Effect of Salt Addition Time on the Nutritional Profile of Thunnus obesus Head Soup and the Formation of Micro/Nano-Sized Particle Structure
Abstract
:1. Introduction
2. Results and Discussion
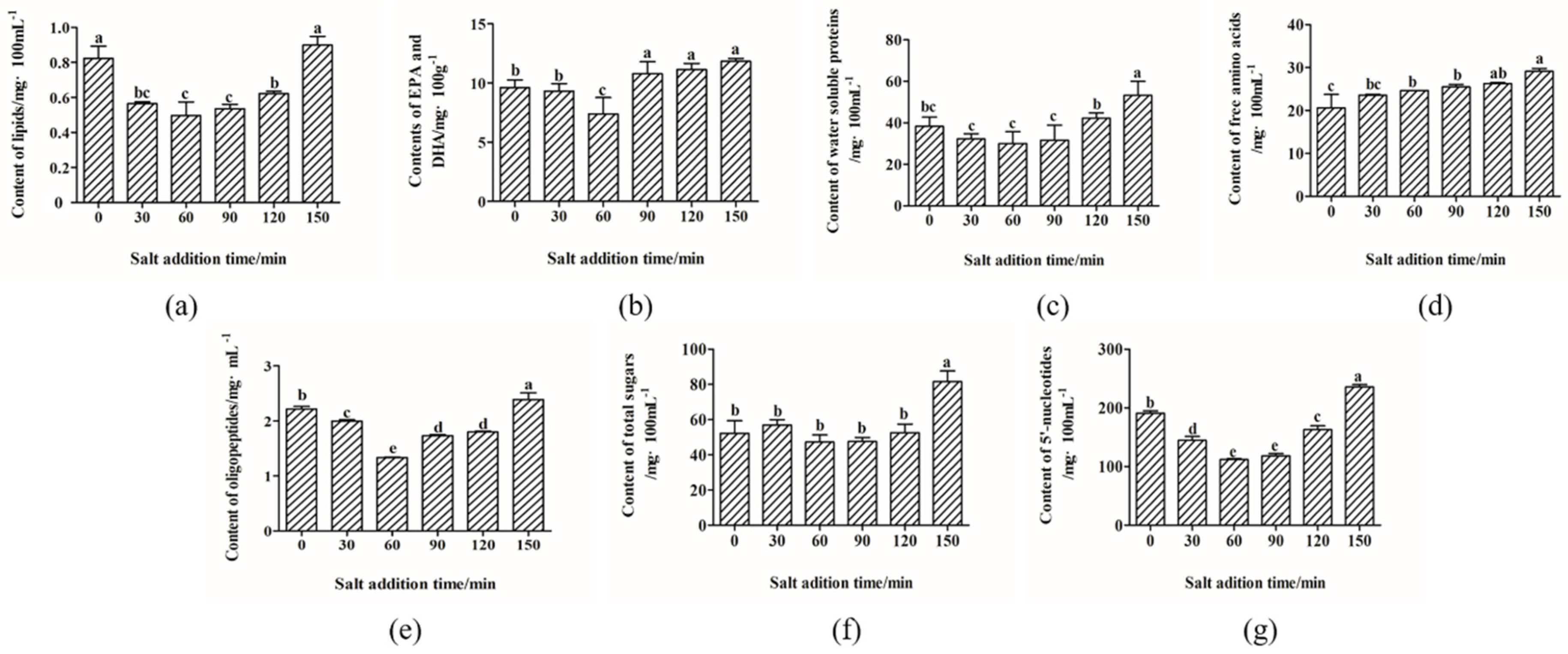
2.1. Nutrients Migration
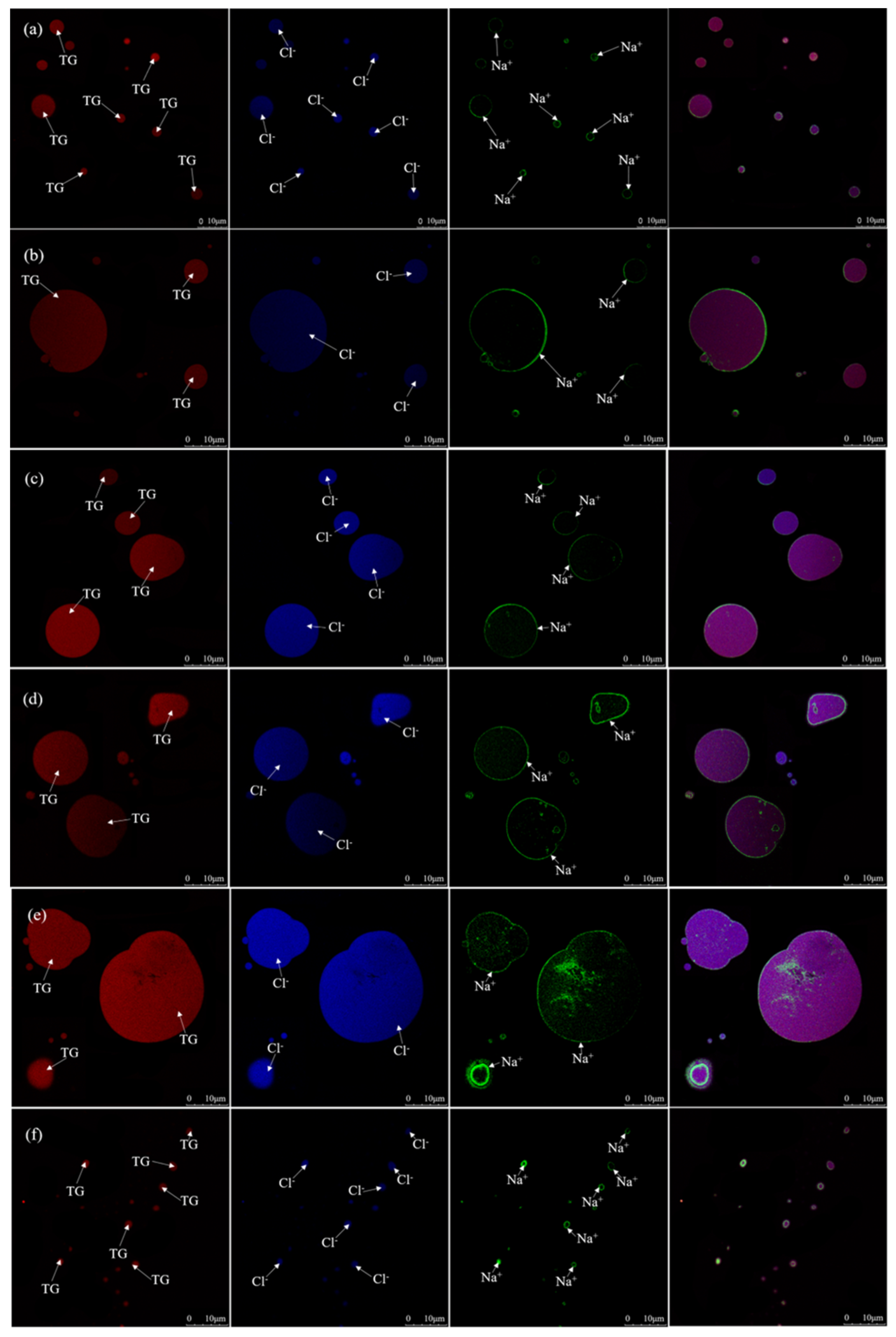
2.2. The Inverted Optical Microscopy was Used to Characterize the Morphological Changes in MNPs Dispersed in Big Eye Tuna Head Soups at Different Salt Addition Times
2.3. The Co-Location of Triglyceride and So On in MNPS
2.4. The Analysis of Umami Taste
3. Materials and Methods
3.1. Materials and Reagents
3.2. Samples Preparation
3.3. Lipid Analysis
3.4. Analysis of EPA and DHA
3.5. Water Soluble Proteins Analysis
3.6. The Analysis of Free Amino Acids
3.7. Oligopeptides Analysis
3.8. Total Sugars Analysis
3.9. 5′-Nucleotides Analysis
3.10. Microstructural Analysis
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohanty, B.P.; Mahanty, A.; Ganguly, S.; Mitra, T.; Karunakaran, D.; Anandan, R. Nutritional composition of food fishes and their importance in providing food and nutritional security. Food Chem. 2019, 293, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Herpandi, N.H.; Rosma, A.; Nadiah, W.A.W. The tuna fishing industry: A new outlook on fish protein hydrolysates. Compr. Rev. Food Sci. Food Saf. 2011, 10, 195–207. [Google Scholar] [CrossRef]
- Kim, J.W.; Samant, S.S.; Seo, Y.; Seo, H.S. Variation in saltiness perception of soup with respect to soup serving temperature and consumer dietary habits. Appetite 2015, 84, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Shen, H.; Zheng, J.Y.; Tao, N.; Gu, S.; Huang, Y.; Wang, M. Identification of key umami-related compounds in yangtze coilia ectenes by combining electronic tongue analysis with sensory evaluation. RSC Adv. 2016, 6, 45689–45695. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamaguchi, K.; Konosu, S. Sensory analysis of taste-active components in the extract of boiled snow crab meat. J. Food Sci. 1981, 46, 479–483. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Zhang, Z.; Tiwari, B.K.; Kerry, J.P.; Burgess, C.M. Salt reduction strategies in processed meat products—A review. Trends Food Sci. Technol. 2017, 59, 70–78. [Google Scholar] [CrossRef]
- Cheema, M.; Smith, P.B.; Patterson, A.D.; Hristov, A.; Harte, F.M. The association of lipophilic phospholipids with native bovine casein micelles in skim milk: Effect of lactation stage and casein micelle size. J. Dairy Sci. 2018, 101, 8672–8687. [Google Scholar] [CrossRef]
- Ren, Q.; Li, L.; Ma, Y. Thermal and structural changes of pasteurized milk fat globules during storage. Food Biosci. 2019, 28, 27–35. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, L.; Wu, Y.; Zhou, P. Changes in milk fat globule membrane proteome after pasteurization in human. Bovine and caprine species. Food Chem. 2019, 279, 209–215. [Google Scholar] [CrossRef]
- Ke, L.; Zhou, J.; Lu, W.; Gao, G.; Rao, P. The power of soups: Super-hero or team-work. Trends Food Sci. Technol. 2011, 22, 492–497. [Google Scholar] [CrossRef]
- Wang, H.; Gao, G.; Ke, L.; Zhou, J.; Rao, P.; Jin, Y.; Lei, H.; Wan, J.; Wang, Q. Isolation of colloidal particles from porcine bone soup and their interaction with murine peritoneal macrophage. J. Funct. Foods 2019, 54, 403–411. [Google Scholar] [CrossRef]
- Qian, X.; Fan, X.; Su, H.; Zhang, J.; Tao, N.; Zhong, J.; Wang, X.; Han, B. Migration of lipid and other components and formation of micro/nano-sized colloidal structure in Tuna (Thunnus obesus) head soup. Food Sci. Technol. 2019, 111, 69–76. [Google Scholar] [CrossRef]
- Cui, L.; Fan, J.; Sun, Y.; Zhu, Z.; Yi, J. The prooxidant activity of salts on the lipid oxidation of lecithin-stabilized oil-in-water emulsions. Food Chem. 2018, 25, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Cho, H.T.; Mcclements, D.J.; Decker, E.A.; Park, Y. Effects of salts on oxidative stability of lipids in tween-20 stabilized oil-in-water emulsions. Food Chem. 2016, 197, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Osbornbarnes, H.T.; Akoh, C.C. Copper—Catalyzed oxidation of a structured lipid-based emulsion containing alpha-tocopherol and citric acid: Influence of pH and NaCl. J. Agric. Food Chem. 2003, 51, 6851–6855. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Chassenieux, C.; Nicolai, T. Kinetics of nacl induced gelation of soy protein aggregates: Effects of temperature, aggregate size, and protein concentration. Food Hydrocoll. 2017, 77, 66–74. [Google Scholar] [CrossRef]
- Unterhaslberger, G.; Schmitt, C.; Sanchez, C.; Appolonia-Nouzille, C.; Raemy, A. Heat denaturation and aggregation of β-lactoglobulin enriched WPI in the presence of arginine HCl, NaCl and guanidinium HCl at pH 4.0 and 7.0. Food Hydrocoll. 2006, 20, 1006–1019. [Google Scholar] [CrossRef]
- Ke, L.J.; Wang, H.Q.; Gao, G.Z.; Rao, P.F.; He, L.; Zhou, J.W. Direct interaction of food derived colloidal micro/nanoparticles with oral macrophages. NPJ Sci. Food 2017, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Jensen, R.G.; Bitman, J.; Carlson, S.E.; Couch, S.C.; Hanosh, M.; Newburg, D.S. Milk Lipids: Human Milk Lipids. In Handbook of Milk Composition; Jensen, R.G., Ed.; Academic Press: Cambridge, UK, 1995; pp. 495–542. [Google Scholar]
- Wang, B.; Wang, L.J.; Li, D.; Adhikari, B.; Shi, J. Effect of gum arabic on stability of oil-in-water emulsion stabilized by flaxseed and soybean protein. Carbohydr. Polym. 2011, 86, 343–351. [Google Scholar] [CrossRef]
- Keenan, T.W.; Patton, S. The Structure of Milk: The Milk Lipid Globule Membrane. In Handbook of Milk Composition; Jensen, R.G., Ed.; Academic Press: Cambridge, UK, 1995; pp. 5–50. [Google Scholar]
- Yamaguchi, S.; Yoshikawa, T.; Ikeda, S.; Ninomiya, T. Measurement of the relative taste intensity of some l-α-amino acids and 5′-nucleotides. J. Food Sci. 1971, 36, 846–849. [Google Scholar] [CrossRef]
- Bellisle, F. Glutamate and the umami taste: Sensory, metabolic, nutritional and behavioural considerations. A review of the literature published in the last 10 years. Neurosci. Biobehav. Rev. 1999, 23, 423–438. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Frey, A.E.; Luboski, J.A.; Foster, M.A.; Erickson, R.P. Taste of glutamate salts in young and elderly subjects: Role of inosine 5′-monophosphate and ions. Physiol. Behav. 1991, 49, 843–854. [Google Scholar] [CrossRef]
- Veciananogues, M.T.; Izquierdopulido, M.; Vidalcarou, M.C. Determination of ATP related compounds in fresh and canned tuna fish by HPLC. Food Chem. 1997, 59, 467–472. [Google Scholar] [CrossRef]
- Kong, Y.; Yang, X.; Ding, Q.; Zhang, Y.; Sun, B.; Chen, H.; Sun, Y. Comparison of non-volatile umami components in chicken soup and chicken enzymatic hydrolysate. Food Res. Int. 2017, 102, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Engel, E.; Nicklaus, S.; Salles, C.; Le, Q.J. Relevance of omission tests to determine flavor-active compounds in food: Application to cheese taste. Food Qual. Prefer. 2002, 13, 505–513. [Google Scholar] [CrossRef]
- Maga, J.A. Flavor potentiators. Crit. Rev. Food Sci. Nutr. 1983, 18, 231–312. [Google Scholar] [CrossRef]
- Folch, J.; Lee, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 22, 477–509. [Google Scholar]
- Zhang, J.; Tao, N.; Wang, M.; Shi, W.; Ye, B.; Wang, X.; Zhu, Q.; Hua, C. Characterization of Phospholipids from Pacific Saury (Cololabis saira) Viscera and Their Neuroprotective Activity. Food Biosci. 2018, 24, 120–126. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Tanimoto, S.; Kawakami, K.; Morimoto, S. Changes in the free amino acid content of the shucked oyster crassostrea gigas stored in salt Water at 3. Fish. Aquat. Sci. 2013, 16, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Qiu, W.; Zhang, R.; Row, K.H.; Jin, Y. Effect of amino acids on microwave dielectric properties of minced antarctic krill (Euphausia superba). Food Bioprocess Technol. 2017, 10, 1809–1823. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, L.; Zeng, Q. Effect of different boiling methods on flavor components of chicken skeleton soup. Sci. Technol. Food Ind. 2015, 36, 314–319. [Google Scholar]
- Morris, D.L. Quantitative determination of carbohydrates with dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Chen, S.; Xie, J.; Qu, Y.; Song, X. Analysis of 10 nucleotides and related compounds in Litopenaeus vannamei during chilled storage by HPLC-DAD. Food Sci. Technol. 2016, 67, 187–193. [Google Scholar] [CrossRef]
- Lopez, C.; Madec, M.N.; Jimenez-Flores, R. Lipid rafts in the bovine milk fat globule membrane revealed by the lateral segregation of phospholipids and heterogeneous distribution of glycoproteins. Food Chem. 2010, 120, 22–33. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.I.; Marunaka, Y. Intracellular chloride ion concentration in differentiating neuronal cell and its role in growing neurite. Biochem. Biophys. Res. Commun. 2016, 479, 338–342. [Google Scholar] [CrossRef]
- Bender, K.J.; Trussell, L.O. Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron 2009, 61, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.T.H.; Ong, L.; Beaucher, E.; Madec, M.N.; Kendish, S.; Gras, S.L.; Lopez, C. Buffalo milk fat globules and their biological membrane: In situ structural investigations. Food Res. Int. 2015, 67, 35–43. [Google Scholar] [CrossRef]
- Martinez, V.; Henary, M. Nile red and Nile blue: Applications and syntheses of structural analogues. Chem. Eur. J. 2016, 22, 13764–13782. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Ong, L.; Lopez, C.; Kentish, S.E.; Gras, S.L. Microstructure and physicochemical properties reveal differences between high moisture buffalo and bovine mozzarella cheeses. Food Res. Int. 2017, 102, 458–467. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Umami Compounds | Taste Threshold/mg·100 mL−1 [25] | Content/mg·100 mL−1 | TAV | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | 150 min | 0 min | 30 min | 60 min | 90 min | 120 min | 150 min | |||
| 5′-nucleotides | 5′-GMP | 12.5 | 42.91 ± 1.66ab | 42.1 ± 4.57ab | 39.82 ± 0.34abc | 34.98 ± 1.45c | 37.01 ± 2.86bc | 45.36 ± 1.41a | 3.430 | 3.370 | 3.185 | 2.798 | 2.960 | 3.629 |
| 5′-IMP | 25 | 54.41 ± 1.02b | 41.25 ± 0.71c | 33.99 ± 1.08d | 28.41 ± 1.13e | 27.7 ± 0.75e | 66.52 ± 1.62a | 2.180 | 1.650 | 1.359 | 1.136 | 1.108 | 2.661 | |
| 5′-AMP | 50 | 93.59 ± 1.17b | 61.22 ± 1.41c | 38.13 ± 0.07e | 54.76 ± 1.37d | 98.2 ± 2.93b | 123.74 ± 3.69a | 1.870 | 1.220 | 0.763 | 1.095 | 1.964 | 2.475 | |
| umami amino acids | Asp | 100 | 0.44 ± 0.10d | 0.33 ± 0.00e | 0.63 ± 0.01c | 0.86 ± 0.01a | 0.92 ± 0.02a | 0.74 ± 0.00b | 0.004 | 0.003 | 0.006 | 0.009 | 0.009 | 0.007 |
| Glu | 30 | 1.57 ± 0.28c | 1.65 ± 0.03bc | 1.68 ± 0.03bc | 1.90 ± 0.03ab | 1.99 ± 0.03a | 1.71 ± 0.01abc | 0.052 | 0.055 | 0.056 | 0.063 | 0.066 | 0.057 | |
| Gly | 100 | 1.14 ± 0.19a | 1.27 ± 0.01a | 1.10 ± 0.02a | 1.21 ± 0.02a | 1.25 ± 0.01a | 1.12 ± 0.01a | 0.011 | 0.013 | 0.011 | 0.012 | 0.012 | 0.011 | |
| EUC/gMSG·100 g−1 | 0.03 | 0.4 ± 0.09b | 0.37 ± 0.01b | 0.35 ± 0.02b | 0.43 ± 0.02b | 0.35 ± 0.01b | 0.54 ± 0.00a | 13.240 | 11.550 | 11.700 | 12.440 | 14.360 | 17.980 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Li, X.; Tao, N.; Zhang, J.; Wang, M.; Qian, X.; Su, H.; Zhong, J. Effect of Salt Addition Time on the Nutritional Profile of Thunnus obesus Head Soup and the Formation of Micro/Nano-Sized Particle Structure. Molecules 2019, 24, 4447. https://doi.org/10.3390/molecules24244447
Fan X, Li X, Tao N, Zhang J, Wang M, Qian X, Su H, Zhong J. Effect of Salt Addition Time on the Nutritional Profile of Thunnus obesus Head Soup and the Formation of Micro/Nano-Sized Particle Structure. Molecules. 2019; 24(24):4447. https://doi.org/10.3390/molecules24244447
Chicago/Turabian StyleFan, Xinyi, Xiaopeng Li, Ningping Tao, Jing Zhang, Mingfu Wang, Xueli Qian, Hong Su, and Jian Zhong. 2019. "Effect of Salt Addition Time on the Nutritional Profile of Thunnus obesus Head Soup and the Formation of Micro/Nano-Sized Particle Structure" Molecules 24, no. 24: 4447. https://doi.org/10.3390/molecules24244447





