Candidate Polyurethanes Based on Castor Oil (Ricinus communis), with Polycaprolactone Diol and Chitosan Additions, for Use in Biomedical Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Obtaining Polyols
2.2. Mechanical Properties of PUs
2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4. Thermal Analysis
2.4.1. Thermogravimetric Analysis
2.4.2. Differential Scanning Calorimetry (DSC)
2.5. Hydrophilic Character
2.5.1. Contact Angle
2.5.2. Water Absorption Rate
2.6. Density Determination
2.7. Dynamo-Mechanical Thermal Analysis (DMTA)
2.8. Field-Emission Scanning Electron Microscopy (FESEM)
2.9. In Vitro Biodegradability Assays
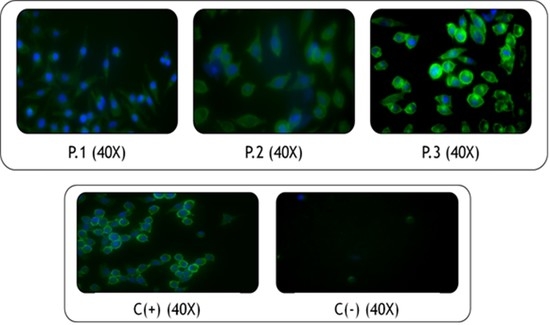
2.10. In Vitro Cell Viability Assay by the MTT Method
2.11. Immunocytochemical Techniques
2.11.1. In Vitro Cell Viability Assay by a Live/Dead Kit
2.11.2. Fixation and Morphological Analysis with Phalloidin and DAPI
2.12. Evaluation of Inflammatory Processes
3. Materials and Methods
3.1. Reagents
3.2. Biological Material
3.3. Obtaining Polyols
3.4. Synthesis of Polyurethanes
3.5. Mechanical Tests
3.6. Fourier-Transform Infrared Spectroscopy (FTIR)
3.7. Thermal Analysis
3.7.1. Thermogravimetric Analysis
3.7.2. Differential Scanning Calorimetry
3.8. Hydrophilic Character
3.8.1. Contact Angle
3.8.2. Water Absorption Rate
3.9. Determination of Density
3.10. Dynamic Mechanical Thermal Analysis (DMTA)
3.11. Field Emission Scanning Electron Microscopy (FESEM)
3.12. In Vitro Biodegradability Tests
3.13. In Vitro Cell Viability Assay by the MTT Method
3.14. Immunocytochemical Techniques
3.15. Differentiation into Macrophages and Inflammation Stimulation
3.16. Evaluation of Inflammatory Processes
3.17. Immunoassay
3.18. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alishiri, M.; Shojaei, A.; Abdekhodaie, M.J.; Yeganeh, H. Synthesis and characterization of biodegradable acrylated polyurethane based on poly(ε-caprolactone) and 1,6-hexamethylene diisocyanate. Mater. Sci. Eng. C 2014, 42, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, H.; Yeganeh, H.; Yari, A.; Nezhad, S.K. Castor oil-based polyurethane coatings containing benzyl triethanol ammonium chloride: Synthesis, characterization, and biological properties. J. Mater. Sci. 2014, 49, 5365–5377. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Gubanska, I.; Janik, H.; Sienkiewicz, M. Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-C.; Hung, K.-C.; Hung, S.-C.; Hsu, S. Evaluation of biodegradable elastic scaffolds made of anionic polyurethane for cartilage tissue engineering. Colloids Surf. B Biointerfaces 2015, 125, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Dean, E.W.; Breuer, C.K. In vivo applications of electrospun tissue-engineered vascular grafts: A review. Tissue Eng. Part B 2014, 20, 628–640. [Google Scholar] [CrossRef]
- Park, H.; Gong, M.-S.; Park, J.-H.; Moon, S.-I.; Wall, I.B.; Kim, H.-W.; Lee, J.H.; Knowles, J.C. Silk fibroin-polyurethane blends: Physical properties and effect of silk fibroin content on viscoelasticity, biocompatibility and myoblast differentiation. Acta Biomater. 2013, 9, 8962–8971. [Google Scholar] [CrossRef]
- Rajan, K.P.; Al-ghamdi, A.; Parameswar, R.; Nando, G.B. Blends of thermoplastic polyurethane and polydimethylsiloxane rubber: Assessment of biocompatibility and suture holding strength of membranes. Int. J. Biomater. 2013, 2013. [Google Scholar] [CrossRef]
- Rodríguez-Galán, A.; Franco, L.; Puiggal, J. Biodegradable Polyurethanes and Poly(ester amide)s. In Handbook of Biodegradable Polymers: Synthesis, Characterization and Applications; Lendlein, A., Sisson, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 133–154. [Google Scholar]
- Adolph, E.J.; Pollins, A.C.; Cardwell, N.L.; Davidson, J.M.; Guelcher, S.A.; Nanney, L.B. Biodegradable lysine-derived polyurethane scaffolds promote healing in a porcine full-thickness excisional wound model. J. Biomater. Sci. Polym. Ed. 2014, 25, 1973–1985. [Google Scholar] [CrossRef] [Green Version]
- Shourgashti, Z.; Khorasani, M.T.; Khosroshahi, S.M.E. Plasma-induced grafting of polydimethylsiloxane onto polyurethane surface: Characterization and in vitro assay. Radiat. Phys. Chem. 2010, 79, 947–952. [Google Scholar] [CrossRef]
- Qiu, H.; Li, D.; Chen, X.; Fan, K.; Ou, W.; Chen, K.C.; Xu, K. Synthesis, characterizations, and biocompatibility of block poly(ester-urethane)s based on biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P3/4HB) and poly(ε-caprolactone). J. Biomed. Mater. Res. A 2013, 101, 75–86. [Google Scholar] [CrossRef]
- Morral-Ruíz, G.; Melgar-Lesmes, P.; García, M.L.; Solans, C.; García-Celma, M.J. Polyurethane and polyurea nanoparticles based on polyoxyethylene castor oil derivative surfactant suitable for endovascular applications. Int. J. Pharm. 2014, 461, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dulińska-Molak, I.; Lekka, M.; Kurzydłowski, K.J. Surface properties of polyurethane composites for biomedical applications. Appl. Surf. Sci. 2013, 270, 553–560. [Google Scholar] [CrossRef]
- Chan-Chan, L.H.; Solis-Correa, R.; Vargas-Coronado, R.F.; Cervantes-Uc, J.M.; Cauich-Rodríguez, J.V.; Quintana, P.; Bartolo-Pérez, P. Degradation studies on segmented polyurethanes prepared with HMDI, PCL and different chain extenders. Acta Biomater. 2010, 6, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Rehman, S.; Zia, F. Chitin and chitosan based polyurethanes: A review of recent advances and prospective biomedical applications. Int. J. Biol. Macromol. 2016, 86, 630–645. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S. Enhanced antibacterial activity, antioxidant and in vitro biocompatibility of modified polycaprolactone-based membranes. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 872–880. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Nair, S.S.; Nair, A.S. Fabrication of a bioadhesive transdermal device from chitosan and hyaluronic acid for the controlled release of lidocaine. Carbohydr. Polym. 2016, 152, 687–698. [Google Scholar] [CrossRef]
- Kaur, G.; Mahajan, M.; Bassi, P. Derivatized Polysaccharides: Preparation, characterization, and application as bioadhesive polymer for drug delivery. Int. J. Polym. Mater. 2013, 62, 475–481. [Google Scholar] [CrossRef]
- Wu, H.; Williams, G.R.; Wu, J.; Wu, J.; Niu, S.; Li, H.; Wang, H.; Zhu, L. Regenerated chitin fibers reinforced with bacterial cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018, 180, 304–313. [Google Scholar] [CrossRef]
- Aranguren, M.I.; González, J.F.; Mosiewicki, M.A. Biodegradation of a vegetable oil based polyurethane and wood flour composites. Polym. Test. 2012, 31, 7–15. [Google Scholar] [CrossRef]
- Guelcher, S.; Srinivasan, A.; Dumas, J. Synthesis, mechanical properties, biocompatibility, and biodegradation of polyurethane networks from lysine polyisocyanates. Biomaterials 2008, 29, 1762–1775. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.; Vreeburg, R.A.M.; Savelkoul, H.F.J.; Wichers, H.J. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: A tool to study inflammation modulating effects of food-derived compounds. Food Funct. 2010, 1, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Sakata, R.K.; Issy, A.M.; Gerola, L.R. Citocinas y dolor. Rev. Bras. Anestesiol. 2011, 61, 137–142. [Google Scholar] [CrossRef]
- Small, A.; Lansdown, N.; Al-Baghdadi, M.; Quach, A.; Ferrante, A. Facilitating THP-1 macrophage studies by differentiating and investigating cell functions in polystyrene test tubes. J. Immunol. Methods 2018, 461, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.E.; To, J.; O’Brien, B.A.; Donnelly, S. The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. J. Immunol. Methods 2016, 430, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ballerini, P.; Diomede, F.; Petragnani, N.; Cicchitti, S.; Merciaro, I.; Cavalcanti, M.F.X.B.; Trubiani, O. Conditioned medium from relapsing-remitting multiple sclerosis patients reduces the expression and release of inflammatory cytokines induced by LPS-gingivalis in THP-1 and MO3.13 cell lines. Cytokine 2017, 96, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Dreskin, S.C.; Thomas, G.W.; Dale, S.N.; Heasley, L.E. Isoforms of Jun kinase are differentially expressed and activated in human monocyte/macrophage (THP-1) cells. J. Immunol. 2001, 166, 5646–5653. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.C.; Thomas, D.; Monaghan, M.; Carroll, O.; Chen, X.; Woodhouse, K.; Brien, T.O.; Pandit, A. An injectable elastin-based gene delivery platform for dose- dependent modulation of angiogenesis and inflammation for critical limb ischemia. Biomaterials 2015, 65, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Yao, Z.; Sato, T.; Keeney, M.; Li, C.; Pajarinen, J.; Yang, F.; Egashira, K.; Goodman, S.B. Suppression of wear-particle-induced pro-inflammatory cytokine and chemokine production in macrophages via NF-κB decoy oligodeoxynucleotide: A preliminary report. Acta Biomater. 2014, 10, 3747–3755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Laube, T.; Weisser, J.; Berger, S.; Börner, S.; Bischoff, S.; Schubert, H.; Gajda, M.; Bräuer, R.; Schnabelrauch, M. In situ foamable, degradable polyurethane as biomaterial for soft tissue repair. Mater. Sci. Eng. C 2017, 78, 163–174. [Google Scholar] [CrossRef]
- Temenoff, J.S.; Mikos, A.G. Biomaterials: The Intersection of Biology and Materials Science, 8th ed.; Prentice Hall, Inc.: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Vannozzi, L.; Ricotti, L.; Santaniello, T.; Terencio, T.; Oropesa-Nunez, R.; Canale, C.; Borghi, F.; Menciassi, A.; Lenardi, C.; Gerges, I. 3D porous polyurethanes featured by different mechanical properties: Characterization and interaction with skeletal muscle cells. J. Mech. Behav. Biomed. Mater. 2017, 75, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Chashmejahanbin, M.R.; Daemi, H.; Barikani, M.; Salimi, A. Noteworthy impacts of polyurethane-urea ionomers as the efficient polar coatings on adhesion strength of plasma treated polypropylene. Appl. Surf. Sci. 2014, 317, 688–695. [Google Scholar] [CrossRef]
- Braun, U.; Lorenz, E.; Weimann, C.; Sturm, H.; Karimov, I.; Ettl, J.; Meier, R.; Wohlgemuth, W.A.; Berger, H.; Wildgruber, M. Mechanic and surface properties of central-venous port catheters after removal: A comparison of polyurethane and silicon rubber materials. J. Mech. Behav. Biomed. Mater. 2016, 64, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Jutrzenka Trzebiatowska, P.; Santamaria Echart, A.; Calvo Correas, T.; Eceiza, A.; Datta, J. The changes of crosslink density of polyurethanes synthesised with using recycled component. Chemical structure and mechanical properties investigations. Prog. Org. Coat. 2018, 115, 41–48. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Castor oil-based hyperbranched polyurethanes as advanced surface coating materials. Prog. Org. Coat. 2013, 76, 157–164. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Isocyanate terminated castor oil-based polyurethane prepolymer: Synthesis and characterization. Prog. Org. Coat. 2015, 80, 39–48. [Google Scholar] [CrossRef]
- Chen, H.; Yu, X.; Zhou, W.; Peng, S.; Zhao, X. Highly toughened polylactide (PLA) by reactive blending with novel polycaprolactone-based polyurethane (PCLU) blends. Polym. Test. 2018, 70, 275–280. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Imran, M.; Lian, Q.; Shehzad, F.K.; Athir, N.; Zhang, J.; Cheng, J. Curcumin incorporated polyurethane urea elastomers with tunable thermo-mechanical properties. React. Funct. Polym. 2018, 128, 97–103. [Google Scholar] [CrossRef]
- Cakić, S.M.; Ristić, I.S.; Cincović, M.M.; Nikolić, N.C.; Nikolić, L.; Cvetinov, M.J. Synthesis and properties biobased waterborne polyurethanes from glycolysis product of PET waste and poly (caprolactone) diol. Prog. Org. Coat. 2017, 105, 111–122. [Google Scholar] [CrossRef]
- Ferreira, P.; Pereira, R.; Coelho, J.F.J.; Silva, A.F.M.; Gil, M.H. Modification of the biopolymer castor oil with free isocyanate groups to be applied as bioadhesive. Int. J. Biol. Macromol. 2007, 40, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arévalo, F.; Uscategui, Y.L.; Diaz, L.; Cobo, M.; Valero, M.F. Effect of the incorporation of chitosan on the physico-chemical, mechanical properties and biological activity on a mixture of polycaprolactone and polyurethanes obtained from castor oil. J. Biomater. Appl. 2016, 31, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Corcuera, M.A.; Rueda, L.; Fernandez d’Arlas, B.; Arbelaiz, A.; Marieta, C.; Mondragon, I.; Eceiza, A. Microstructure and properties of polyurethanes derived from castor oil. Polym. Degrad. Stab. 2010, 95, 2175–2184. [Google Scholar] [CrossRef]
- Uscátegui, Y.L.; Arévalo-Alquichire, S.J.; Gómez-Tejedor, J.A.; Vallés-Lluch, A.; Díaz, L.E.; Valero, M.F. Polyurethane-based bioadhesive synthesized from polyols derived from castor oil (Ricinus communis) and low concentration of chitosan. J. Mater. Res. 2017, 32, 3699–3711. [Google Scholar] [CrossRef]
- Saikia, A.; Karak, N. Renewable resource based thermostable tough hyperbranched epoxy thermosets as sustainable materials. Polym. Degrad. Stab. 2017, 135, 8–17. [Google Scholar] [CrossRef]
- Thakur, S.; Hu, J. Polyurethane: A Shape Memory Polymer (SMP). In Aspects of Polyurethanes; Yilmaz, F., Ed.; InTechOpen: London, UK, 2017; pp. 53–71. [Google Scholar]
- Sáenz-Pérez, M.; Lizundia, E.; Laza, J.M.; García-Barrasa, J.; Vilas, J.L.; León, L.M. Methylene diphenyl diisocyanate (MDI) and toluene diisocyanate (TDI) based polyurethanes: Thermal, shape-memory and mechanical behavior. RSC Adv. 2016, 6, 69094–69102. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, H.; Qu, W.; Xu, Z.; Han, Z. Biomedical segmented polyurethanes based on polyethylene glycol, poly(ε-caprolactone-co-d,l-lactide), and diurethane diisocyanates with uniform hard segment: Synthesis and properties. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 947–956. [Google Scholar] [CrossRef]
- Mi, H.Y.; Jing, X.; Hagerty, B.S.; Chen, G.; Huang, A.; Turng, L.S. Post-crosslinkable biodegradable thermoplastic polyurethanes: Synthesis, and thermal, mechanical, and degradation properties. Mater. Des. 2017, 127, 106–114. [Google Scholar] [CrossRef]
- Gossart, A.; Battiston, K.G.; Gand, A.; Pauthe, E.; Santerre, J.P. Mono vs multilayer fibronectin coatings on polar/hydrophobic/ionic polyurethanes: Altering surface interactions with human monocytes. Acta Biomater. 2018, 66, 129–140. [Google Scholar] [CrossRef]
- Marques, D.S.; Santos, J.M.C.; Ferreira, P.; Correia, T.R.; Correia, I.J.; Gil, M.H.; Baptista, C.M.S.G. Photocurable bioadhesive based on lactic acid. Mater. Sci. Eng. C 2016, 58, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, Z.; Khan, A.S.; Roohpour, N.; Glogauer, M.; Rehman, I.U. Protein adsorption capability on polyurethane and modified-polyurethane membrane for periodontal guided tissue regeneration applications. Mater. Sci. Eng. C 2016, 68, 267–275. [Google Scholar] [CrossRef]
- Conejero-García, Á.; Gimeno, H.R.; Sáez, Y.M.; Vilariño-Feltrer, G.; Ortuño-Lizarán, I.; Vallés-Lluch, A. Correlating synthesis parameters with physicochemical properties of poly(glycerol sebacate). Eur. Polym. J. 2017, 87, 406–419. [Google Scholar] [CrossRef]
- Carriço, C.S.; Fraga, T.; Pasa, V.M.D. Production and characterization of polyurethane foams from a simple mixture of castor oil, crude glycerol and untreated lignin as bio-based polyols. Eur. Polym. J. 2016, 85, 53–61. [Google Scholar] [CrossRef]
- Fuentes, L.E.; Pérez, S.; Martínez, S.I.; García, Á.R. Redes poliméricas interpenetradas de poliuretano a partir de aceite de ricino modificado y poliestireno: Miscibilidad y propiedades mecánicas en función de la composición. Revisata Ion. 2011, 24, 45–50. [Google Scholar]
- Das, B.; Konwar, U.; Mandal, M.; Karak, N. Sunflower oil based biodegradable hyperbranched polyurethane as a thin film material. Ind. Crops Prod. 2013, 44, 396–404. [Google Scholar] [CrossRef]
- Cherng, J.Y.; Hou, T.Y.; Shih, M.F.; Talsma, H.; Hennink, W.E. Polyurethane-based drug delivery systems. Int. J. Pharm. 2013, 450, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Spontón, M.; Casis, N.; Mazo, P.; Raud, B.; Simonetta, A.; Ríos, L.; Estenoz, D. Biodegradation study by Pseudomonas sp. of flexible polyurethane foams derived from castor oil. Int. Biodeterior. Biodegradation 2013, 85, 85–94. [Google Scholar] [CrossRef]
- Gogoi, S.; Barua, S.; Karak, N. Biodegradable and thermostable synthetic hyperbranched poly(urethane-urea)s as advanced surface coating materials. Prog. Org. Coat. 2014, 77, 1418–1427. [Google Scholar] [CrossRef]
- Calvo-Correas, T.; Santamaria-Echart, A.; Saralegi, A.; Martin, L.; Valea, Á.; Corcuera, M.A.; Eceiza, A. Thermally-responsive biopolyurethanes from a biobased diisocyanate. Eur. Polym. J. 2015, 70, 173–185. [Google Scholar] [CrossRef]
- Reddy, T.T.; Kano, A.; Maruyama, A.; Takahara, A. Synthesis, characterization and drug release of biocompatible/biodegradable non-toxic poly(urethane urea)s based on poly(epsilon-caprolactone)s and lysine-based diisocyanate. J. Biomater. Sci. Polym. Ed. 2010, 21, 1483–1502. [Google Scholar] [CrossRef] [PubMed]
- Coakley, D.N.; Shaikh, F.M.; O’Sullivan, K.; Kavanagh, E.G.; Grace, P.A.; McGloughlin, T.M. In vitro evaluation of acellular porcine urinary bladder extracellular matrix—A potential scaffold in tissue engineered skin. Wound Med. 2015, 10–11, 9–16. [Google Scholar] [CrossRef]
- Shahrousvand, M.; Sadeghi, G.M.M.; Shahrousvand, E.; Ghollasi, M.; Salimi, A. Superficial physicochemical properties of polyurethane biomaterials as osteogenic regulators in human mesenchymal stem cells fates. Colloids Surf. B Biointerfaces 2017, 156, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Ortuno-Lizarán, I.; Vilarino-Feltrer, G.; Martinez-Ramos, C.; Pradas, M.M.; Vallés-Lluch, A. Influence of synthesis parameters on hyaluronic acid hydrogels intended as nerve conduits. Biofabrication 2016, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Mi, H.Y.; Huang, H.X.; Turng, L.S. Shape memory thermoplastic polyurethane (TPU)/poly(ε-caprolactone) (PCL) blends as self-knotting sutures. J. Mech. Behav. Biomed. Mater. 2016, 64, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.; Bauler, T.J.; Malik-Kale, P.; Steele-Mortimer, O. The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. PLoS ONE 2018, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Jung, H.S.; Yang, H.I.; Yoo, M.C.; Kim, C.; Kim, K.S. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm. Res. 2007, 56, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Gómez Estrada, H.A.; González Ruiz, K.N.; Medina, J.D. Actividad antiinflamatoria de productos naturales. Bol. Latinoam. Caribe Plantas Med. Aromat. 2011, 10, 182–217. [Google Scholar]
- González, R.; Zamora, Z.; Alonso, Y. Citocinas anti-inflamatorias y sus acciones y efectos en la sepsis y el choque séptico. REDVET Rev. Electrónica Vet. 2009, 10, 1–11. [Google Scholar]
- Valero, M.F.; Ortegón, Y. Polyurethane elastomers-based modified castor oil and poly(e-caprolactone) for surface-coating applications: Synthesis, characterization, and in vitro degradation. J. Elastomers Plast. 2015, 47, 360–369. [Google Scholar] [CrossRef]
- Valero, M.F.; Pulido, J.E.; Ramírez, Á.; Cheng, Z. Determinación de la densidad de entrecruzamiento de poliuretanos obtenidos a partir de aceite de ricino modificado por transesterificación. Polímeros 2009, 19, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Valero, M.F. Poliuretanos elastoméricos obtenidos a partir de aceite de ricino y almidón de yuca original y modificado con anhidrido propiónico: Síntesis, propiedades fisicoquímicas y fisicomecánicas. Quim. Nov. 2010, 33, 850–854. [Google Scholar] [CrossRef]
- Simón-Allué, R.; Pérez-López, P.; Sotomayor, S.; Peña, E.; Pascual, G.; Bellón, J.M.; Calvo, B. Short- and long-term biomechanical and morphological study of new suture types in abdominal wall closure. J. Mech. Behav. Biomed. Mater. 2014, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Jiang, H.; Kim, M.J.; Vink, J.; Cremers, S.; Paik, D.; Wapner, R.; Mahendroo, M.; Myers, K. Quantitative evaluation of collagen crosslinks and corresponding tensile mechanical properties in mouse cervical tissue during normal pregnancy. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Mekewi, M.A.; Ramadan, A.M.; ElDarse, F.M.; Abdel Rehim, M.H.; Mosa, N.A.; Ibrahim, M.A. Preparation and characterization of polyurethane plasticizer for flexible packaging applications: Natural oils affirmed access. Egypt. J. Pet. 2017, 26, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Hormaiztegui, M.E.V.; Aranguren, M.I.; Mucci, V.L. Synthesis and characterization of a waterborne polyurethane made from castor oil and tartaric acid. Eur. Polym. J. 2018, 102, 151–160. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Członka, S.; Bertino, M.F.; Strzelec, K. Rigid polyurethane foams reinforced with industrial potato protein. Polym. Test. 2018, 68, 135–145. [Google Scholar] [CrossRef]
- Basak, P.; Adhikari, B. Effect of the solubility of antibiotics on their release from degradable polyurethane. Mater. Sci. Eng. C 2012, 32, 2316–2322. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Zhang, L.; Qin, P.; Wang, P. One-step surface modification of polyurethane using affinity binding peptides for enhanced fouling resistance. J. Biomater. Sci. Polym. Ed. 2015, 26, 459–467. [Google Scholar] [CrossRef]
- Rezvanain, M.; Ahmad, N.; Mohd Amin, M.C.I.; Ng, S.F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef]
- Vilariño Feltrer, G.; Martínez Ramos, C.; Monleon De La Fuente, A.; Vallés Lluch, A.; Moratal Pérez, D.; Barcia Albacar, J.; Monleón Pradas, M. Schwann-cell cylinders grown inside hyaluronic-acid tubular scaffolds with gradient porosity. Acta Biomater. 2016, 30, 199–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |



















| Polymeric Material | Tg (°C) | ||
|---|---|---|---|
| P.1 | P.2 | P.3 | |
| 0%Ch-0%PCL | −14.8 | −12.8 | 14.8 |
| 3%Ch-0%PCL | −13.3 | −1.0 | 13.7 |
| 0%Ch-15%PCL | −25.3 | −10.2 | 4.7 |
| 3%Ch-15%PCL | −25.4 | −14.8 | 2.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uscátegui, Y.L.; Díaz, L.E.; Gómez-Tejedor, J.A.; Vallés-Lluch, A.; Vilariño-Feltrer, G.; Serrano, M.A.; Valero, M.F. Candidate Polyurethanes Based on Castor Oil (Ricinus communis), with Polycaprolactone Diol and Chitosan Additions, for Use in Biomedical Applications. Molecules 2019, 24, 237. https://doi.org/10.3390/molecules24020237
Uscátegui YL, Díaz LE, Gómez-Tejedor JA, Vallés-Lluch A, Vilariño-Feltrer G, Serrano MA, Valero MF. Candidate Polyurethanes Based on Castor Oil (Ricinus communis), with Polycaprolactone Diol and Chitosan Additions, for Use in Biomedical Applications. Molecules. 2019; 24(2):237. https://doi.org/10.3390/molecules24020237
Chicago/Turabian StyleUscátegui, Yomaira L., Luis E. Díaz, José A. Gómez-Tejedor, Ana Vallés-Lluch, Guillermo Vilariño-Feltrer, María A. Serrano, and Manuel F. Valero. 2019. "Candidate Polyurethanes Based on Castor Oil (Ricinus communis), with Polycaprolactone Diol and Chitosan Additions, for Use in Biomedical Applications" Molecules 24, no. 2: 237. https://doi.org/10.3390/molecules24020237
APA StyleUscátegui, Y. L., Díaz, L. E., Gómez-Tejedor, J. A., Vallés-Lluch, A., Vilariño-Feltrer, G., Serrano, M. A., & Valero, M. F. (2019). Candidate Polyurethanes Based on Castor Oil (Ricinus communis), with Polycaprolactone Diol and Chitosan Additions, for Use in Biomedical Applications. Molecules, 24(2), 237. https://doi.org/10.3390/molecules24020237