An Efficient Approach to Phosphorylated Isoindoline Fused with Triazoles via Zn-Catalyzed Cascade Cyclization of 2–Propynol Benzyl Azides and Diarylphosphine Oxides
Abstract
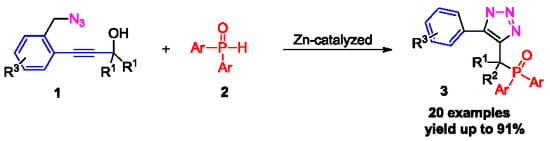
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Berger, D.; Citarella, R.; Dutia, M.; Greenberger, L.; Hallett, W.; Paul, R.; Powell, D. Novel Multidrug Resistance Reversal Agents. J. Med. Chem. 1999, 42, 2145–2161. [Google Scholar] [CrossRef]
- Goethem, S.V.; Matheeussen, V.; Joossens, J.; Lambeir, A.-M.; Chen, X.; Meester, I.D.; Haemers, A.; Augustyns, K.; der Veken, P.V. Structure-Activity Relationship Studies on Isoindoline Inhibitors of Dipeptidyl Peptidases 8 and 9 (DPP8, DPP9): Is DPP8-Selectivity an Attainable Goal? J. Med. Chem. 2011, 54, 5737–5746. [Google Scholar] [CrossRef] [PubMed]
- Das Adhikary, N.; Chattopadhyay, P. Design and Synthesis of 1,2,3-Triazole-Fused Chiral Medium-Ring Benzo-Heterocycles, Scaffolds Mimicking Benzolactams. J. Org. Chem. 2012, 77, 5399–5405. [Google Scholar] [CrossRef] [PubMed]
- Kallander, L.S.; Lu, Q.; Chen, W.; Tomaszek, T.; Yang, G.; Tew, D.; Meek, T.D.; Hofmann, G.A.; Schulz-Pritchard, C.K.; Smith, W.W.; et al. 4-Aryl-1,2,3-triazole: A Novel Template for a Reversible Methionine Aminopeptidase 2 Inhibitor, Optimized To Inhibit Angiogenesis in Vivo. J. Med. Chem. 2005, 48, 5644–5647. [Google Scholar] [CrossRef]
- Greenblatt, D.J.; Harmatz, J.S.; Shapiro, L.; Engelhardt, N.; Gouthro, T.A.; Shader, R.I. Sensitivity to Triazolam in the Elderly. N. Engl. J. Med. 1991, 324, 1691–1698. [Google Scholar] [CrossRef]
- Tatsuta, K.; Ikeda, Y.; Miura, S. Synthesis and Glycosidase Inhibitory Activities of Nagstatin Triazole Analogs. J. Antibiot. 1996, 49, 836. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hseih, H.-Y.; Lee, W.-C.; Chandru, G.C.; Hu, W.-P.; Liang, J.-J.; Tsai, T.-R.; Chou, Y.-W.; Kuo, K.-K.; Chen, C.-Y.; Wang, J.J.; et al. Discovery, Synthetic Methodology, and Biological Evaluation for Antiphotoaging Activity of Bicyclic[1,2,3]triazoles: In Vitro and in Vivo Studies. J. Med. Chem. 2013, 56, 5422–5435. [Google Scholar] [CrossRef]
- Shafran, E.A.; Bakulev, V.A.; Rozin, Y.A.; Shafran, Y.M. Condensed 1,2,3-triazoles. Chem. Heterocycl. Compd. 2008, 44, 1040–1069. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ray, K. Synthesis of 1,2,3-Triazole-Fused Heterocycles via Intramolecular Azide-Alkyne Cycloaddition Reactions. Synthesis 2011, 3767–3783. [Google Scholar] [CrossRef]
- Choudhury, C.; Mandal, S.B.; Achari, B. Palladium-copper catalysed heteroannulation of acetylenic compounds: An expeditious synthesis of isoindoline fused with triazoles. Tetrahedron Lett. 2005, 46, 8531–8534. [Google Scholar] [CrossRef]
- Fiandanese, V.; Marchese, G.; Punzi, A.; Iannone, F.; Rafaschieri, G.C. An easy synthetic approach to 1,2,3-triazole-fused heterocycles. Tetrahedron 2010, 66, 8846–8853. [Google Scholar] [CrossRef]
- Fiandanese, V.; Marino, I.; Punzi, A. An easy access to 4-(1,2,3-triazolylalkyl)-1,2,3-triazole-fused dihydroisoquinolines and dihydroisoindoles. Tetrahedron 2012, 68, 10310–10317. [Google Scholar] [CrossRef]
- Brahma, K.; Achari, B.; Choudhury, C. Facile Synthesis of [1,2,3]-Triazole-Fused Isoindolines, Tetrahydroisoquinolines, Benzoazepines and Benzoazocines by Palladium-Copper Catalysed Heterocyclisation. Synthesis 2013, 45, 545–555. [Google Scholar]
- Ramachary, D.B.; Ramakumar, K.; Narayana, V.V. Amino Acid-Catalyzed Cascade [3+2]-Cycloaddition/Hydrolysis Reactions Based on the Push–Pull Dienamine Platform: Synthesis of Highly Functionalized NH-1,2,3-Triazoles. Chem. Eur. J. 2008, 14, 9143–9147. [Google Scholar] [CrossRef] [PubMed]
- Ramachary, D.B.; Shashank, A.B. Organocatalytic Triazole Formation, Followed by Oxidative Aromatization: Regioselective Metal-Free Synthesis of Benzotriazoles. Chem. Eur. J. 2013, 19, 13175–13181. [Google Scholar] [CrossRef] [PubMed]
- Belkheira, M.; Abed, D.E.; Pons, J.-M.; Bressy, C. Organocatalytic Synthesis of 1,2,3-Triazoles from Unactivated Ketones and Arylazides. Chem. Eur. J. 2011, 17, 12917–12921. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, S.Y.; Danence, L.J.T.; Gao, Y.; Wang, J. Amine-Catalyzed [3+2] Huisgen Cycloaddition Strategy for the Efficient Assembly of Highly Substituted 1,2,3-Triazoles. Chem. Eur. J. 2012, 18, 6088–6093. [Google Scholar] [CrossRef]
- Seus, N.; Goncalves, L.C.; Deobald, A.M.; Savegnago, L.; Alves, D.; Paixao, M.W. Synthesis of arylselanyl-1H-1,2,3-triazole-4-carboxylates by organocatalytic cycloaddition of azidophenyl arylselenides with β-keto-esters. Tetrahedron 2012, 68, 10456–10463. [Google Scholar] [CrossRef]
- Li, W.; Jia, Q.; Du, Z.; Wang, J. Direct access to triazole-olefins through catalytic cycloaddition of azides to unsaturated aldehydes. Chem. Commun. 2013, 49, 10187–10189. [Google Scholar]
- Yeung, D.K.J.; Gao, T.; Huang, J.; Sun, S.; Guo, H.; Wang, J. Organocatalytic 1,3-dipolar cycloaddition reactions of ketones andazides with water as a solvent. Green Chem. 2013, 15, 2384–2388. [Google Scholar] [CrossRef]
- Seus, N.; Goldani, B.; Lenardão, E.J.; Savegnago, L.; Paixão, M.W.; Alves, D. Organocatalytic Synthesis of (Arylselanyl)phenyl-1H-1,2,3-triazole-4-carboxamides by Cycloaddition between Azidophenyl Arylselenides and β-Oxo-amides. Eur. J. Org. Chem. 2013, 2014, 1059–1065. [Google Scholar] [CrossRef]
- Li, W.; Du, Z.; Huang, J.; Jia, Q.; Zhang, K.; Wang, J. Direct access to 1,2,3-triazoles through organocatalytic 1,3-dipolar cycloaddition reaction of allyl ketones with azides. Green Chem. 2014, 16, 3003–3006. [Google Scholar] [CrossRef]
- Li, W.; Du, Z.; Zhang, K.; Wang, J. Organocatalytic 1,3-dipolar cycloaddition reaction of α,β-unsaturated ketones with azides through iminium catalysis. Green Chem. 2015, 17, 781–784. [Google Scholar] [CrossRef]
- Ramachary, D.B.; Shashank, A.B.; Karthik, S. An Organocatalytic Azide–Aldehyde [3+2] Cycloaddition: High-Yielding Regioselective Synthesis of 1,4-Disubstituted 1,2,3-Triazoles. Angew. Chem. Int. Ed. 2014, 53, 10420–10424. [Google Scholar] [CrossRef] [PubMed]
- Shashank, A.B.; Karthik, S.; Madhavachary, R.; Ramachary, D.B. An Enolate-Mediated Organocatalytic Azide–Ketone [3+2]-Cycloaddition Reaction: Regioselective High-Yielding Synthesis of Fully Decorated 1,2,3-Triazoles. Chem. Eur. J. 2014, 20, 16877–16881. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J. Lewis Base Catalyzed Aerobic Oxidative Intermolecular Azide–Zwitterion Cycloaddition. Angew. Chem. Int. Ed. 2014, 53, 14186–14190. [Google Scholar] [CrossRef] [PubMed]
- Ramasastry, S.S.V. Enamine/Enolate-Mediated Organocatalytic Azide–Carbonyl [3+2] Cycloaddition Reactions for the Synthesis of Densely Functionalized 1,2,3-Triazoles. Angew. Chem. Int. Ed. 2014, 53, 14310–14312. [Google Scholar] [CrossRef]
- Ali, A.; Corrêa, A.G.; Alves, D.; Zukerman-Schpector, J.; Westermann, B.; Ferreira, M.A.B.; Paixao, M.W. An efficient one-pot strategy for the highly regioselective metal-free synthesis of 1,4-disubstituted-1,2,3-triazoles. Chem. Commun. 2014, 50, 11926–11929. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, X. New Chiral Phosphorus Ligands for Enantioselective Hydrogenation. Chem. Rev. 2003, 103, 3029–3070. [Google Scholar] [CrossRef]
- George, A.; Veis, A. Phosphorylated Proteins and Control over Apatite Nucleation, Crystal Growth, and Inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef]
- Queffélec, C.; Petit, M.; Janvier, P.; Knight, D.A.; Bujoli, B. Surface Modification Using Phosphonic Acids and Esters. Chem. Rev. 2012, 112, 3777–3807. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Garner, R.C.; Nicholson, S.; Kissling, C.J.; Mayers, D. Microdose Pharmacokinetics of IDX899 and IDX989, Candidate HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors, Following Oral and Intravenous Administration in Healthy Male Subjects. J. Clin. Pharmacol. 2009, 49, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- La Regina, G.; Coluccia, A.; Silvestri, R. Antiviral. Chem. Chemother. 2010, 20, 213–237. [Google Scholar]
- Alexandre, F.R.; Amador, A.; Bot, S.; Caillet, C.; Convard, T.; Jakubik, J.; Musiu, C.; Poddesu, B.; Vargiu, L.; Liuzzi, M.; et al. Synthesis and Biological Evaluation of Aryl-phospho-indole as Novel HIV-1 Non-nucleoside Reverse Transcriptase Inhibitors. J. Med. Chem. 2011, 54, 392–395. [Google Scholar] [CrossRef]
- Muzart, J. Gold-catalysed reactions of alcohols: Isomerisation, inter- and intramolecular reactions leading to C–C and C–heteroatom bonds. Tetrahedron 2008, 64, 5815–5849. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Yao, M.-L. Direct Propargylic Substitution of Hydroxyl Group in Propargylic Alcohols. Curr. Org. Synth. 2008, 5, 28–32. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, L.; Lu, P.; Wang, Y. Recent Advances on the Lewis Acid-Catalyzed Cascade Rearrangements of Propargylic Alcohols and Their Derivatives. ACS Catal. 2014, 4, 1911–1925. [Google Scholar] [CrossRef]
- Song, X.-R.; Qiu, Y.-F.; Liu, X.-Y.; Liang, Y.-M. Recent advances in the tandem reaction of azides with alkynes or alkynols. Org. Biomol. Chem. 2016, 14, 11317–11331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tanimoto, H.; Morimoto, T.; Nishiyama, Y.; Kakiuchi, K. Regioselective Rapid Synthesis of Fully Substituted 1,2,3-Triazoles Mediated by Propargyl Cations. Org. Lett. 2013, 15, 5222–5225. [Google Scholar] [CrossRef]
- Zhang, H.; Tanimoto, H.; Morimoto, T.; Nishiyama, Y.; Kakiuchi, K. Acid-mediated synthesis of fully substituted 1,2,3-triazoles: Multicomponent coupling reactions, mechanistic study, synthesis of serine hydrolase inhibitor and its derivatives. Tetrahedron 2014, 70, 9828–9835. [Google Scholar] [CrossRef]
- Yang, T.; Ding, H.; Li, R.; Jin, F.; Song, X.-R.; Chen, X.; Bai, J.; Xiao, Q.; Liang, Y.-M. para-TsOH-Promoted Cascade Reaction of ortho-Propynol Phenyl Azides for the Synthesis of 4-Methoxy Quinolines and Propargyl Methyl Ethers: Insight on Mechanism of Propargylic Alcohols. Asian J. Org. Chem. 2019, 8, 391–398. [Google Scholar] [CrossRef]
- Li, R.; Jin, F.; Song, X.-R.; Yang, T.; Ding, H.; Yang, R.; Xiao, Q.; Liang, Y.-M. Acid-promoted cyclization of 2-propynolphenols leading to 4-tosyloxy-2H-chromenes. Tetrahedron Lett. 2019, 60, 331–334. [Google Scholar] [CrossRef]
- Li, R.; Song, X.-R.; Chen, X.; Ding, H.; Xiao, Q.; Liang, Y.-M. Copper-Catalyzed Cascade Cyclization of 2-Propynolphenols: Access to 4-Phosphorylated 2H-Chromenes. Adv. Synth. Catal. 2017, 359, 3962–3967. [Google Scholar] [CrossRef]
- Song, X.-R.; Li, R.; Ding, H.; Chen, X.; Yang, T.; Jiang, B.; Xiao, Q.; Liang, Y.-M. An efficient approach to 4-chloro quinolines via TMSCl-mediated cascade cyclization of ortho-propynol phenyl azides. Org. Chem. Front. 2018, 5, 1537–1541. [Google Scholar] [CrossRef]
- Song, X.-R.; Li, R.; Yang, T.; Chen, X.; Ding, H.; Xiao, Q.; Liang, Y.-M. Novel and Efficient Access to Flavones under Mild Conditions: Aqueous HI-Mediated Cascade Cyclization/Oxidative Radical Reaction of 2-Propynolphenols. Eur. J. Org. Chem. 2018, 40, 5548–5552. [Google Scholar] [CrossRef]
- Yang, T.; Kou, P.; Jin, F.; Song, X.-R.; Bai, J.; Ding, H.; Xiao, Q.; Liang, Y.-M. TFA-Promoted Sulfonation/Cascade Cyclization of 2-Propynolphenols with Sodium Sulfinates to 4-Sulfonyl 2H-Chromenes under Metal-free Conditions. Org. Chem. Front. 2019, 6, 3162–3166. [Google Scholar] [CrossRef]
- CCDC 1949212 (compound 3a) Contains the Supplementary Cystallographic Data for This Paper. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 26 August 2019).
- Song, X.-R.; Li, R.; Ding, H.; Yang, R.; Xiao, Q.; Liang, Y.-M. Highly efficient access to 4-chloro-2H-chromenes and 1,2-dihydroquinolines under mild conditions: TMSCl-mediated cyclization of 2-propynolphenols/anilines. Tetrahedron Lett. 2016, 57, 4519–4524. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, G.; Hong, D.; Lu, P.; Wang, Y. Tandem Reaction of Propargylic Alcohol, Sulfonamide, and N-Iodosuccinimide: Synthesis of N-(2-Iodoinden-1-yl)arenesulfonamide. Org. Lett. 2011, 13, 1024–1027. [Google Scholar] [CrossRef]
Sample Availability: Samples of the final compounds are available from the authors. |






| Entry | Catalyst (x mol%) | Solvent | T [°C] | Yield [%] |
|---|---|---|---|---|
| 1 | Sc(OTf)3 (30) | DCE | 100 | 42 |
| 2 | Zn(OTf)2 (30) | DCE | 100 | 53 |
| 3 | Cu(OTf)2 (30) | DCE | 100 | trace |
| 4 | Cu(OAc)2 (30) | DCE | 100 | trace |
| 5 | CuCl2 (30) | DCE | 100 | <5 |
| 6 | AgOTf (30) | DCE | 100 | trace |
| 7 | Zn(OTf)2 (30) | MeNO2 | 100 | 61 |
| 8 | Zn(OTf)2 (30) | CH3CN | 100 | 81 |
| 9 | Zn(OTf)2 (30) | 1,4-dioxane | 100 | trace |
| 10 | Zn(OTf)2 (30) | DCM | 40 | 32 |
| 11 | Zn(OTf)2 (30) | CH3CN | 80 | 75 |
| 12 | Zn(OTf)2 (30) | CH3CN | 110 | 80 |
| 13 | Zn(OTf)2 (20) | CH3CN | 100 | 82 |
| 14 | Zn(OTf)2 (10) | CH3CN | 100 | 67 |
| 15 b | Zn(OTf)2 (20) | CH3CN | 100 | 71 |
| 16 c | Zn(OTf)2 (20) | CH3CN | 100 | 86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Song, X.-R.; Yang, R.; Ding, H.; Bai, J.; Xiao, Q. An Efficient Approach to Phosphorylated Isoindoline Fused with Triazoles via Zn-Catalyzed Cascade Cyclization of 2–Propynol Benzyl Azides and Diarylphosphine Oxides. Molecules 2019, 24, 3526. https://doi.org/10.3390/molecules24193526
Yang T, Song X-R, Yang R, Ding H, Bai J, Xiao Q. An Efficient Approach to Phosphorylated Isoindoline Fused with Triazoles via Zn-Catalyzed Cascade Cyclization of 2–Propynol Benzyl Azides and Diarylphosphine Oxides. Molecules. 2019; 24(19):3526. https://doi.org/10.3390/molecules24193526
Chicago/Turabian StyleYang, Tao, Xian-Rong Song, Ruchun Yang, Haixin Ding, Jiang Bai, and Qiang Xiao. 2019. "An Efficient Approach to Phosphorylated Isoindoline Fused with Triazoles via Zn-Catalyzed Cascade Cyclization of 2–Propynol Benzyl Azides and Diarylphosphine Oxides" Molecules 24, no. 19: 3526. https://doi.org/10.3390/molecules24193526
APA StyleYang, T., Song, X.-R., Yang, R., Ding, H., Bai, J., & Xiao, Q. (2019). An Efficient Approach to Phosphorylated Isoindoline Fused with Triazoles via Zn-Catalyzed Cascade Cyclization of 2–Propynol Benzyl Azides and Diarylphosphine Oxides. Molecules, 24(19), 3526. https://doi.org/10.3390/molecules24193526