Observations on the Effects of Residualization and Dehalogenation on the Utility of N-Succinimidyl Ester Acylation Agents for Radioiodination of the Internalizing Antibody Trastuzumab
Abstract
:1. Introduction
2. Results
2.1. Radiolabeling and Quality Control
2.2. Uptake, Internalization, and Cellular Processing on BT474 Cells In Vitro
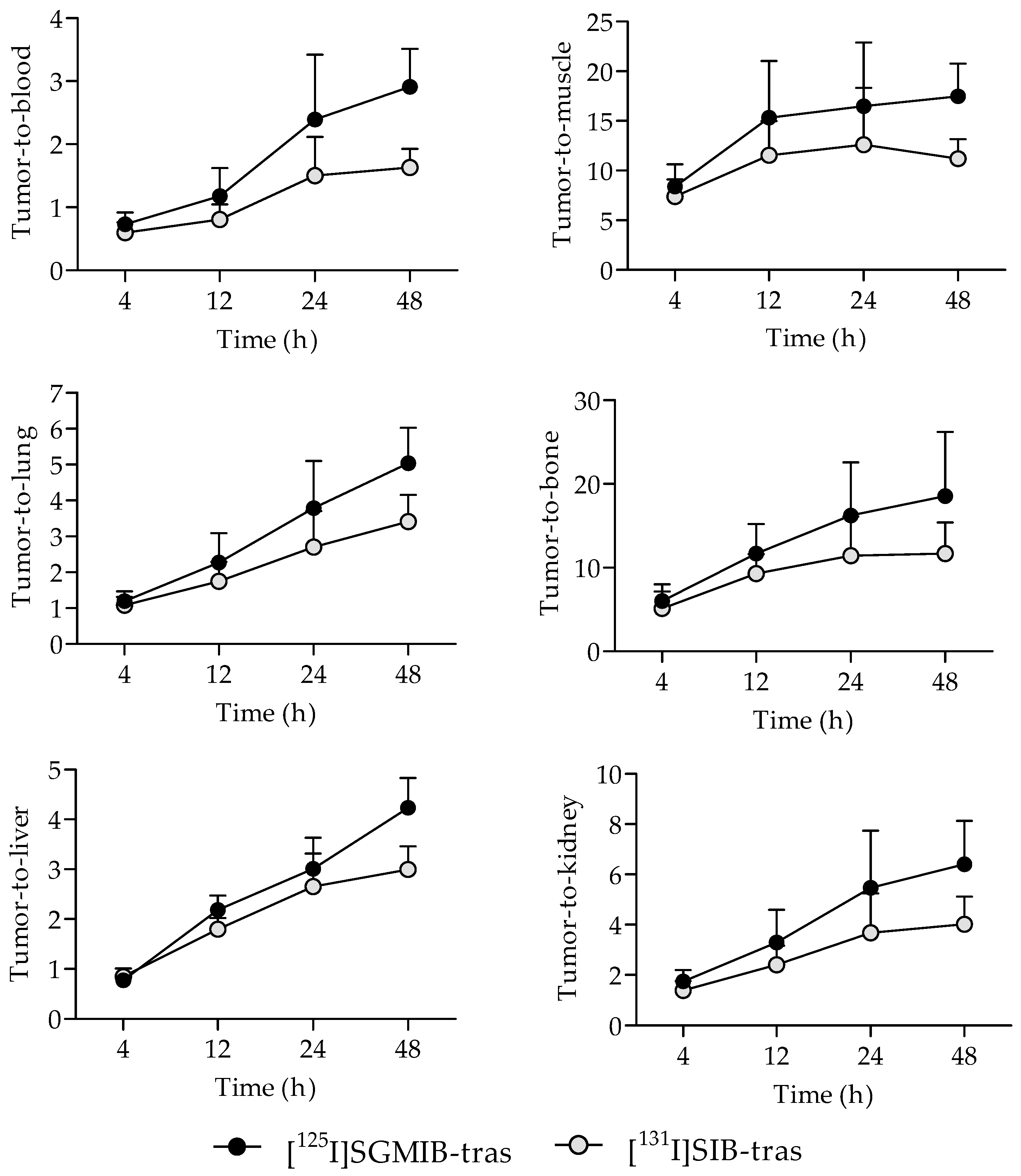
2.3. Tissue Distribution in Mice with BT474M1 Tumor Xenografts
3. Discussion
4. Materials and Methods
4.1. General
4.2. Synthesis of N-Succinimidyl 3-[131I]iodobenzoate ([131I]SIB)
4.3. Synthesis of N-Succinimidyl 4-guanidinomethyl 3-[125I]iodobenzoate ([125I]SGMIB)
4.4. Conjugation of [125I]SGMIB and [131I]SIB to Trastuzumab
4.5. Uptake and Internalization of Labeled Trastuzumab Conjugates in HER2-Expressing BT474 Cells
4.6. Evaluation of the Labeled Trastuzumab Conjugates in BT474M1 Tumor Xenografts In Vivo
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ross, J.S.; Slodkowska, E.A.; Symmans, W.F.; Pusztai, L.; Ravdin, P.M.; Hortobagyi, G.N. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 2009, 14, 320–368. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; Mcguire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Presta, L.; Gorman, C.M.; Ridgway, J.B.B.; Henner, D.; Wong, W.L.T.; Rowland, A.M.; Kotts, C.; Carver, M.E.; Shepard, H.M. Humanization of an anti-P185HER2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. USA 1992, 89, 4285–4289. [Google Scholar] [CrossRef]
- Sauter, G.; Lee, J.; Bartlett, J.M.S.; Slamon, D.J.; Press, M.F. Guidelines for human epidermal growth factor receptor 2 testing: Biologic and methodologic considerations. J. Clin. Oncol. 2009, 27, 1323–1333. [Google Scholar] [CrossRef]
- Perik, P.J.; Lub-De Hooge, M.N.; Gietema, J.A.; van der Graaf, W.T.A.; de Korte, M.A.; Jonkman, S.; Kosterink, J.G.W.; van Veldhuisen, D.J.; Sleijfer, D.T.; Jager, P.L.; et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 2006, 24, 2276–2282. [Google Scholar] [CrossRef]
- Dijkers, E.C.F.; Kosterink, J.G.W.; Rademaker, A.P.; Perk, L.R.; van Dongen, G.A.M.S.; Bart, J.; de Jong, J.R.; de Vries, E.G.E.; Lub-de Hooge, M.N. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J. Nucl. Med. 2009, 50, 974–981. [Google Scholar] [CrossRef]
- Paudyal, P.; Paudyal, B.; Hanaoka, H.; Oriuchi, N.; Iida, Y.; Yoshioka, H.; Tominaga, H.; Watanabe, S.; Watanabe, S.; Ishioka, N.S.; et al. Imaging and biodistribution of HER2/neu expression in non-small cell lung cancer xenografts with 64Cu-labeled trastuzumab PET. Cancer Sci. 2010, 101, 1045–1050. [Google Scholar] [CrossRef]
- Orlova, A.; Wullberg, H.; Stone-Elander, S.; Tolmachev, V. On the selection of a tracer for PET imaging of HER2-expressing tumors: Direct comparison of a 124I-labeled affibody molecule and trastuzumab in a murine xenograft model. J. Nucl. Med. 2009, 50, 417–425. [Google Scholar] [CrossRef]
- Boskovitz, A.; McLendon, R.E.; Okamura, T.; Sampson, J.H.; Bigner, D.D.; Zalutsky, M.R. Treatment of HER2-positive breast carcinomatous meningitis with intrathecal administration of alpha-particle-emitting 211At-labeled trastuzumab. Nucl. Med. Biol. 2009, 36, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, E.C.; Munnink, T.H.O.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schroder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.E.; Bading, J.R.; Colcher, D.M.; Conti, P.S.; Frankel, P.H.; Carroll, M.I.; Tong, S.; Poku, E.; Miles, J.K.; Shively, J.E.; et al. Functional imaging of human epidermal growth factor receptor 2-positive metastatic breast cancer using 64Cu-DOTA-trastuzumab PET. J. Nucl. Med. 2014, 55, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Caldas-Lopes, E.; Divilov, V.; Longo, V.A.; Taldone, T.; Zatorska, D.; Chiosis, G.; Lewis, J.S. Measuring the pharmacodynamic effects of a novel Hsp90 inhibitor on HER2/neu expression in mice using 89Zr-DFO-trastuzumab. PLoS ONE 2010, 5, e8859. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; McDougald, D.; Choi, J.; Koumarianou, E.; Weitzel, D.; Osada, T.; Lyerly, H.K.; Zalutsky, M.R. Preclinical evaluation of 18F-labeled anti-HER2 nanobody conjugates for imaging HER2 receptor expression by immuno-PET. J. Nucl. Med. 2016, 57, 967–973. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; Vincke, C.; Xavier, C.; Aerts, A.; Impens, N.; Baatout, S.; De Raeve, H.; Muyldermans, S.; Caveliers, V.; Devoogdt, N.; et al. Targeted radionuclide therapy with a 177Lu-labeled anti-HER2 nanobody. Theranostics 2014, 4, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Girish, S.; Gupta, M.; Wang, B.; Lu, D.; Krop, I.E.; Vogel, C.L.; Burris, H.A.; LoRusso, P.M.; Yi, J.H.; Saad, O.; et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): An antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother. Pharmacol. 2012, 69, 1229–1240. [Google Scholar] [CrossRef]
- Vivier, D.; Sharma, S.K.; Zeglis, B.M. Understanding the in vivo fate of radioimmunoconjugates for nuclear imaging. J. Labelled Comp. Radiopharm. 2018, 61, 672–692. [Google Scholar] [CrossRef]
- Lub-de Hooge, M.N.; Kosterink, J.G.W.; Perik, P.J.; Nijnuis, H.; Tran, L.; Bart, J.; Suurmeijer, A.J.H.; de Jong, S.; Jager, P.L.; de Vries, E.G.E. Preclinical characterisation of 111In-DTPA-trastuzumab. Br. J. Pharmacol. 2004, 143, 99–106. [Google Scholar] [CrossRef]
- Garg, P.K.; Alston, K.L.; Zalutsky, M.R. Catabolism of radioiodinated murine monoclonal-antibody F(ab’)2 fragment labeled using N-succinimidyl 3-iodobenzoate and Iodogen methods. Bioconjugate Chem. 1995, 6, 493–501. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Narula, A.S. A Method for the radiohalogenation of proteins resulting in decreased thyroid uptake of radioiodine. Appl. Radiat. Isot. 1987, 38, 1051–1055. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Noska, M.A.; Colapinto, E.V.; Garg, P.K.; Bigner, D.D. Enhanced tumor localization and in vivo stability of a monoclonal antibody radioiodinated using N-succinimidyl 3-(tri-n-butylstannyl)benzoate. Cancer Res. 1989, 49, 5543–5549. [Google Scholar] [PubMed]
- Vaidyanathan, G.; Affleck, D.J.; Li, J.; Welsh, P.; Zalutsky, M.R. A polar substituent containing acylation agent for the radioiodination of internalizing monoclonal antibodies: N-succinimidyl 4-guanidinomethyl-3-[131I]iodobenzbate ([131I]SGMIB). Bioconjugate Chem. 2001, 12, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; Alston, K.L.; Bigner, D.D.; Zalutsky, M.R. Nε-(3-[*I]iodobenzoyl)-Lys5-Nα-maleimido-Gly1-GEEEK ([*I]IB-Mal-D-GEEEK): A radioiodinated prosthetic group containing negatively charged d-glutamates for labeling internalizing monoclonal antibodies. Bioconjugate Chem. 2006, 17, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Pruszynski, M.; Koumarianou, E.; Vaidyanathan, G.; Chitneni, S.; Zalutsky, M.R. D-Amino acid peptide residualizing agents bearing N-hydroxysuccinimido- and maleimido-functional groups and their application for trastuzumab radioiodination. Nucl. Med. Biol. 2015, 42, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Howell, R.W. Radiation spectra for Auger-electron emitting radionuclides: Report No. 2 of AAPM Nuclear Medicine Task Group No. 6. Med. Phys. 1992, 19, 1371–1383. [Google Scholar] [CrossRef]
- Akabani, G.; Carlin, S.; Welsh, P.; Zalutsky, M.R. In vitro cytotoxicity of 211At-labeled trastuzumab in human breast cancer cell lines: Effect of specific activity and HER2 receptor heterogeneity on survival fraction. Nucl. Med. Biol. 2006, 33, 333–347. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Zalutsky, M.R. Preparation of N-succinimidyl 3-[*I]iodobenzoate: An agent for the indirect radioiodination of proteins. Nat. Protoc. 2006, 1, 707–713. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Zalutsky, M.R. Synthesis of N-succinimidyl 4-guanidinomethyl-3-[*I]iodobenzoate: A radio-iodination agent for labeling internalizing proteins and peptides. Nat. Protoc. 2007, 2, 282–286. [Google Scholar] [CrossRef]
- Yang, Z.X.; Cao, H.; Xing, C.G.; Wei, S.H.; Jiang, G.Q.; Liu, Z.L. Visualization and body distribution of [131I]-herceptin in nude mice with BT-474 breast carcinoma. Genet. Mol. Res. 2014, 13, 6804–6812. [Google Scholar] [CrossRef]
- Ferreira, C.L.; Yapp, D.T.T.; Crisp, S.; Sutherland, B.W.; Ng, S.S.W.; Gleave, M.; Bensimon, C.; Jurek, P.; Kiefer, G.E. Comparison of bifunctional chelates for 64Cu antibody imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Tinianow, J.N.; Gill, H.S.; Ogasawara, A.; Flores, J.E.; Vanderbilt, A.N.; Luis, E.; Vandlen, R.; Darwish, M.; Junutula, J.R.; Williams, S.P.; et al. Site-specifically 89Zr-labeled monoclonal antibodies for immunoPET. Nucl. Med. Biol. 2010, 37, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.J.; Desilva, R.; Jain, S.; Lears, K.; Rogers, B.; Lapi, S. 89Zr-Radiolabeled trastuzumab imaging in orthotopic and metastatic breast tumors. Pharmaceuticals 2012, 5, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Vivier, D.; Sharma, S.K.; Adumeau, P.; Rodriguez, C.; Fung, K.; Zeglis, B.M. The impact of FcγRI binding on immuno-PET. J. Nucl. Med. 2019, 60, 1174–1182. [Google Scholar] [CrossRef]
- McKnight, B.N.; Viola-Villegas, N.T. Monitoring Src status after dasatinib treatment in HER2+ breast cancer with 89Zr-trastuzumab PET imaging. Breast Cancer Res. 2018, 20, 130. [Google Scholar] [CrossRef]
- Abou, D.S.; Ku, T.; Smith-Jones, P.M. In vivo biodistribution and accumulation of 89Zr in mice. Nucl. Med. Biol. 2011, 38, 675–681. [Google Scholar] [CrossRef]
- Wu, Q.; Li, J.; Zhu, S.; Wu, J.; Chen, C.; Liu, Q.; Wei, W.; Zhang, Y.; Sun, S. Breast cancer subtypes predict the preferential site of distant metastases: A SEER based study. Oncotarget 2017, 8, 27990–27996. [Google Scholar] [CrossRef]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-Trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef]
- Dehdashti, F.; Wu, N.; Bose, R.; Naughton, M.J.; Ma, C.X.; Marquez-Nostra, B.V.; Diebolder, P.; Mpoy, C.; Rogers, B.E.; Lapi, S.E.; et al. Evaluation of [89Zr]trastuzumab-PET/CT in differentiating HER2-positive from HER2-negative breast cancer. Breast Cancer Res. Treat. 2018, 169, 523–530. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Hyman, D.M.; Lyashchenko, S.K.; Lewis, J.S.; Carrasquillo, J.A. 89Zr-Trastuzumab PET/CT for detection of human epidermal growth factor receptor 2-positive metastases in patients with human epidermal growth factor receptor 2-negative primary breast cancer. Clin. Nucl. Med. 2017, 42, 912–917. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds N-succinimidyl 3-iodobenzoate (SIB) and N-succinimidyl 4-guanidinomethyl 3-iodobenzoate (SGMIB) are available from the authors. |



| Organ/Tissue | 4 h 1 | 12 h 1 | 24 h 1 | 48 h 1 | ||||
|---|---|---|---|---|---|---|---|---|
| SGMIB | SIB | SGMIB | SIB | SGMIB | SIB | SGMIB | SIB | |
| Liver | 17.2 ± 2.0 | 14.5 ± 2.9 | 9.3 ± 1.6 | 8.6 ± 1.4 | 8.2 ± 3.8 | 7.2 ± 4.0 | 5.0 ± 2.4 | 4.7 ± 2.4 |
| Spleen | 19.8 ± 9.0 | 19.1 ± 9.1 | 14.0 ± 8.2 | 12.8 ± 7.5 | 7.7 ± 3.1 | 7.0 ± 3.1 | 8.9 ± 6.4 | 8.3 ± 6.1 |
| Lungs | 11.1 ± 2.0 | 10.8 ± 2.0 | 9.1 ± 1.5 | 9.0 ± 1.7 | 5.8 ± 1.6 | 5.8 ± 1.7 | 4.1 ± 1.7 | 3.9 ± 1.6 |
| Heart | 6.9 ± 1.8 | 7.1 ± 2.0 | 5.7 ± 1.2 | 5.9 ± 1.3 | 3.4 ± 1.6 | 3.5 ± 1.6 | 2.5 ± 1.2 | 2.6 ± 1.3 |
| Kidneys | 7.6 ± 1.2 | 8.5 ± 0.9 | 6.5 ± 1.1 | 6.5 ± 1.0 | 4.1 ± 1.4 | 4.4 ± 1.4 | 3.3 ± 1.5 | 3.4 ± 1.5 |
| Bladder | 2.0 ± 0.6 | 2.2 ± 0.7 | 3.6 ± 0.6 | 3.6 ± 0.5 | 4.0 ± 2.1 | 4.2 ± 2.6 | 3.6 ± 1.4 | 3.6 ± 1.3 |
| Stomach | 2.7 ± 0.7 | 3.0 ± 0.7 | 1.8 ± 0.4 | 1.8 ± 0.5 | 1.7 ± 0.5 | 2.1 ± 0.6 | 1.1 ± 0.9 | 1.3 ± 1.0 |
| Thyroid 2 | 0.6 ± 0.4 | 0.6 ± 0.4 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.2 | 0.3 ± 0.2 |
| Bone | 2.3 ± 0.4 | 2.4 ± 0.4 | 1.7 ± 0.3 | 1.7 ± 0.5 | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.2 ± 0.7 | 1.2 ± 0.7 |
| Muscle | 1.6 ± 0.3 | 1.6 ± 0.3 | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.5 | 1.3 ± 0.5 | 1.1 ± 0.3 | 1.1 ± 0.3 |
| Blood | 18.4 ± 4.5 | 19.9 ± 4.6 | 18.0 ± 3.0 | 19.3 ± 3.0 | 9.6 ± 3.6 | 10.6 ± 3.5 | 7.0 ± 2.9 | 8.0 ± 3.1 |
| Brain | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Tumor 3 | 13.0 ± 2.3 | 11.5 ± 2.3 | 20.3 ± 6.4 | 15.2 ± 3.7 | 20.7 ± 7.0 | 14.6 ± 4.3 | 20.1 ± 7.4 | 12.8 ± 4.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitneni, S.K.; Koumarianou, E.; Vaidyanathan, G.; Zalutsky, M.R. Observations on the Effects of Residualization and Dehalogenation on the Utility of N-Succinimidyl Ester Acylation Agents for Radioiodination of the Internalizing Antibody Trastuzumab. Molecules 2019, 24, 3907. https://doi.org/10.3390/molecules24213907
Chitneni SK, Koumarianou E, Vaidyanathan G, Zalutsky MR. Observations on the Effects of Residualization and Dehalogenation on the Utility of N-Succinimidyl Ester Acylation Agents for Radioiodination of the Internalizing Antibody Trastuzumab. Molecules. 2019; 24(21):3907. https://doi.org/10.3390/molecules24213907
Chicago/Turabian StyleChitneni, Satish K., Eftychia Koumarianou, Ganesan Vaidyanathan, and Michael R. Zalutsky. 2019. "Observations on the Effects of Residualization and Dehalogenation on the Utility of N-Succinimidyl Ester Acylation Agents for Radioiodination of the Internalizing Antibody Trastuzumab" Molecules 24, no. 21: 3907. https://doi.org/10.3390/molecules24213907





