Antioxidant Activity of Blueberry (Vaccinium spp.) Cultivar Leaves: Differences across the Vegetative Stage and the Application of Near Infrared Spectroscopy
Abstract
:1. Introduction
2. Results
2.1. Cultivars Antioxidant Activity
2.1.1. Total Phenolic Content (TPC)
2.1.2. Total Flavonoid Content (TFC)
2.1.3. Total Antioxidant Capacity (TAC)
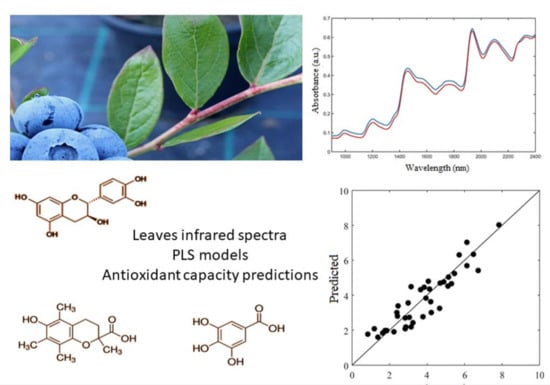
2.2. Antioxidant Activity Prediction by NIR Spectroscopy
3. Discussion
4. Material and Methods
4.1. Reagents and Solutions
4.2. Vaccinium Cultivar Leaves
4.3. Antioxidant Activity
4.3.1. Total Phenolic Content (TPC)
4.3.2. Total flavonoid Content (TFC)
4.3.3. Total Antioxidant Capacity (TAC)
4.4. Near Infrared Spectroscopy
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cutler, B.R.; Gholami, S.; Chua, J.S.; Kuberan, B.; Babu, P.V.A. Blueberry metabolites restore cell surface glycosaminoglycans and attenuate endothelial inflammation in diabetic human aortic endothelial cells. Int. J. Cardiol. 2018, 261, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Norberto, S.; Silva, S.; Meireles, M.; Faria, A.; Pintado, M.; Calhau, C. Blueberry anthocyanins in health promotion: A metabolic overview. J. Funct. Foods 2013, 5, 1518–1528. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqyist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry Supplementation Improves Memory in Older Adults. J. Agr. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, E.D.; Ren, Z.H.; DeFuria, J.; Obin, M.S.; Meydani, S.N.; Wu, D.Y. Dietary supplementation with blueberry partially restores T-cell-mediated function in high-fat-diet-induced obese mice. Br. J. Nutr. 2018, 119, 1393–1399. [Google Scholar] [CrossRef] [Green Version]
- Available online: http://www.who.int/dietphysicalactivity/fruit/en (accessed on 4 June 2019).
- Ehlenfeldt, M.K.; Prior, R.L. Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry. J. Agr. Food Chem. 2010, 49, 2222–2227. [Google Scholar] [CrossRef]
- Harris, C.S.; Burt, A.J.; Saleem, A.; Le, P.M.; Martineau, L.C.; Haddad, P.S.; Bennett, S.A.L.; Arnason, J.T. A single HPLC-PAD-APCI/MS method for the quantitative comparison of phenolic compounds found in leaf, stem, root and fruit extracts of Vaccinium angustifolium. Phytochem. Anal. 2007, 18, 161–169. [Google Scholar] [CrossRef]
- Li, C.; Feng, J.; Huang, W.; An, X. Composition of Polyphenols and Antioxidant Activity of Rabbiteye Blueberry (Vaccinium ashei) in Nanjing. J. Agr. Food Chem. 2013, 61, 523–531. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Tan, J.; Wang, B. Influence of harvest season on antioxidant activity and constituents of rabbiteye blueberry (Vaccinium ashei) leaves. J. Agr. Food Chem. 2013, 61, 11477–11483. [Google Scholar] [CrossRef]
- Cezarotto, V.S.; Giacomelli, S.R.; Vendruscolo, M.H.; Vestena, A.S.; Cezarotto, C.S.; Cruz, R.C.; Maurer, L.H.; Ferreira, L.M.; Emanuelli, T.; Cruz, L. Influence of harvest season and cultivar on the variation of phenolic compounds composition and antioxidant properties in Vaccinium ashei leaves. Molecules 2017, 22, 1603. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J.; Wang, H.; Li, S.; Zheng, X.; Du, H.; Xu, Y.; Wang, L. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods 2015, 16, 295–304. [Google Scholar] [CrossRef]
- Kim, S.M.; Um, B. Evaluation of the antioxidant activity of phenolic compounds among blueberry cultivars by HPLC-ESI/MS and on-line HPLC-ABTS system. J. Med. Plant Res. 2011, 5, 5008–5016. [Google Scholar]
- Routray, W.; Orsat, V. Variation of phenolic activity and antioxidant activity of North American highbush blueberry leaves with variation of time of harvest and cultivar. Ind. Crops Prod. 2014, 62, 147–155. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Barnackas, Š.; Kazernavičiūtė, R.; Maždžierienė, R.; Pukalskas, A.; Šipailienė, A.; Labokas, J.; Ložienė, K.; Abrutienė, G. Variations in antioxidant capacity and phenolics in leaf extracts isolated by different polarity solvents from seven blueberry (Vaccinium L.) genotypes at three phenological stages. Acta Physiol Plant. 2016, 38, 33. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Almeida, M.I.G.S.; Barreiros, L.; Reis, S.; Segundo, M.A. Automatic Aluminum chloride method for routine estimation of total flavonoids in red wines and teas. Food Anal. Meth. 2012, 5, 530–539. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Barreiros, L.; Maia, M.A.; Reis, S.; Segundo, M.A. Rapid assessment of endpoint antioxidant capacity of red wines through microchemical methods using a kinetic matching approach. Talanta 2012, 97, 473–483. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Machado, S.; Segundo, M.A.; Lopes, J.A.; Páscoa, R.N.M.J. Rapid assessment of bioactive phenolics and methylxanthines in spent coffee grounds by FT-NIR spectroscopy. Talanta 2016, 147, 460–467. [Google Scholar] [CrossRef]
- Páscoa, R.N.M.J.; Machado, S.; Magalhães, L.M.; Lopes, J.A. Value adding to red grape pomace exploiting eco-friendly FT-NIR spectroscopy technique. Food Bioprocess Tech. 2015, 8, 865–874. [Google Scholar] [CrossRef]
- Xiong, C.; Liu, C.; Pan, W.; Ma, F.; Xiong, C.; Qi, L.; Chen, F.; Lu, X.; Yang, J.; Zheng, L. Non-destructive determination of total polyphenols content and classification of storage periods of Iron Buddha tea using multispectral imaging system. Food Chem. 2015, 176, 130–136. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Shen, Y.; Chen, J.; Xiao, P.; Bao, J.S. Nondestructive prediction of total phenolics, flavonoid contents, and antioxidant capacity of rice grain using near-infrared spectroscopy. J. Agric. Food Chem. 2008, 56, 8268–8272. [Google Scholar] [CrossRef]
- Wu, D.; Chen, J.Y.; Lu, B.Y.; Xiong, L.N.; He, Y.; Zhang, Y. Application of near infrared spectroscopy for the rapid determination of antioxidant activity of bamboo leaf extract. Food Chem. 2012, 135, 2147–2156. [Google Scholar] [CrossRef]
- Arslan, M.; Zou, X.B.; Tahir, H.E.; Hu, X.T.; Rakha, A.; Zareef, M.; Seweh, E.A.; Basheer, S. NIR spectroscopy coupled chemometric algorithms for rapid antioxidants activity assessment of Chinese dates (Zizyphus Jujuba Mill.). Int. J. Food Eng. 2019, 15, 20180148. [Google Scholar] [CrossRef]
- Zareef, M.; Chen, Q.S.; Ouyang, Q.; Arslan, M.; Hassan, M.M.; Ahmad, W.; Viswadevarayalu, A.; Wang, P.Y.; Wang, A.C. Rapid screening of phenolic compounds in congou black tea (Camellia sinensis) during in vitro fermentation process using portable spectral analytical system coupled chemometrics. J. Food Process. Pres. 2019, 43, e13996. [Google Scholar] [CrossRef]
- Piljac-Žegarac, J.; Belščak, A.; Piljac, A. Antioxidant capacity and polyphenolic content of blueberry (Vaccinium corymbosum L.) leaf infusions. J. Med. Food. 2009, 12, 608–614. [Google Scholar]
- Laxa, M.; Liebthal, M.; Relman, W.; Chibani, K.; Dietz, K.J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, E94. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Su, T.-J.; Cai, S.-M.; Wu, W. Fungal endophyte-derived Fritillaria unibracteata var wabuensis: diversity, antioxidant capacities in vitro and relations to phenolic, flavonoid or saponin compounds. Sci. Rep. 2017, 7, 42008. [Google Scholar] [PubMed]
- AACC. Near-Infrared Methods—Guidelines for Model Development and Maintenance; AACC: St. Paul, MN, USA, 1999. [Google Scholar]
- Lu, X.; Wang, J.; Al-Qadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Páscoa, R.N.M.J.; Teixeira, A.M.; Sousa, C. Antioxidant capacity of Camellia japonica cultivars assessed by near- and mid-infrared spectroscopy. Planta 2019, 249, 1053–1062. [Google Scholar]
- Geladi, P.; Kowalsky, B.R. Partial least-squares regression: a tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1616–1627. [Google Scholar] [CrossRef]
Sample Availability: Samples of the cultivar leaves are available from the authors. |

| Collecting Date | 31th May 2017 | 1st September 2017 | 1st December 2017 | |||
|---|---|---|---|---|---|---|
| Collecting Region | Region A | Region B | Region C | Region A | Region A | |
| Cultivar | Legacy | 106.2 ± 2.8 | 102.2 ± 3.2 | - | 64.3 ± 1.2 | 208.8 ± 3.6 |
| Ozarkblue | 106.5 ± 2.5 | - | - | 78.9 ± 3.3 | - | |
| Misty | 157.6 ± 8.1 | - | - | 177.6 ± 6.2 | 176.5 ± 3.6 | |
| Star | 99.1 ± 2.4 | - | - | - | 170.1 ± 1.6 | |
| Ochlochonee | 127.3 ± 7.9 | 117.2 ± 5.2 | - | 217.9 ± 3.2 | - | |
| Drapler | 134.4 ± 3.8 | 55.2 ± 1.8 | - | - | 207.5 ± 7.9 | |
| Bluecrop | 127.8 ± 4.2 | 95.6 ± 2.9 | - | 120.4 ± 5.5 | 131.0 ± 2.1 | |
| Duke | 82.8 ± 11.6 | 74.3 ± 4.1 | - | 93.0 ± 1.3 | 118.6 ± 4.3 | |
| Powderblue | 161.2 ± 2.8 | 131.2 ± 4.8 | - | - | 219.7 ± 6.1 | |
| O’neal | 72.7 ±1.1 | - | - | - | 162.5 ± 1.1 | |
| Chandler | 123.8 ± 2.1 | 83.0 ± 9.4 | - | 166.5 ± 2.6 | 199.4 ± 5.9 | |
| Bluejay | 127.3 ± 5.4 | - | - | - | - | |
| Camellia | 104.2 ± 8.8 | 81.9 ± 12.5 | - | 69.3 ± 3.1 | 141.9 ± 2.6 | |
| Goldtraube | 114.2 ± 3.2 | - | - | 154.1 ±5.7 | 152.5 ± 3.5 | |
| Titan | 153.2 ± 2.6 | - | - | 40.8 ± 1.3 | 272.8 ± 4.0 | |
| Huron | 75.5 ± 2.2 | - | - | 39.6 ± 1.8 | 192.2 ± 3.9 | |
| Aurora | 110.9 ± 3.3 | - | - | 191.2 ± 6.3 | 227.4 ± 9.9 | |
| Liberty | 117.9 ± 14.8 | - | - | 157.3 ± 1.6 | 176.9 ± 6.0 | |
| Bluegold | - | 69.8 ± 3.9 | - | - | - | |
| Elliott | - | - | - | - | 199.0 ± 1.3 | |
| Patriot | - | - | - | - | 155.5 ± 5.2 | |
| Biloxi | - | - | - | - | 150.7 ± 3.9 | |
| Alix blue | - | - | 97.9 ± 5.9 | - | - | |
| New hanover | - | - | 92.7 ± 4.1 | - | - | |
| Sunset blue | - | - | 98.8 ± 5.0 | - | - | |
| Gupton | - | - | 96.6 ± 5.2 | - | - | |
| Cipria | - | - | 137.3 ± 4.4 | - | - | |
| Collecting Date | 31th May 2017 | 1st September 2017 | 1st December 2017 | |||
|---|---|---|---|---|---|---|
| Collecting Region | Region A | Region B | Region C | Region A | Region A | |
| Cultivar | Legacy | 181.3 ± 6.2 | 113.0 ± 2.9 | - | 60.5 ± 1.4 | 128.1 ± 3.7 |
| Ozarkblue | 177.7 ± 2.9 | - | - | 59.2 ± 2.9 | - | |
| Misty | 269.1 ± 7.4 | - | - | 163.7 ± 10.0 | 143.0 ± 4.6 | |
| Star | 97.4 ± 3.9 | - | - | - | 125.1 ± 2.4 | |
| Ochlochonee | 181.1 ± 7.9 | 67.7 ± 2.9 | - | 192.7 ± 4.5 | - | |
| Drapler | 204.6 ± 20.6 | 71.0 ± 6.2 | - | - | 156.0 ± 2.3 | |
| Bluecrop | 198.1 ± 9.8 | 73.3 ± 3.9 | - | 96.4 ± 4.0 | 86.4 ± 2.3 | |
| Duke | 79.6 ± 9.7 | 108.9 ± 5.1 | - | 71.3 ± 2.2 | 87.5 ± 3.2 | |
| Powderblue | 248.8 ± 20.4 | 72.2 ± 5.1 | - | - | 189.1 ± 6.0 | |
| O’neal | 111.3 ± 4.4 | - | - | - | 106.2 ± 2.4 | |
| Chandler | 119.2 ± 3.5 | 74.9 ± 4.0 | - | 133.9 ± 4.4 | 156.7 ± 7.6 | |
| Bluejay | 184.5 ± 5.4 | - | - | - | - | |
| Camellia | 79.0 ± 6.5 | 45.2 ± 2.3 | - | 47.3 ± 2.0 | 101.4 ± 2.2 | |
| Goldtraube | 110.7 ± 4.9 | - | - | 115.1 ± 4.4 | 118.1 ± 5.7 | |
| Titan | 244.2 ± 7.5 | - | - | 47.5 ± 4.6 | 242.5 ± 6.9 | |
| Huron | 103.0 ± 5.6 | - | - | 41.2 ± 4.8 | 133.8 ± 6.0 | |
| Aurora | 103.4 ± 4.2 | - | - | 141.0 ± 5.9 | 150.8 ± 6.6 | |
| Liberty | 78.8 ± 5.8 | - | - | 50.0 ± 4.6 | 126.4 ± 4.2 | |
| Bluegold | - | 94.2 ± 3.5 | - | - | - | |
| Elliott | - | - | - | - | 131.9 ± 4.1 | |
| Patriot | - | - | - | - | 89.6 ± 5.6 | |
| Biloxi | - | - | - | - | 96.0 ± 3.1 | |
| Alix blue | - | - | 88.8 ± 5.2 | - | - | |
| New hanover | - | - | 96.2 ± 6.9 | - | - | |
| Sunset blue | - | - | 92.5 ± 8.4 | - | - | |
| Gupton | - | - | 97.3 ± 4.8 | - | - | |
| Cipria | - | - | 129.2 ± 4.0 | - | - | |
| Collecting Date | 31th May 2017 | 1st September 2017 | 1st December 2017 | |||
|---|---|---|---|---|---|---|
| Collecting Region | Region A | Region B | Region C | Region A | Region A | |
| Cultivar | Legacy | 53.8 ± 1.2 | 56.3 ± 1.0 | - | 34.4 ± 1.3 | 81.4 ± 0.7 |
| Ozarkblue | 50.3 ± 0.8 | - | - | 43.1 ± 2.7 | - | |
| Misty | 63.7 ± 2.0 | - | - | 99.6 ± 3.3 | 72.6 ± 1.9 | |
| Star | 46.5 ± 1.5 | - | - | - | 76.4 ± 0.9 | |
| Ochlochonee | 62.8 ± 0.8 | 56.3 ± 0.9 | - | 101.1 ± 1.4 | - | |
| Drapler | 60.8 ± 1.7 | 34.5 ± 2.2 | - | - | 107.8 ± 3.8 | |
| Bluecrop | 57.0 ± 1.2 | 51.7 ± 1.5 | - | 55.6 ± 1.8 | 61.1 ± 2.2 | |
| Duke | 39.3 ± 1.5 | 40.0 ± 1.3 | - | 47.6 ± 1.7 | 61.0 ± 1.8 | |
| Powderblue | 65.5 ± 1.0 | 68.2 ± 2.1 | - | - | 115.8 ± 3.5 | |
| O’neal | 40.3 ± 0.3 | - | - | - | 66.7 ± 0.9 | |
| Chandler | 55.8 ± 0.8 | 43.0 ± 3.7 | - | 92.1 ± 0.9 | 107.3 ± 0.9 | |
| Bluejay | 59.1 ± 1.7 | - | - | - | - | |
| Camellia | 50.8 ± 0.9 | 44.5 ± 4.0 | - | 39.2 ± 1.2 | 62.2 ± 1.5 | |
| Goldtraube | 50.2 ± 3.3 | - | - | 86.2 ± 1.2 | 70.6 ± 2.1 | |
| Titan | 63.1 ± 1.3 | - | - | 23.2 ± 1.1 | 124.8 ± 2.5 | |
| Huron | 41.7 ± 1.3 | - | - | 22.6 ± 0.7 | 113.4 ± 3.1 | |
| Aurora | 54.9 ± 3.9 | - | - | 100.0 ± 2.6 | 120.0 ± 1.2 | |
| Liberty | 61.9 ± 4.4 | - | - | 23.3 ± 1.6 | 73.3 ± 1.7 | |
| Bluegold | - | 44.7 ± 2.5 | - | - | - | |
| Elliott | - | - | - | - | 78.2 ± 0.9 | |
| Patriot | - | - | - | - | 64.5 ± 1.7 | |
| Biloxi | - | - | - | - | 62.2 ± 1.5 | |
| Alix blue | - | - | 42.9 ± 1.2 | - | - | |
| New hanover | - | - | 43.0 ± 1.9 | - | - | |
| Sunset blue | - | - | 48.6 ± 4.6 | - | - | |
| Gupton | - | - | 46.4 ± 3.8 | - | - | |
| Cipria | - | - | 56.0 ± 5.0 | - | - | |
| Parameter | Data Set | Spectral Range | LVs | RMSEC | R2C | RMSECV | R2CV | RMSEP | R2P | RER |
|---|---|---|---|---|---|---|---|---|---|---|
| Total Phenolic Content | 1 | 6315–5390 and 4925–4073 | 5 | 12.3 | 0.94 | 13.1 | 0.93 | 12.6 | 0.93 | 18.5 |
| 2 | 6315–5390 and 4925–4073 | 7 | 12.6 | 0.94 | 13.9 | 0.92 | 13.5 | 0.94 | 17.3 | |
| 3 | 6315–5390 and 4925–4073 | 5 | 8.0 | 0.91 | 9.1 | 0.88 | 8.8 | 0.89 | 12.0 | |
| 4 | 6315–5390 and 4925–4073 | 5 | 4.7 | 0.97 | 5.8 | 0.95 | 5.7 | 0.94 | 15.0 | |
| 5 | 6315–5390 and 4925–4073 | 5 | 6.3 | 0.99 | 7.9 | 0.98 | 6.9 | 0.99 | 25.5 | |
| 6 | 6315–5390 and 4925–4073 | 6 | 8.4 | 0.95 | 10.2 | 0.93 | 8.8 | 0.93 | 17.4 | |
| Total Flavonoids Content | 1 | 6315–5390 and 4925–4073 | 9 | 16.4 | 0.86 | 18.1 | 0.83 | 16.6 | 0.88 | 12.1 |
| 2 | 6315–5390 and 4925–4073 | 9 | 12.1 | 0.95 | 13.9 | 0.93 | 12.5 | 0.94 | 18.3 | |
| 3 | 6315–5390 and 4925–4073 | 9 | 17.3 | 0.89 | 21.8 | 0.83 | 18.7 | 0.84 | 12.0 | |
| 4 | 6315–5390 and 4925–4073 | 9 | 15.5 | 0.92 | 22.8 | 0.82 | 16.8 | 0.94 | 11.4 | |
| 5 | 6315–5390 and 4925–4073 | 6 | 5.8 | 0.98 | 7.4 | 0.97 | 6.7 | 0.98 | 22.7 | |
| 6 | 6315–5390 and 4925–4073 | 6 | 7.2 | 0.97 | 8.6 | 0.95 | 7.3 | 0.95 | 21.4 | |
| Total Antioxidant Capacity | 1 | 6315–5390 and 4925–4073 | 7 | 6.2 | 0.92 | 6.8 | 0.91 | 6.4 | 0.93 | 16.0 |
| 2 | 6315–5390 and 4925–4073 | 7 | 6.5 | 0.93 | 7.1 | 0.92 | 7.1 | 0.92 | 14.3 | |
| 3 | 6315–5390 and 4925–4073 | 7 | 3.0 | 0.90 | 3.5 | 0.86 | 3.0 | 0.88 | 11.2 | |
| 4 | 6315–5390 and 4925–4073 | 7 | 1.9 | 0.95 | 2.4 | 0.92 | 1.9 | 0.94 | 13.5 | |
| 5 | 6315–5390 and 4925–4073 | 7 | 2.3 | 0.99 | 3.8 | 0.98 | 2.9 | 0.99 | 26.8 | |
| 6 | 6315–5390 and 4925–4073 | 6 | 3.5 | 0.98 | 4.5 | 0.96 | 3.7 | 0.98 | 17.4 |
| Species | Cultivar | Nº | May | June | July | August | September | October | November | December | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||
| V. corymbosum | Biloxi | 1 | X | |||||||||||||||||||||||||||||||
| New hanover | 1 | X | ||||||||||||||||||||||||||||||||
| Camellia | 2 | X | X | X | ||||||||||||||||||||||||||||||
| O’neal | 1 | X | X | |||||||||||||||||||||||||||||||
| Misty | 1 | X | X | X | ||||||||||||||||||||||||||||||
| Alix blue | 1 | X | ||||||||||||||||||||||||||||||||
| Star | 1 | X | X | |||||||||||||||||||||||||||||||
| Gupton | 1 | X | ||||||||||||||||||||||||||||||||
| Cipria | 1 | X | ||||||||||||||||||||||||||||||||
| Goldtraube | 1 | X | X | X | ||||||||||||||||||||||||||||||
| Legacy | 2 | X | X | X | ||||||||||||||||||||||||||||||
| Ozarkblue | 1 | X | X | |||||||||||||||||||||||||||||||
| Bluegold | 1 | X | ||||||||||||||||||||||||||||||||
| Draper | 2 | X | X | |||||||||||||||||||||||||||||||
| Chandler | 2 | X | X | X | ||||||||||||||||||||||||||||||
| Liberty | 1 | X | X | X | ||||||||||||||||||||||||||||||
| Bluecrop | 2 | X | X | X | ||||||||||||||||||||||||||||||
| Sunset blue | 1 | X | ||||||||||||||||||||||||||||||||
| Duke | 2 | X | X | |||||||||||||||||||||||||||||||
| Patriot | 1 | X | X | |||||||||||||||||||||||||||||||
| Bluejay | 1 | X | ||||||||||||||||||||||||||||||||
| Huron | 1 | X | X | X | ||||||||||||||||||||||||||||||
| Aurora | 1 | X | X | X | ||||||||||||||||||||||||||||||
| Elliott | 1 | X | X | |||||||||||||||||||||||||||||||
| V. ashei | Titan | 1 | X | X | X | |||||||||||||||||||||||||||||
| Ochlochonee | 2 | X | X | |||||||||||||||||||||||||||||||
| Powderblue | 2 | X | X | |||||||||||||||||||||||||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Páscoa, R.N.M.J.; Gomes, M.J.; Sousa, C. Antioxidant Activity of Blueberry (Vaccinium spp.) Cultivar Leaves: Differences across the Vegetative Stage and the Application of Near Infrared Spectroscopy. Molecules 2019, 24, 3900. https://doi.org/10.3390/molecules24213900
Páscoa RNMJ, Gomes MJ, Sousa C. Antioxidant Activity of Blueberry (Vaccinium spp.) Cultivar Leaves: Differences across the Vegetative Stage and the Application of Near Infrared Spectroscopy. Molecules. 2019; 24(21):3900. https://doi.org/10.3390/molecules24213900
Chicago/Turabian StylePáscoa, Ricardo N.M.J., Maria João Gomes, and Clara Sousa. 2019. "Antioxidant Activity of Blueberry (Vaccinium spp.) Cultivar Leaves: Differences across the Vegetative Stage and the Application of Near Infrared Spectroscopy" Molecules 24, no. 21: 3900. https://doi.org/10.3390/molecules24213900