Anthocyanins and Their C6-C3-C6 Metabolites in Humans and Animals
Abstract
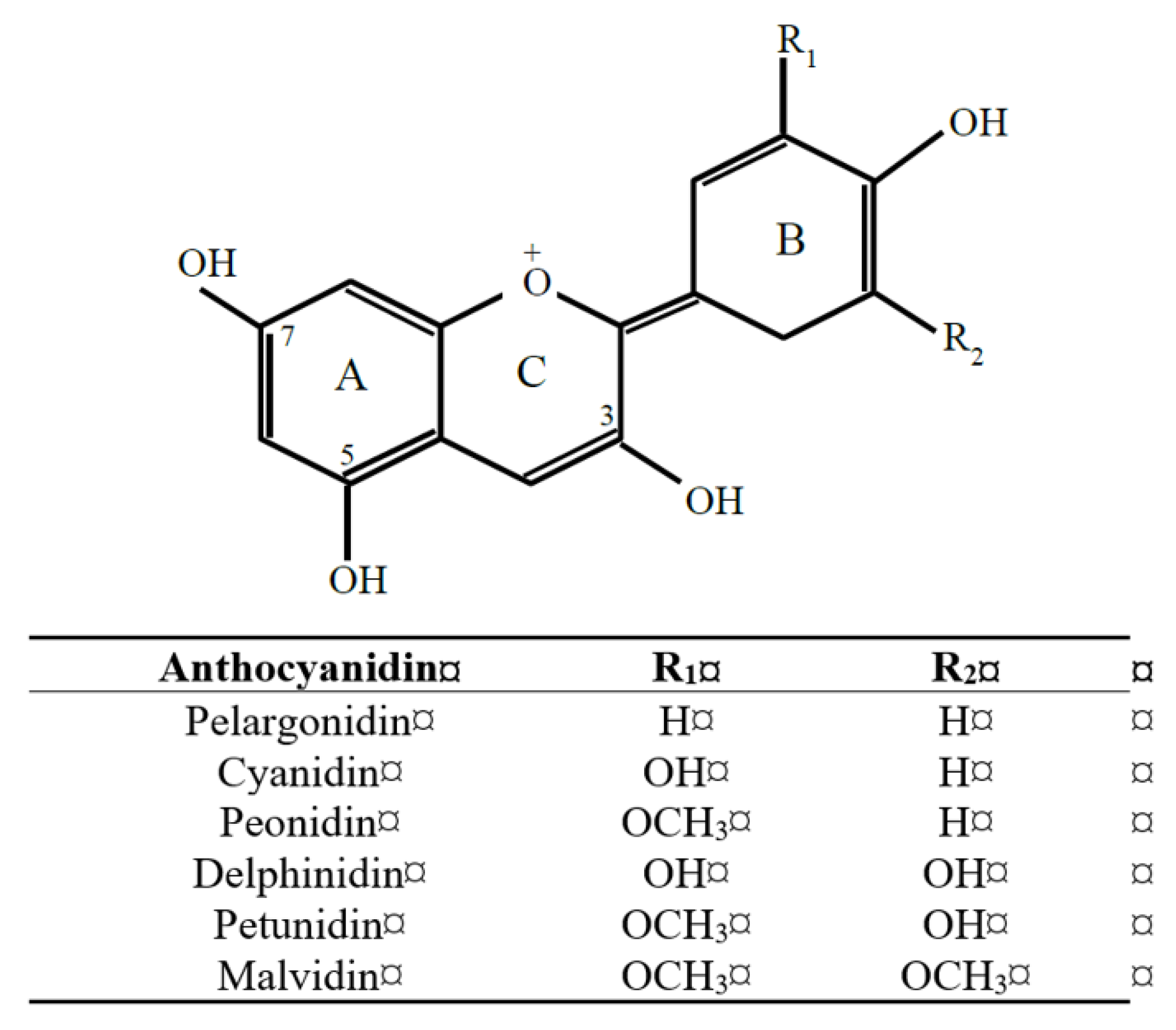
:1. Introduction
2. Anthocyanin Properties in Vivo
2.1. Water Activity and Anthocyanin Stability
2.2. Membrane Solubility of Anthocyanins
2.3. Anthocyanin Association with Human Serum Albumin
2.4. Anthocyanins in the Intestinal Tract
3. Uptake of Parent Anthocyanins on the Bilitranslocase
4. Parent Anthocyanin Conversion into C6-C3-C6 Products in Vivo
4.1. Deglycosylation
4.2. First-Pass Metabolism
5. Enterohepatic Circulation
5.1. Factors Affecting EHC Uptake
5.2. Flavonoids in EHC
5.3. Anthocyanins and EHC
6. Structural Specificity in Anthocyanin’s Behavior in Vivo
6.1. Cyanidin Metabolism and Products
6.2. Malvidin Metabolism and Products
6.3. Pelargonidin Metabolism and Products
7. Anthocyanin Retention in Membranes and Tissue
7.1. Anthocyanins in Tissues
7.2. Anthocyanin Beyond the Blood Brain Barrier
8. How Anthocyanin-Derived C6-C3-C6 Products May Work in Vivo
8.1. The Pool of Anthocyanin Products in the Body
8.2. Slow Clearance of C6-C3-C6 Metabolites from Human Urine
8.3. How C6-C3-C6 Products from Anthocyanins May Work in Vivo
9. Methods to Study Anthocyanin Bioavailability
10. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ADME | absorption, digestion, metabolism and excretion |
| C3g | cyanidin-3-glucoside |
| EHC | enterohepatic circulation |
| GIT | gastrointestinal tract |
| Mal3g | malvidin-3-glucoside |
| MEKC | micellar electrokinetic capacity |
| Peo3g | peonidin-3-glucoside |
References
- Ivey, K.L.; Jensen, M.K.; Hodgson, J.M.; Eliassen, A.H.; Cassidy, A.; Rimm, E.B. Association of flavonoid-rich foods and flavonoids with risk of all-cause mortality. Br. J. Nutr. 2017, 117, 1470–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, E.; Kandaswami, C. The impact of plant flavonoids on mammalian biology: Implications for immunity, inflammation and cancer. In The Flavonoids Advances in Research; Routledge: London, UK, 1993; pp. 619–652. [Google Scholar] [CrossRef]
- Morais, C.A.; de Rosso, V.V.; Estadella, D.; Pisani, L.P. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J. Nutr Biochem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovanucci, E.L. Dietary flavonoid and lignan intake and mortality in prospective cohort studies: Systematic review and dose-response meta-analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 1–11. [Google Scholar] [CrossRef]
- Goetz, M.E.; Judd, S.E.; Safford, M.M.; Hartman, T.J.; McClellan, W.M.; Vaccarino, V. Dietary flavonoid intake and incident coronary heart disease: The REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am. J. Clin. Nutr. 2016, 104, 1236–1244. [Google Scholar] [CrossRef]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Li, L.; Bennett, D.; Guo, Y.; Key, T.J.; Bian, Z.; Sherliker, P.; Gao, H.; Chen, Y.; Yang, L.; et al. Fresh fruit consumption and major cardiovascular disease in China. J. Med. 2016, 374, 1332–1343. [Google Scholar] [CrossRef]
- Yang, L.; Ling, W.; Du, Z.; Chen, Y.; Li, D.; Deng, S.; Liu, Z.; Yang, L. Effects of anthocyanins on cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2017, 8, 684–693. [Google Scholar] [CrossRef]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.-M.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; MacGregor, A.; et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.; O’Reilly, E.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.P.; Curham, G.; Rimm, E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; van Dam, R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ 2016, 352, i17. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.; Hee, K.J.; Breteler, M.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.; Botting, N.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef]
- de Ferrars, R.M.; Cassidy, A.; Curtis, P.; Kay, C.D. Phenolic metabolites of anthocyanins following a dietary intervention study in post-menopausal women. Mol. Nutr. Food Res. 2014, 58. [Google Scholar] [CrossRef]
- Woodward, G.; Kroon, P.; Cassidy, A.; Kay, C. Anthocyanin stability and recovery: Implications for the analysis of clinical and experimental samples. J. Agric. Food Chem. 2009, 57, 5271–5278. [Google Scholar] [CrossRef]
- Wu, X.; Pittman, H.E.; Prior, R.L. Fate of anthocyanins and antioxidant capacity in contents of the gastrointestinal tract of weanling pigs following black raspberry consumption. J. Agric. Food Chem. 2006, 54, 583–589. [Google Scholar] [CrossRef]
- He, J.; Wallace, T.C.; Keatley, K.E.; Failla, M.L.; Giusti, M.M. Stability of black raspberry anthocyanins in the digestive tract lumen and transport efficiency into gastric and small intestinal tissues in the rat. J. Agric. Food Chem. 2009, 57, 3141–3148. [Google Scholar] [CrossRef] [PubMed]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.-L.; Remesey, C. Anthocyanins are efficiently absorbed from the small intestine in rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A.; Wrolstad, R.E. The stability of pelargonidin-based anthocyanins at varying water activity. Food Chem. 2001, 75, 185–196. [Google Scholar] [CrossRef]
- Lima, J.C.; Vautier-Giongo, C.; Lopes, A.; Melo, E.; Quina, F.H.; Maçanita, A.L. Color stabilization of anthocyanins: Effect of SDS micelles on the acid−base and hydration kinetics of malvidin 3-glucoside (Oenin). J. Phys. Chem A. 2002, 106, 5851–5859. [Google Scholar] [CrossRef]
- Mulinacci, N.; Romani, A.; Pinelli, P.; Gallori, S.; Giaccherini, C.; Vincieri, F.F. Stabilisation of natural anthocyanins by micellar systems. Int J. Pharm. 2001, 216, 23–31. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Van Camp, J. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure-activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef]
- Müller, L.; Bednář, P.; Barták, P.; Lemr, K.; Ševčík, J. Estimation of partition coefficients by MEKC Part 2: Anthocyanins. J. Sep. Sci. 2005, 28, 1285–1290. [Google Scholar] [CrossRef]
- Kay, C.D. Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutr. Res. Rev. 2006, 19, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Kaldas, M.I.; Walle, U.K.; van der Woude, H.; McMillan, J.M.; Walle, T. Covalent binding of the flavonoid quercetin to human serum albumin. J. Agric. Food Chem. 2005, 53, 4194–4197. [Google Scholar] [CrossRef]
- Cahyana, Y.; Gordon, M.H. Interaction of anthocyanins with human serum albumin: Influence of pH and chemical structure on binding. Food Chem. 2013, 141, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Murkovic, M.; Adam, U.; Pfannhauser, W. Analysis of anthocyane glycosides in human serum. Fresenius J. Anal. Chem. 2000, 366, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.-L.; Rémésy, C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Liu, Y.; Fillmore, S.A.E. Human anthocyanin bioavailability: Effect of intake duration and dosing. Food Funct. 2017, 8, 4563–4569. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Seymour, E.M.; Wolforth, J.; McNish, R.; Kaufman, P.B.; Bolling, S.F. Tissue bioavailability of anthocyanins from whole tart cherry in healthy rats. Food Chem. 2015, 171, 26–31. [Google Scholar] [CrossRef]
- Del Bò, C.; Ciappellano, S.; Klimis-Zacas, D.; Martini, D.; Gardana, C.; Riso, P.; Porrini, M. Anthocyanin absorption, metabolism, and distribution from a wild blueberry-enriched diet (Vaccinium angustifolium) is affected by diet duration in the Sprague−Dawley rat. J. Agric. Food Chem. 2010, 58, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Macierzanka, A.; Rigby, N.M.; Corfield, A.P.; Wellner, N.; Bottger, F.; Clare Mills, E.N.; Mackie, A.R. Adsorption of bile salts to particles allows penetration of intestinal mucus. Soft Matter 2011, 7, 8077–8084. [Google Scholar] [CrossRef]
- Chen, I.-L.; Tsai, Y.-J.; Huang, C.-M.; Tsai, T.-H. Lymphatic absorption of quercetin and rutin in rat and their pharmacokinetics in systemic plasma. J. Agric. Food Chem. 2010, 58, 546–551. [Google Scholar] [CrossRef]
- Murota, K.; Terao, J. Quercetin appears in the lymph of unanesthetized rats as its phase II metabolites after administered into the stomach. FEBS Lett. 2005, 579, 5343–5346. [Google Scholar] [CrossRef] [Green Version]
- Mullen, W.; Edwards, C.A.; Serafini, M.; Crozier, A. Bioavailability of pelargonidin-3-O-glucoside and its metabolites in humans following the ingestion of strawberries with and without cream. J. Agric. Food Chem. 2008, 56, 713–719. [Google Scholar] [CrossRef]
- Lesser, S.; Cermak, R.; Wolffram, S. Bioavailability of quercetin in pigs is influenced by the dietary fat content. J. Nutr. 2004, 134, 1508. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The interaction of anthocyanins with bilitranslocase. Biochem Biophys. Res. Commun. 2002, 296, 631–636. [Google Scholar] [CrossRef]
- Passamonti, S.; Terdoslavich, M.; Franca, R.; Vanzo, A.; Tramer, F.; Braidor, E.; Petrussa, E.; Vianello, A. Bioavailability of flavonoids: A review of their membrane transport and the function of bilitranslocase in animal and plant organisms. Curr. Drug Metab. 2009, 10, 369–394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zuo, Z.; Lin, G. Intestinal and hepatic glucuronidation of flavonoids. Mol. Pharm. 2007, 4, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa: Extensive presystemic metabolism reduces apparent bioavailability. J. Agric. Food Chem. 2014, 62, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- Kamonpatana, K.; Giusti, M.M.; Chitchumroonchokchai, C.; Moreno-Cruz, M.; Riedl, K.M.; Kumar, P.; Failla, M.L. Susceptibility of anthocyanins to ex vivo degradation in human saliva. Food Chem. 2012, 135, 738–747. [Google Scholar] [CrossRef] [Green Version]
- Day, A.J.; DuPonta, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.C.; Morgan, M.R.A.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver β-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef]
- Németh, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Charron, C.S.; Kurilich, A.C.; Clevidence, B.A.; Simon, P.W.; Harrison, D.J.; Britz, S.J.; Baer, D.J.; Novotny, J.A. Bioavailability of anthocyanins from purple carrot juice: Effects of acylation and plant matrix. J. Agric. Food Chem. 2009, 57, 1226–1230. [Google Scholar] [CrossRef]
- Kurilich, A.C.; Clevidence, B.A.; Britz, S.J.; Simon, P.W.; Novotny, J.A. Plasma and urine responses are lower for acylated vs. nonacylated anthocyanins from raw and cooked purple carrots. J. Agric. Food Chem. 2005, 53, 6537–6542. [Google Scholar] [CrossRef]
- He, J.; Magnuson, B.A.; Lala, G.; Tian, Q.; Schwartz, S.J.; Giusti, M.M. Intact anthocyanins and metabolites in rat urine and plasma after 3 months of anthocyanin supplementation. Nutr. Cancer. 2006, 54, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Cooney, J.M.; Jensen, D.J.; McGhie, T.K. LC-MS identification of anthocyanins in boysenberry extract and anthocyanin metabolites in human urine following dosing. J. Sci. Food Agric. 2004, 84, 237–245. [Google Scholar] [CrossRef]
- Matuschek, M.C.; Hendriks, W.H.; McGhie, T.K.; Reynolds, G.W. The jejunum is the main site of absorption for anthocyanins in mice. J. Nutr. Biochem. 2006, 17, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, T.H.; Cooke, D.; Brown, K.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and metabolism of the putative cancer chemopreventive agent cyanidin-3-glucoside in mice. Cancer Chemother Pharmacol. 2009, 64, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; McDonald, J.E.; Liu, Y.; Fillmore, S.A.E. Flavonoid metabolites in human urine during blueberry anthocyanin intake. J. Agric. Food Chem. 2017, 65, 1582–1591. [Google Scholar] [CrossRef]
- Vanzo, A.; Terdoslavich, M.; Brandoni, A.; Torres, A.M.; Vrhovsek, U.; Passamonti, S. Uptake of grape anthocyanins into the rat kidney and the involvement of bilitranslocase. Mol. Nutr. Food Res. 2008, 52, 1106–1116. [Google Scholar] [CrossRef]
- Felgines, C.; Texier, O.; Garcin, P.; Besson, C.; Lamaison, J.L.; Scalbert, A. Tissue distribution of anthocyanins in rats fed a blackberry anthocyanin-enriched diet. Mol. Nutr. Food Res. 2009, 53, 1098–1103. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Shida, Y.; Rahman, M.M.; Hatano, Y.; Konishi, T. Extended glucuronidation is another Major path of cyanidin 3-O-β-d-glucopyranoside metabolism in rats. J. Agric. Food Chem. 2005, 53, 7312–7319. [Google Scholar] [CrossRef]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef]
- Kalt, W.; Liu, Y.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E. Anthocyanin metabolites are abundant and persistent in human urine. J. Agric. Food Chem. 2014, 62, 3926–3934. [Google Scholar] [CrossRef]
- Wu, B.; Kulkarni, K.; Basu, S.; Zhang, S.; Hu, M. First-pass metabolism via UDP-glucuronosyltransferase: A barrier to oral bioavailability of phenolics. J. Pharm. Sci. 2011, 100, 3655–3681. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, M. Natural polyphenol disposition via coupled metabolic pathways. Expert Opin Drug Metab. Toxicol. 2007, 3, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.; Magnusson, B.; Burczynski, F.; Weiss, M. Enterohepatic circulation. Clin. Pharmacokinet. 2002, 41, 751–790. [Google Scholar] [CrossRef] [PubMed]
- Lafay, S.; Morand, C.; Manach, C.; Besson, C.; Scalbert, A. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Br. J. Nutr. 2006, 96, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Morand, C.; Besson, C.; Cotelle, N.; Vezin, H.; Demigne, C.; Remesey, C. The splanchnic metabolism of flavonoids highly differed according to the nature of the compound. Am. J. Gastro. Liver Physiol. 2003, 284, G980–G988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sfakianos, J.; Coward, L.; Kirk, M.; Barnes, S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J. Nutr. 1997, 127, 1260–1268. [Google Scholar] [CrossRef]
- Zeng, M.; Sun, R.; Basu, S.; Ma, Y.; Ge, S.; Yin, T.; Gao, S.; Zhang, J.; Hu, M. Disposition of flavonoids via recycling: Direct biliary excretion of enterically or extrahepatically derived flavonoid glucuronides. Mol. Nutr. Food Res. 2016, 60, 1006–1019. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, S.; Li, L.; Jiang, H. Metabolism of flavonoids in humans: A comprehensive review. Curr. Drug Metab. 2014, 15, 48–61. [Google Scholar] [CrossRef]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Gastrointestinal stability and bioavailability of (poly)phenolic compounds following ingestion of Concord grape juice by humans. Mol. Nutr. Food Res. 2012, 56, 497–509. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ichiyanagi, T.; Iida, H.; Ito, K.; Tsuda, T.; Hirayama, M.; Konishi, T. Ingested delphinidin-3-rutinoside is primarily excreted to urine as the intact form and to bile as the methylated forms in rats. J. Agric. Food Chem. 2006, 54, 578–582. [Google Scholar] [CrossRef]
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Fulvio, M. The stomach as a site for anthocyanins absorption from food. FEBS Lett. 2003, 544, 210–213. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Shida, Y.; Rahman, M.M.; Hatano, Y.; Matsumoto, H.; Hirayama, M.; Konishi, T. Metabolic pathway of cyanidin 3-O-β-d-glucopyranoside in rats. J. Agric. Food Chem. 2005, 53, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Krisa, S.; Mauray, A.; Besson, C.; Lamaison, J.-L.; Scalbert, A.; Merillon, J.-M.; Texier, O. Radiolabelled cyanidin 3-O-glucoside is poorly absorbed in the mouse. Br. J. Nutr. 2010, 103, 1738–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felgines, C.; Talavera, S.; Texier, O.; Gil-Izquierdo, A.; Lamaison, J.L.; Remesy, C. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans. J. Agric. Food Chem. 2005, 53, 7721–7727. [Google Scholar] [CrossRef] [PubMed]
- Fornasaro, S.; Ziberna, L.; Gasperotti, M.; Tramer, F.; Vrhovsek; Mattivi, F.; Passamonti, S. Determination of cyanidin 3-glucoside in rat brain, liver and kidneys by UPLC/MS-MS and its application to a short-term pharmacokinetic study. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, A.; Vrhovsek, U.; Tramer, F.; Mattivi, F.; Passamonti, S. Exceptionally fast uptake and metabolism of cyanidin 3-glucoside by rat kidneys and liver. J. Nat. Prod. 2011, 74, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Fernández-Castillejo, S.; Rubió, L.; Macià, A.; Solà, R. Anthocyanin tissue bioavailability in animals: Possible implications for human health. A systematic review. J. Agric. Food Chem. 2018, 66, 11531–11543. [Google Scholar] [CrossRef]
- Chen, T.Y.; Kritchevsky, J.; Hargett, K.; Feller, K.; Klobusnik, R.; Song, B.J.; Cooper, B.; Jouni, Z.; Ferruzzi, M.G.; Janle, E.M. Plasma bioavailability and regional brain distribution of polyphenols from apple/grape seed and bilberry extracts in a young swine model. Mol. Nutr Food Res. 2015, 59, 2432–2447. [Google Scholar] [CrossRef]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 705–712. [Google Scholar] [CrossRef]
- Milbury, P.E.; Kalt, W. Xenobiotic metabolism and berry flavonoid transport across the blood brain barrier. J. Agric. Food Chem. 2010, 58, 3950–3956. [Google Scholar] [CrossRef]
- Sakakibara, H.; Ogawa, T.; Koyanagi, A.; Kobayasho, S.; Goda, T.; Kumazawa, S.; Kobayashi, H.; Shimoi, K. Distribution and excretion of bilberry anthocyanins in mice. J. Agric. Food Chem. 2009, 57, 7681–7686. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Walle, T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispos. 2006, 34, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Zimman, A.; Waterhouse, A.L. Enzymatic synthesis of [3‘-O-methyl-3H]malvidin-3-glucoside from petunidin-3-glucoside. J. Agric. Food Chem. 2002, 50, 2429–2431. [Google Scholar] [CrossRef] [PubMed]
- Carkeet, C.; Clevidence, B.A.; Novotny, J.A. Anthocyanin excretion by humans increases linearly with increasing strawberry dose. J. Nutr. 2008, 138, 897–902. [Google Scholar] [CrossRef]
- El Mohsen, M.A.; Marks, J.; Kuhnle, G.; Moore, K.; Debnam, E.; Srai, S.K.; Rice-Evans, C.; Spencer, J.P.E. Absorption, tissue distribution and excretion of pelargonidin and its metabolites following oral administration to rats. Br. J. Nutr. 2006, 95, 51. [Google Scholar] [CrossRef]
- Prior, R.L. Anthocyanins: Understanidng their absorption and metabolism. In Flavonoids and Related Compounds: Bioavailability and Function; Spencer, J.P., Crozier, A., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 79–92. [Google Scholar]
- Kalt, W.; McDonald, J.E.; Ricker, R.D.; Lu, X. Anthocyanin content and profile within and among blueberry species. Can. J. Plant. Sci. 1999, 79, 617–623. [Google Scholar] [CrossRef]
- Aqil, F.; Vadhanam, M.V.; Jeyabalan, J.; Cai, J.; Singh, I.P.; Gupta, R.C. Detection of anthocyanins/anthocyanidins in animal tissues. J. Agric. Food Chem. 2014, 62, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, T.; Shida, Y.; Rahman, M.M.; Hatano, Y.; Konishi, T. Bioavailability and tissue distribution of anthocyanins in bilberry (Vaccinium myrtillus L.) extract in rats. J. Agric. Food Chem. 2006, 54, 6578–6587. [Google Scholar] [CrossRef]
- Borges, G.; Roowi, S.; Rouanet, J.-M.; Duthie, G.G.; Lean, M.E.J.; Crozier, A. The bioavailability of raspberry anthocyanins and ellagitannins in rats. Mol. Nutr. Food Res. 2007, 51, 714–725. [Google Scholar] [CrossRef]
- Del Bo’, C.; Roursgaard, M.; Porrini, M.; Loft, S.; Møller, P.; Riso, P. Different effects of anthocyanins and phenolic acids from wild blueberry (Vaccinium angustifolium) on monocytes adhesion to endothelial cells in a TNF-α stimulated proinflammatory environment. Mol. Nutr. Food Res. 2016, 60. [Google Scholar] [CrossRef]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Gil-Izquierdo, A.; Lamaison, J.-L.; Remesey, C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J. Agric. Food Chem. 2005, 53, 3902–3908. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. Fast access of some grape pigments to the brain. J. Agric. Food Chem. 2005, 53, 7029–7034. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-Y.; Ferruzzi, M.G.; Wu, Q.-L.; Simon, J.E.; Talcott, S.T.; Wang, J.; Ho, L.; Todd, G.; Cooper, B.; Pasinetti, G.M.; et al. Influence of diabetes on plasma pharmacokinetics and brain bioavailability of grape polyphenols and their phase II metabolites in the Zucker diabetic fatty rat. Mol. Nutr. Food Res. 2017, 61, 1700111. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Hanneken, A.; Milbury, P.; Tremblay, F. Recent research on polyphenolics in vision and eye health. J. Agric. Food Chem. 2010, 58, 4001–4007. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Nakamura, Y.; Iida, H.; Ito, K.; Ohguro, H. Comparative assessment of distribution of blackcurrant anthocyanins in rabbit and rat ocular tissues. Exp. Eye Res. 2006, 83, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Kroon, P.A.; Cassidy, A. The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Mol. Nutr. Food Res. 2009, 53, S92–S101. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and flavanones are more bioavailable than previously perceived: A review of recent evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef]
- Forester, S.C.; Waterhouse, A.L. Metabolites are key to understanding health effects of wine polyphenolics. J. Nutr. 2009, 138, 1824S–1831S. [Google Scholar] [CrossRef]
- Forester, S.C.; Waterhouse, A.L. Identification of cabernet sauvignon anthocyanin gut microflora metabolites. J. Agric. Food Chem. 2008, 56, 9299–9304. [Google Scholar] [CrossRef]
- Halliwell, B. Dietary polyphenols: Good, bad, or indifferent for your health? Cardiovasc Res. 2007, 73, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Košinová, P.; Berka, K.; Wykes, M.; Otyepka, M.; Trouillas, P. Positioning of antioxidant quercetin and its metabolites in lipid bilayer membranes: Implication for their lipid-peroxidation inhibition. J. Phys. Chem. B. 2012, 116, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Fabre, G.; Bayach, I.; Berka, K.; Paloncyova, M.; Starok, M.; Rossi, C.; Duroux, J.-L.; Otyepka, M.; Trouillas, P. Synergism of antioxidant action of vitamins E, C and quercetin is related to formation of molecular associations in biomembranes. Chem. Comm. 2015, 51, 7713–7716. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Kay, C.D.; Crozier, A. The bioavailability, transport, and bioactivity of dietary flavonoids: A review from a historical perspective. Compr Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]

| Site | Event | Effect | Reference |
|---|---|---|---|
| Mouth | Deglycosylation Association with saliva proteins | Polarity decrease; membrane solubility increase Decline in free parent anthocyanin | [22,47] |
| Stomach | Uptake on bilitranslocase Stabilization by acidic pH | Rapid absorption and distribution of parent anthocyanins | [44,77] |
| Small intestine | Association with food matrix Association with intestinal mucin High concentration in small intestine Enteric transport and phase 2 metabolism | Increased stability Delayed, impeded absorption Antioxidant effect in small intestine Distribution and formation of phase 2 conjugates | [22,27,103] |
| Liver | Hepatic phase 2 metabolism | Formation of phase 2 conjugates | [106] |
| Large intestine | Survival in colon | Darkening and purple coloration of feces (e.g., birds and bears) Antioxidant protection in colon | [103] |
| Bile | Dissolution of anthocyanins and their metabolites in amphipathic bile | Capacity for enterohepatic circulation due to anthocyanin properties Complex pool of anthocyanin isomers | [56] |
| Plasma | Association with serum albumin Preferred protein association with anthocyanin glycosides | Increased anthocyanin stability Depot for protein-associated anthocyanin | [32] |
| Tissues | Association with tissues | Long-term retention and possible protection of membranes and other structures | [78,104] |
| Liposomes and micelles | Reduced water activity | Increased anthocyanin stability Differential distribution and mobility in membranes | [25,26] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalt, W. Anthocyanins and Their C6-C3-C6 Metabolites in Humans and Animals. Molecules 2019, 24, 4024. https://doi.org/10.3390/molecules24224024
Kalt W. Anthocyanins and Their C6-C3-C6 Metabolites in Humans and Animals. Molecules. 2019; 24(22):4024. https://doi.org/10.3390/molecules24224024
Chicago/Turabian StyleKalt, Wilhelmina. 2019. "Anthocyanins and Their C6-C3-C6 Metabolites in Humans and Animals" Molecules 24, no. 22: 4024. https://doi.org/10.3390/molecules24224024





