules 24, no. 3: 411. https://doi.org/10.3390/molecules24030411





