Concise and Gram-Scale Total Synthesis of Lansiumamides A and B and Alatamide
Abstract
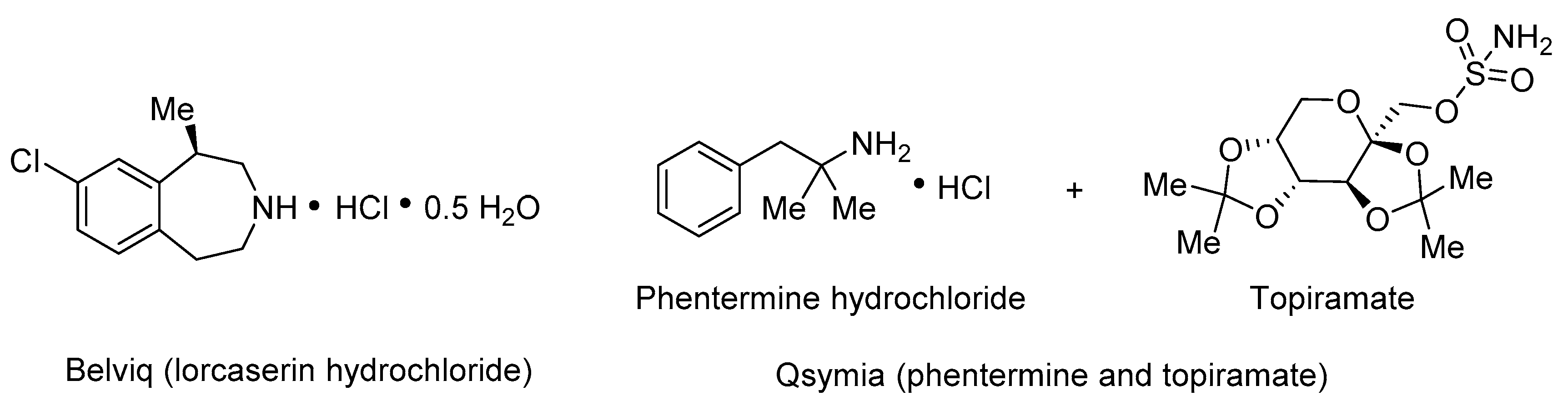
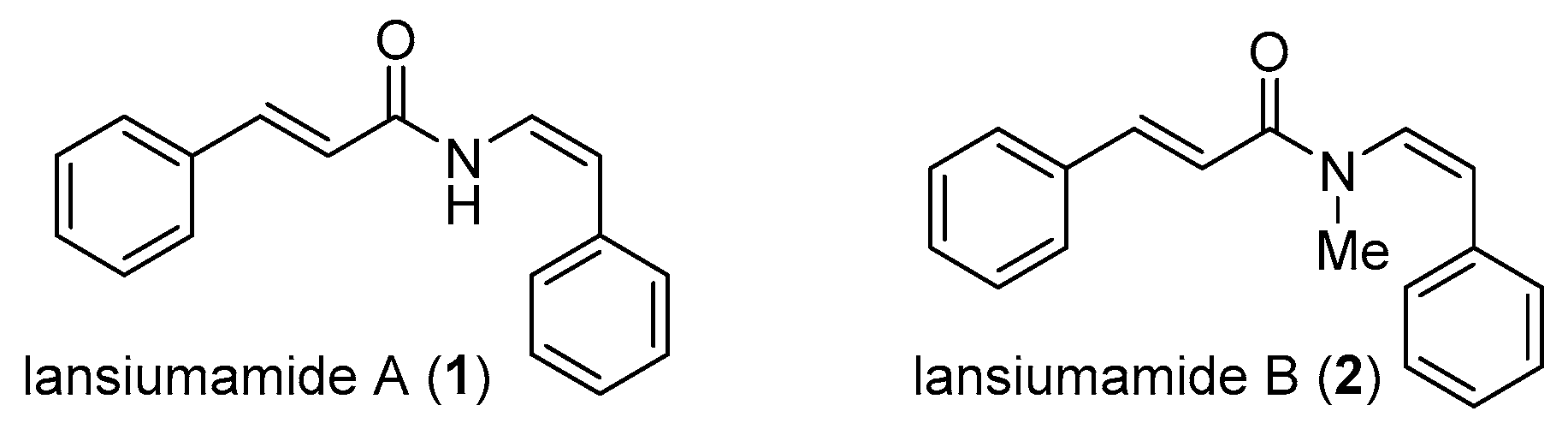
:1. Introduction
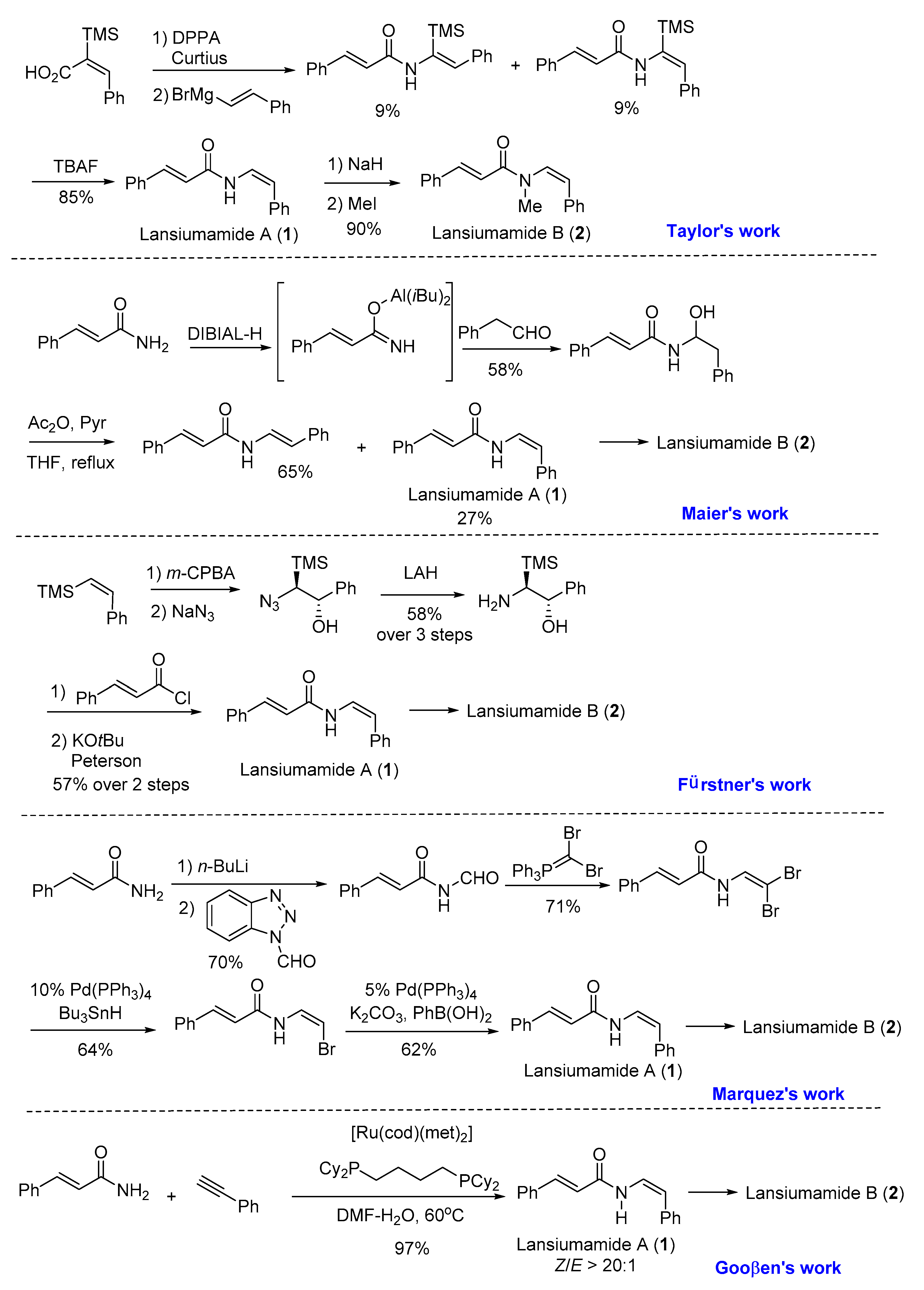
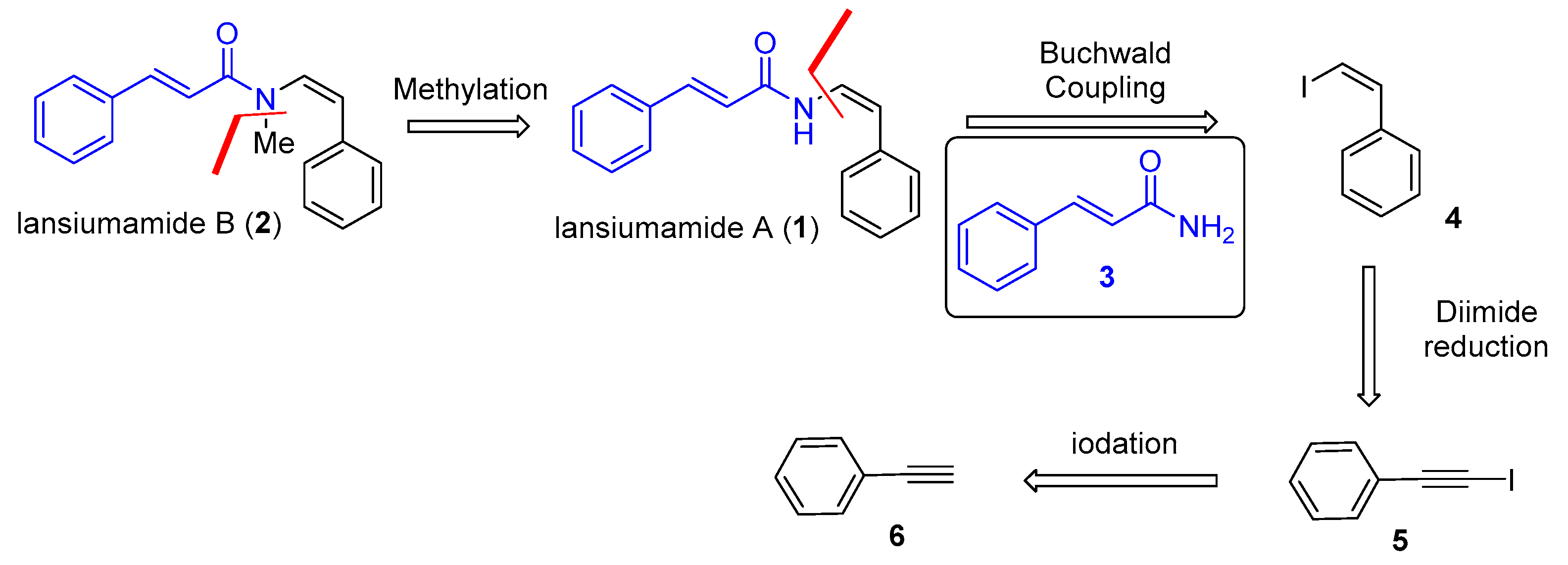
2. Results and Discussion
3. Materials and Methods
3.1. General
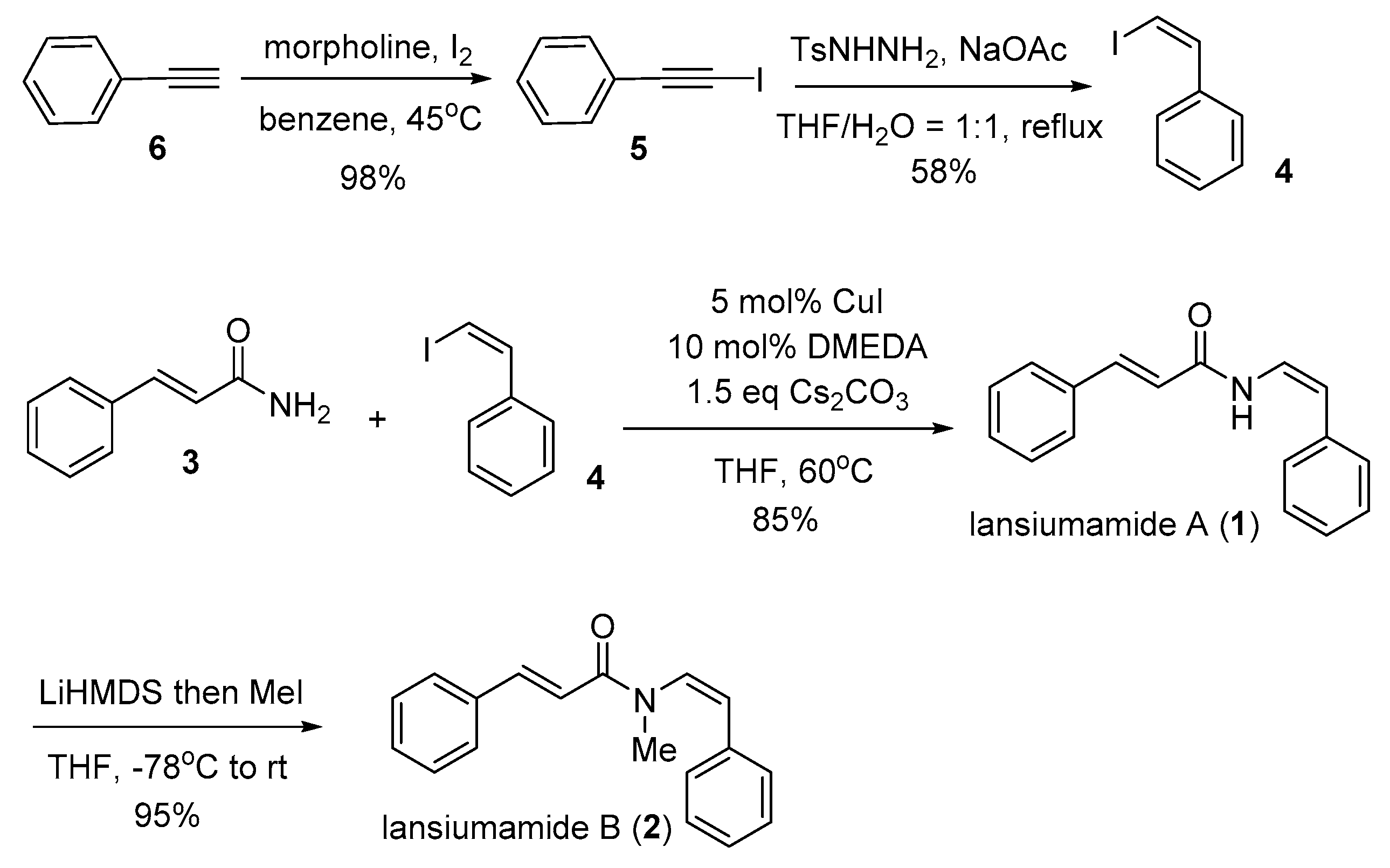
3.2. Total Synthesis of Lansiumamides A and B
3.2.1. Synthesis of (Iodoethynyl)Benzene (5)
3.2.2. Synthesis of (Z)-(2-Iodovinyl)Benzene (4)
3.2.3. Synthesis of Lansiumamide A (1)
3.2.4. Synthesis of Lansiumamide B (2)
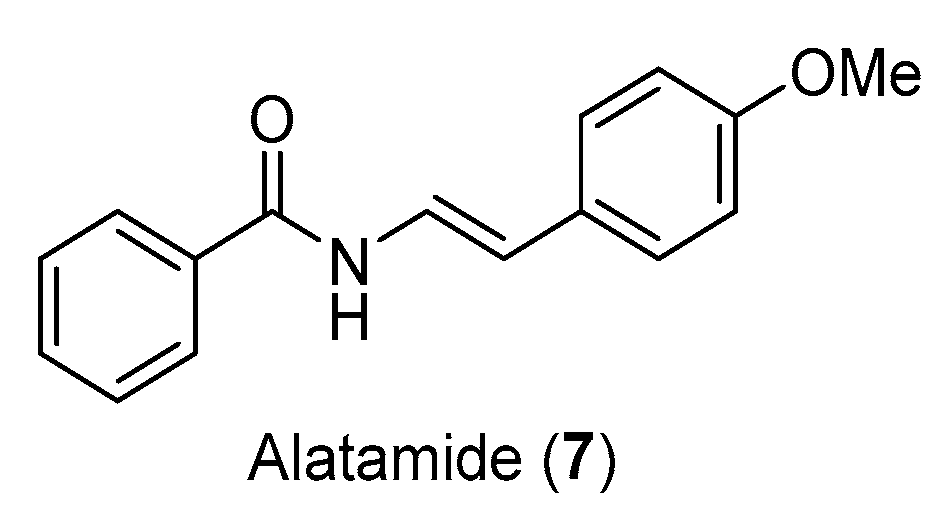
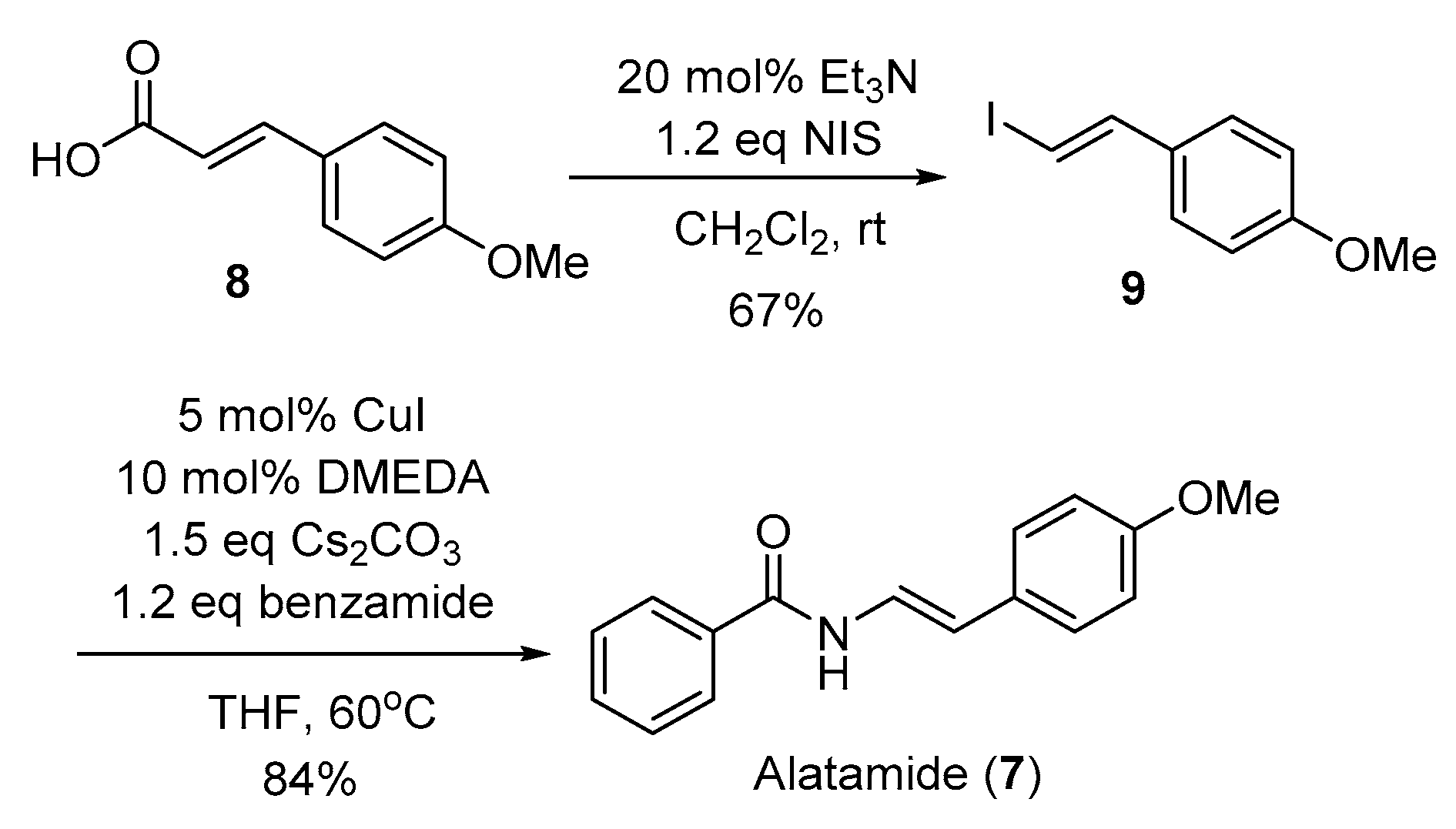
3.3. Total Synthesis of Alatamide
3.3.1. Synthesis of (E)-1-(2-iodovinyl)-4-methoxybenzene (9)
3.3.2. Synthesis of Alatamide (7)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Obesity: Preventing and Controlling the Global Epidemic; Roca: São Paulo, Brazil, 2004. [Google Scholar]
- Reaven, G.M. Pathophysiology of insulin resistance in human disease. Physiol. Rev. 1995, 75, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Must, A.; Spadano, J.; Coakley, E.H.; Field, A.E.; Colditz, G.; Dietz, W.H. The disease burden associated with overweight and obesity. J. Am. Med. Assoc. 1999, 282, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, D.; Xu, Y.S.; Feng, Z.L.; Meng, F.C.; Zhang, Q.W.; Gan, L.S.; Lin, L.G. Clausoxamine, an alkaloid possessing a 1,3-oxazine-4-one ring from the seeds of Clausena lansium and the anti-obesity effect of lansiumamide B. RSC Adv. 2017, 7, 46900–46905. [Google Scholar] [CrossRef]
- Adebajo, A.C.; Iwalewa, E.O.; Obuotor, E.M.; Ibikunle, G.F.; Omisore, N.O.; Adewunmi, C.O.; Obaparusi, O.O.; Klaes, M.; Adetogun, G.E.; Schmidt, T.J.; et al. Pharmacological properties of the extract and some isolated compounds of Clausena lansiumstem bark: Anti-trichomonal, antidiabetic, anti-inflammatory, hepatoprotective and antioxidant effects. J. Ethnopharmacol. 2009, 122, 10–19. [Google Scholar] [CrossRef]
- Daneschvar, H.L.; Aronson, M.D.; Smetana, G.W. FDA-Approved Anti-obesity drugs in the United States. Am. J. Med. 2016, 129, 879.e1–879.e6. [Google Scholar] [CrossRef]
- Lin, J.H. Cinnamamide derivatives from Clausena lansium. Phytochemistry 1989, 28, 621–622. [Google Scholar] [CrossRef]
- Matsui, T.; Ito, C.; Furukawa, H.; Okada, T.; Itoigawa, M. Lansiumamide B and SB-204900 isolated from Clausena lansium inhibit histamine and TNF-a release from RBL-2H3 cells. Inflamm. Res. 2013, 62, 333–341. [Google Scholar] [CrossRef]
- Liu, Y.; Staerk, D.; Nielsen, M.N.; Nyberg, N.; Jäger, A.K. High-resolution hyaluronidase inhibition profiling combined with HPLC–HRMS–SPE–NMR for identification of anti-necrosis constituents in Chinese plants used to treat snakebite. Phytochemistry 2015, 119, 62–69. [Google Scholar] [CrossRef]
- Stefanuti, I.; Smith, S.A.; Taylor, R.J.K. Unsaturated enamides via organometallic addition to isocyanates: The synthesis of Lansamide-I, Lansiumamides A–C and SB-204900. Tetrahedron Lett. 2000, 41, 3735–3738. [Google Scholar] [CrossRef]
- Bayer, A.; Maier, M.E. Synthesis of enamides from aldehydes and amides. Tetrahedron 2004, 60, 6665–6677. [Google Scholar] [CrossRef]
- Fürstner, A.; Brehm, C.; Cancho-Grande, Y. Stereoselective synthesis of enamides by a Peterson reaction manifold. Org. Lett. 2001, 3, 3955–3957. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, A.E.; Ferrari, F.D.; Crawford, J.J.; Marquez, R. Total synthesis of lansiumamides A and B and alatamide. Tetrahedron Lett. 2014, 55, 6042–6043. [Google Scholar] [CrossRef]
- Gooβen, L.J.; Blanchot, M.; Arndt, M.; Salih, K.S.M. Synthesis of botryllamides and lansiumamides via ruthenium-catalyzed hydroamidation of alkynes. Synlett 2010, 2010, 1685–1687. [Google Scholar]
- Xu, H.; Chen, T.; Huang, L.; Shen, Q.; Lian, Z.; Shi, Y.; Ouyang, M.A.; Song, L. Synthesis and fungicidal activity of lansiumamide A and B and their derivatives. Molecules 2018, 23, 1499. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Job, G.E.; Klapars, A.; Buchwald, S.L. Copper-catalyzed coupling of amides and carbamates with vinyl halides. Org. Lett. 2003, 5, 3667–3669. [Google Scholar] [CrossRef] [PubMed]
- Hamersma, J.W.; Snyder, E.I. Diimide reduction using potassium azodicarboxylate. J. Org. Chem. 1965, 30, 3985–3988. [Google Scholar] [CrossRef]
- Picard, J.; Lubin-Germain, N.; Uziel, J.; Augé, J. Indium-mediated alkynylation in C-glycoside synthesis. Synthesis 2006, 2006, 979–982. [Google Scholar]
- Chatterjee, A.; Chakrabarty, M.; Kundu, A.B. Constituents of Pleiospermium alatum: Alatamide and N-benzoyltyramine methyl ether. Aust. J. Chem. 1975, 28, 457–460. [Google Scholar] [CrossRef]
- Feng, C.; Loh, T.P. Rhodium(III)-catalyzed cross-coupling of alkenylboronic acids and N-pivaloyloxylamides. Org. Lett. 2014, 16, 3444–3447. [Google Scholar] [CrossRef]
- Pandey, A.K.; Sharma, R.; Shivahare, R.; Arora, A.; Rastogi, N.; Gupta, S.; Chauhan, P.M.S. Synthesis of perspicamide A and related diverse analogues: Their bioevaluation as potent antileishmanial agents. J. Org. Chem. 2013, 78, 1534–1546. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, D.; Huang, J.; Maruoka, K. Hypervalent iodine mediated chemoselective iodination of alkynes. J. Org. Chem. 2017, 82, 11865–11871. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, J.J.; Bull, J.A.; Charette, A.B. Copper-catalyzed direct alkenylation of N-iminopyridinium ylides. Angew. Chem. Int. Ed. 2010, 49, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Carpita, A.; Ribecai, A.; Rossi, R.; Stabile, P. Synthesis of the racemic forms of carbon-carbon double bond locked analogues of strobilurins which are characterized by a 2-arylcyclopropane ring cis-substituted at C-1 by the methyl (E)-3-methoxypropenoate unit. Tetrahedron 2002, 58, 3673–3680. [Google Scholar] [CrossRef]
- Lee, G.C.M.; Tobias, B.; Holmes, J.M.; Harcourt, D.A.; Garst, M.E. A new synthesis of substituted fulvenes. J. Am. Chem. Soc. 1990, 112, 9330–9336. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1, 2 and 7 are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, R.; Lin, X.; Su, Q.; Guo, B.; Huang, Y.; Ouyang, M.-A.; Song, L.; Xu, H. Concise and Gram-Scale Total Synthesis of Lansiumamides A and B and Alatamide. Molecules 2019, 24, 3764. https://doi.org/10.3390/molecules24203764
Lin R, Lin X, Su Q, Guo B, Huang Y, Ouyang M-A, Song L, Xu H. Concise and Gram-Scale Total Synthesis of Lansiumamides A and B and Alatamide. Molecules. 2019; 24(20):3764. https://doi.org/10.3390/molecules24203764
Chicago/Turabian StyleLin, Ran, Xi Lin, Qian Su, Binbin Guo, Yanqin Huang, Ming-An Ouyang, Liyan Song, and Huiyou Xu. 2019. "Concise and Gram-Scale Total Synthesis of Lansiumamides A and B and Alatamide" Molecules 24, no. 20: 3764. https://doi.org/10.3390/molecules24203764




