Thermal Decomposition Study on Li2O2 for Li2NiO2 Synthesis as a Sacrificing Positive Additive of Lithium-Ion Batteries
Abstract
:1. Introduction
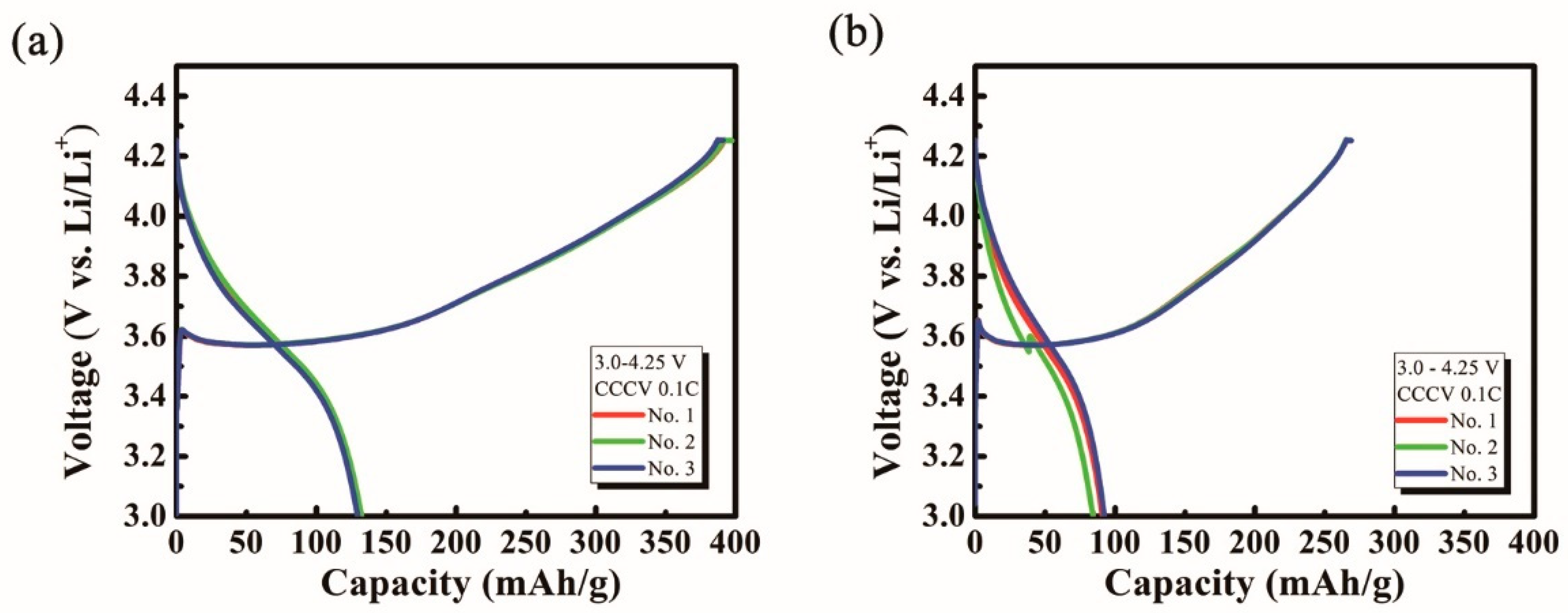
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Lithium Peroxide (Li2O2)
3.2. Thermal Decomposition of Lithium Peroxide (Li2O2) to Lithium Oxide (Li2O)
3.3. Preparation of the L2N
3.4. Material Characterization
3.5. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aurbach, D. Recent studies on the correlation between surface chemistry, morphology, three-dimensional structures and performance of Li and Li-C intercalation anodes in several important electrolyte systems. J. Power Sources 1997, 68, 91–98. [Google Scholar] [CrossRef]
- Besenhard, J.; Winter, M.; Yang, J.; Biberacher, W. Filming mechanism of lithium-carbon anodes in organic and inorganic electrolytes. J. Power Sources 1995, 54, 228–231. [Google Scholar] [CrossRef]
- Park, H.; Yoon, T.; Kim, Y.-U.; Ryu, J.H.; Oh, S.M. Li2NiO2 as a sacrificing positive additive for lithium-ion batteries. Electrochim. Acta. 2013, 108, 591–595. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Menachem, C.; Bar-Tow, D. An advanced tool for the selection of electrolyte components for rechargeable lithium batteries. J. Electrochem. Soc. 1998, 145, 3482–3486. [Google Scholar] [CrossRef]
- Kim, M.G.; Cho, J. Air stable Al2O3-coated Li2NiO2 cathode additive as a surplus current consumer in a Li-ion cell. J. Mater. Chem. 2008, 48, 5880–5887. [Google Scholar] [CrossRef]
- Lee, H. Li2NiO2 as a Novel Cathode Additive for Overdischarge Protection of Li-Ion Batteries. Chem. Mater. 2008, 1, 5–7. [Google Scholar] [CrossRef]
- Hwang, K.; Sohn, H.; Yoon, S. Mesostructured niobium-doped titanium oxide-carbon (Nb-TiO2-C) composite as an anode for high-performance lithium-ion batteries. J. Power Sources 2018, 378, 225–234. [Google Scholar] [CrossRef]
- Jo, C. Tracking the confinement effect of highly dispersive carbon in a tungsten oxide/carbon nanocomposite: Conversion anode materials in lithium ion batteries. J. Mater. Chem. A 2017, 5, 24782–24789. [Google Scholar] [CrossRef]
- Kim, H.-S. Novel silicon–tungsten oxide-carbon composite as advanced negative electrode for lithium-ion batteries. Solid State Ion. 2017, 314, 41–45. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.; Ryu, J.H.; Yoon, S. One pot synthesis of ordered mesoporous carbon-silica-titania with parallel alignment against graphene as advanced anode material in lithium ion batteries. J. Indus. Eng. Chem. 2019, 71, 93–98. [Google Scholar] [CrossRef]
- Kim, Y.; Jo, C.; Lee, J.; Lee, C.W.; Yoon, S. An ordered nanocomposite of organic radical polymer and mesocellular carbon foam as cathode material in lithium ion batteries. J. Mater. Chem. 2012, 22, 1453–1458. [Google Scholar] [CrossRef] [Green Version]
- Sohn, H.; Kim, D.; Lee, J.; Yoon, S. Facile synthesis of a mesostructured TiO2-graphitized carbon (TiO2-gC) composite through the hydrothermal process and its application as the anode of lithium ion batteries. RSC Adv. 2016, 6, 39484–39491. [Google Scholar] [CrossRef]
- Zhou, Y.; Jo, C.; Lee, J.; Lee, C.W.; Qao, G.; Yoon, S. Development of novel mesoporous C-TiO2-SnO2 nanocomposites and their application to anode materials in lithium ion secondary batteries. Microporous Mesoporous Mater. 2011, 151, 172–179. [Google Scholar] [CrossRef]
- Zhou, Y.; Lee, C.W.; Kim, S.-K.; Yoon, S. Ordered mesoporous carbon/MoO2 nanocomposites as stable supercapacitor electrodes. ECS Electrochem. Lett. 2012, 1, A17–A20. [Google Scholar] [CrossRef]
- Zhou, Y.; Yoon, S. Interconnected carbon-decorated Tio2 nanocrystals with enhanced lithium storage performance. Electrochem. Commun. 2013, 40, 54–57. [Google Scholar] [CrossRef]
- Kim, T.; Park, S.; Oh, S.M. Solid-state NMR and electrochemical dilatometry study on Li+ uptake/extraction mechanism in SiO electrode. J. Electrochem. Soc. 2007, 154, A1112–A1117. [Google Scholar] [CrossRef]
- Yoshio, M.; Brodd, R.J.; Kozawa, A. Lithium-ion Batteries; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kang, K.; Chen, C.-H.; Hwang, B.J.; Ceder, G. Synthesis, electrochemical properties, and phase stability of Li2NiO2 with the Immm structure. Chem. Mater. 2009, 16, 2685–2690. [Google Scholar] [CrossRef]
- Moeller, T. Inorganic Syntheses; McGraw Hill Book Company, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Hart, W.A.; Beumel, O.; Whaley, T.P. The Chemistry of Lithium, Sodium, Potassium, Rubidium, Cesium and Francium: Pergamon Texts in Inorganic Chemistry; Elsevier: New York, NY, USA, 2013. [Google Scholar]
- Johnson, R.T.; Biefeld, R.M.; Keck, J.D. Ionic conductivity in Li5AlO4 and LiOH. Mater. Res. Bull. 1977, 12, 577–587. [Google Scholar] [CrossRef]
- Kubota, M.; Matsumoto, S.; Matsuda, H.; Huang, H.Y.; He, Z.H.; Yang, X.X. Chemical Heat Storage with LiOH/LiOH·H2O Reaction for Low-Temperature Heat below 373 K. Adv. Mater. Res. 2014, 2, 757–760. [Google Scholar] [CrossRef]
- Beyer, H.; Meini, S.; Tsiouvaras, N.; Piana, M.; Gasteiger, H.A. Thermal and electrochemical decomposition of lithium peroxide in non-catalyzed carbon cathodes for Li-air batteries. Phy. Chem. Chem. Phy. 2013, 15, 11025–11037. [Google Scholar] [CrossRef]
- Lewis, R.A. Hawley’s Condensed Chemical Dictionary; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Kiat, J.M.; Boemare, G.; Rieu, B.; Aymes, D. Structural evolution of LiOH: Evidence of a solid-solid transformation toward Li2O close to the melting temperature. Solid State Commun. 1998, 108, 241–245. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
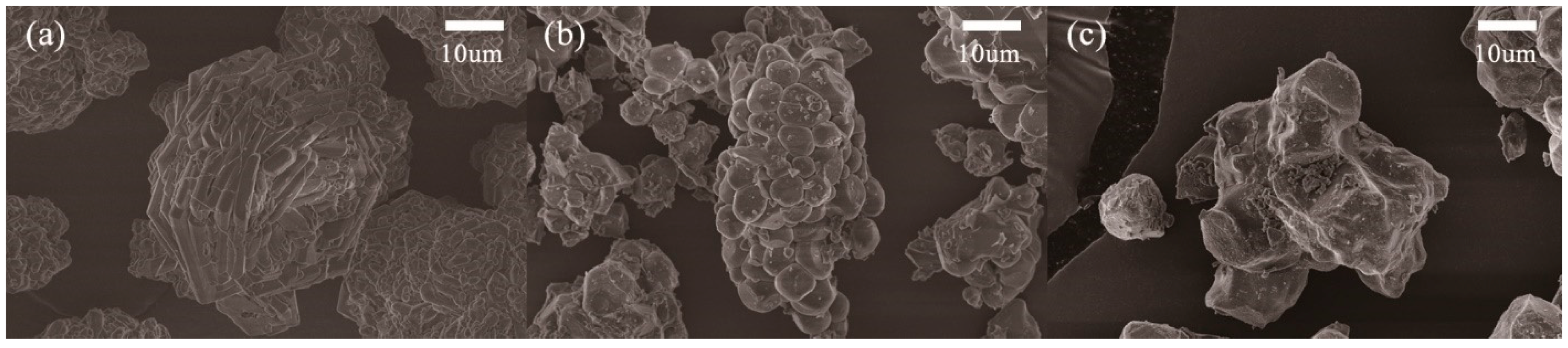
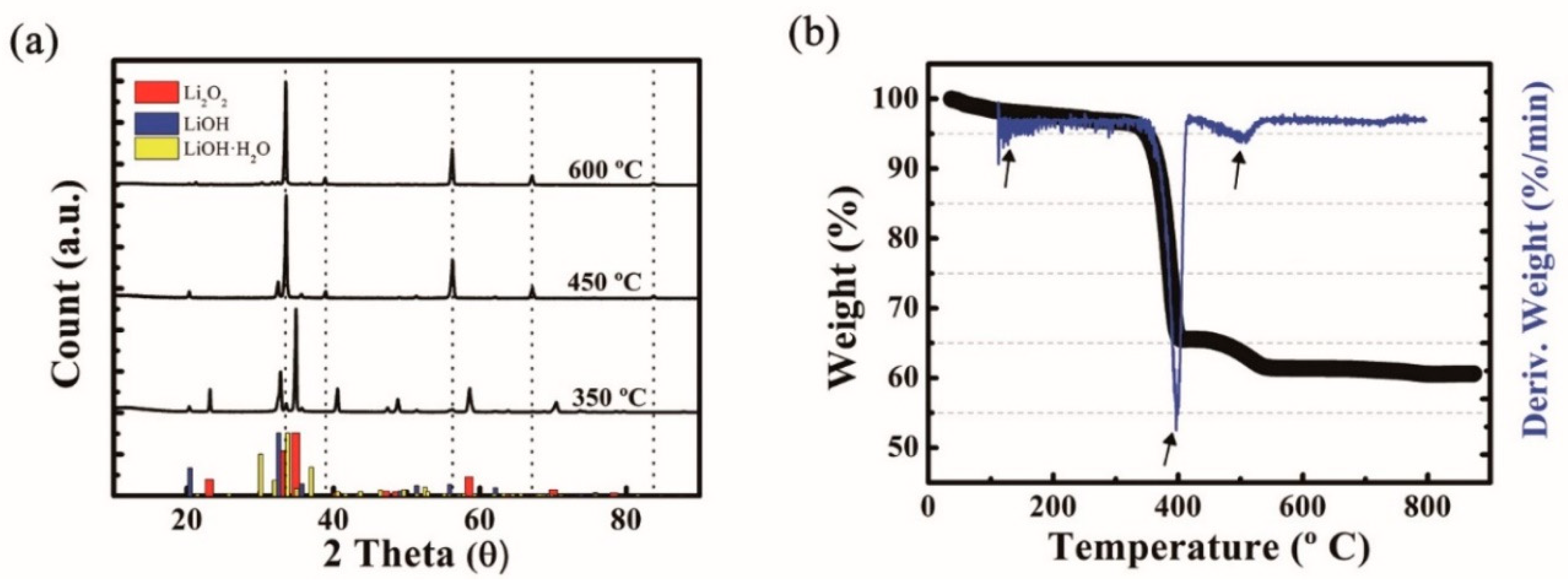
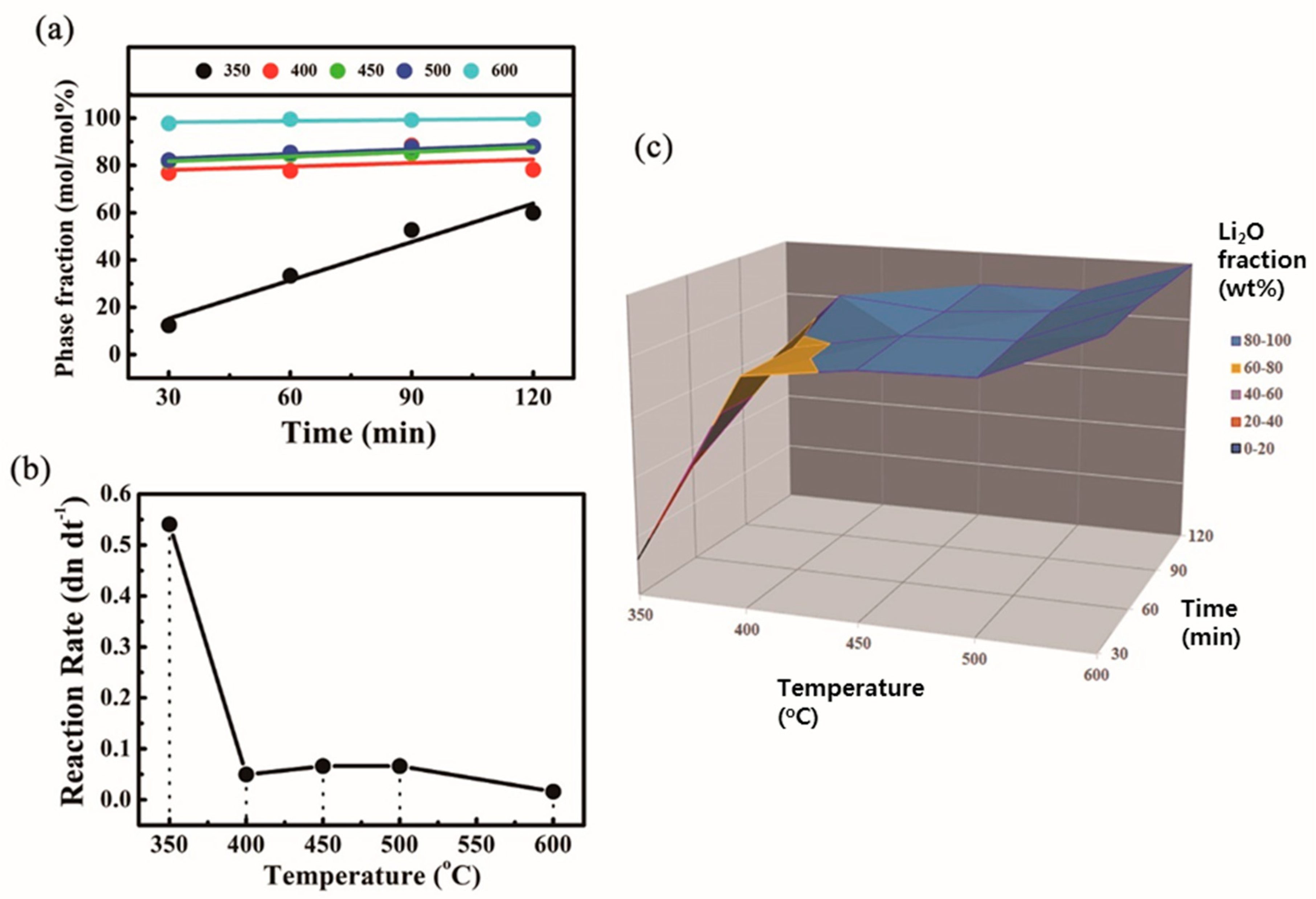
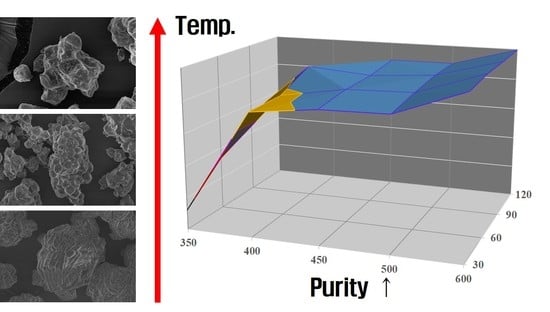
| Temp (°C) | Time (min) | Li2O2 | Li2O | LiOH | LiOH·H2O | Li2CO3 | Sum (wt%) |
|---|---|---|---|---|---|---|---|
| 350 | 30 | 85.7 | 2.1 | 11.7 | 0.5 | 0 | 100 |
| 60 | 84.7 | 5.8 | 9.2 | 0.2 | 0 | 99.9 | |
| 90 | 75.9 | 14.3 | 10.1 | 0.3 | 0 | 100 | |
| 120 | 70.7 | 18.9 | 9.9 | 0.4 | 0 | 99.9 | |
| 400 | 30 | 25.8 | 59.5 | 14.0 | 0.5 | 0.2 | 100 |
| 60 | 0.1 | 80.1 | 17.4 | 2.3 | 0.2 | 100.1 | |
| 90 | 0.2 | 89.2 | 7.8 | 2.6 | 0.3 | 100.1 | |
| 120 | 0 | 81 | 16.9 | 2.1 | 0 | 100 | |
| 450 | 30 | 0 | 82.4 | 11.3 | 6.4 | 0 | 100.1 |
| 60 | 0 | 83.5 | 12.2 | 0.3 | 0 | 100 | |
| 90 | 0 | 87.5 | 12.2 | 0.3 | 0 | 100 | |
| 120 | 0 | 89.5 | 9 | 1.5 | 0 | 100 | |
| 500 | 30 | 0 | 82.8 | 10.5 | 6.7 | 0 | 100 |
| 60 | 0 | 85.9 | 8.7 | 5.4 | 0 | 100 | |
| 90 | 0 | 87.7 | 6.3 | 6 | 0 | 100 | |
| 120 | 0 | 87.7 | 5.9 | 6.4 | 0 | 100 | |
| 600 | 30 | 0 | 97.9 | 1.6 | 0 | 0.5 | 100 |
| 60 | 0 | 99.4 | 0.2 | 0.4 | 0 | 100 | |
| 90 | 0 | 99.1 | 0.2 | 0.8 | 0 | 100.1 | |
| 120 | 0 | 99.5 | 0.2 | 0.3 | 0 | 100 |
| 350 °C | 400 °C | 450 °C | 500 °C | 600 °C | |
|---|---|---|---|---|---|
| 30 min | 12.3 | 76.8 | 81.5 | 82.2 | 97.8 |
| 60 min | 33.3 | 77.7 | 84.4 | 85.4 | 99.5 |
| 90 min | 52.7 | 88.4 | 85.0 | 87.8 | 99.2 |
| 120 min | 59.9 | 78.2 | 87.9 | 88.0 | 99.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kang, H.; Hwang, K.; Yoon, S. Thermal Decomposition Study on Li2O2 for Li2NiO2 Synthesis as a Sacrificing Positive Additive of Lithium-Ion Batteries. Molecules 2019, 24, 4624. https://doi.org/10.3390/molecules24244624
Kim J, Kang H, Hwang K, Yoon S. Thermal Decomposition Study on Li2O2 for Li2NiO2 Synthesis as a Sacrificing Positive Additive of Lithium-Ion Batteries. Molecules. 2019; 24(24):4624. https://doi.org/10.3390/molecules24244624
Chicago/Turabian StyleKim, Jaekwang, Hyunchul Kang, Keebum Hwang, and Songhun Yoon. 2019. "Thermal Decomposition Study on Li2O2 for Li2NiO2 Synthesis as a Sacrificing Positive Additive of Lithium-Ion Batteries" Molecules 24, no. 24: 4624. https://doi.org/10.3390/molecules24244624