Validation of Cell-Based Assay for Quantification of Sesamol Uptake and Its Application for Measuring Target Exposure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Analytical Method
2.2. Validation of Analytical Method
2.2.1. Selectivity and Carryover Effect
2.2.2. Linearity, LOD, LOQ, and LLOQ
2.2.3. Recovery
2.2.4. Precision and Accuracy
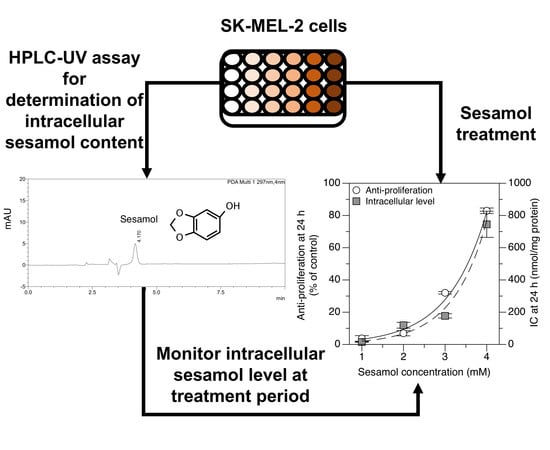
2.3. Applying the Method: Monitoring the Intracellular Uptake of Sesamol and Correlating It with Its Antiproliferative Effect in SK-MEL-2 Cells
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. SK-MEL-2 Cell Culture
3.3. HPLC Stock Solution and Calibration Standards Preparation
3.4. Preparation of Quality Control (QC) Sample
3.5. Chromatographic Analytical Procedure
3.6. Method Validation
3.6.1. Selectivity and Carryover Effect
3.6.2. Recovery
3.6.3. Linearity of the Calibration Curves
3.6.4. Limit of Detection and Lowest Limit of Quantification
3.6.5. Precision and Accuracy
3.7. Determination of Intracellular Accumulation of Sesamol in SK-MEL-2 Cells
3.8. Correlation between the Intracellular Accumulation of Sesamol and the Antiproliferative Effect in the SK-MEL-2 Cells
3.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-C.; Zur, A.A.; Maurer, T.S.; Yee, S.W.; Tolsma, J.; Jasper, P.; Scott, D.O.; Giacomini, K.M. Rapid method to determine intracellular drug concentrations in cellular uptake assays: Application to metformin in organic cation transporter 1-transfected human embryonic kidney 293 Cells. Drug Metab. Dispos. 2016, 44, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Mateus, A.; Treyer, A.; Wegler, C.; Karlgren, M.; Matsson, P.; Artursson, P. Intracellular drug bioavailability: A new predictor of system dependent drug disposition. Sci. Rep. 2017, 7, 43047. [Google Scholar] [CrossRef] [PubMed]
- Konsoula, Z.; Liakopoulou-Kyriakides, M. Effect of endogenous antioxidants of sesame seeds and sesame oil to the thermal stability of edible vegetable oils. LWT Food Sci. Technol. 2010, 43, 1379–1386. [Google Scholar] [CrossRef]
- Ansari, M.A.; Fatima, Z.; Hameed, S. Sesamol: A natural phenolic compound with promising anticandidal potential. J. Pathog. 2014, 2014, 895193. [Google Scholar] [CrossRef]
- Figueiredo, P.S.; Candido, C.J.; Jaques, J.A.; Nunes, Â.A.; Caires, A.R.; Michels, F.S.; Almeida, J.A.; Filiú, W.F.; Hiane, P.A.; Nascimento, V.A.; et al. Oxidative stability of sesame and flaxseed oils and their effects on morphometric and biochemical parameters in an animal model. J. Sci. Food Agric. 2017, 97, 3359–3364. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Sanyal, S.; Vaiphei, K.; Kakkar, V.; Kaur Deol, P.; Pal Kaur, I.; Kaur, T. Sesamol induces apoptosis by altering expression of Bcl-2 and Bax proteins and modifies skin tumor development in Balb/c mice. Anti-Cancer Agents Med. Chem. 2017, 17, 726–733. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, B.; Wang, Y.; Zou, C.; Qiao, Q.; Diao, Z.; Mi, Y.; Zhu, D.; Liu, X. Sesamol induces human hepatocellular carcinoma cells apoptosis by impairing mitochondrial function and suppressing autophagy. Sci. Rep. 2017, 7, 45728. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Chen, F.-Y.; Lee, A.-S.; Ting, K.-H.; Chang, C.-M.; Hsu, J.-F.; Lee, W.-S.; Sheu, J.-R.; Chen, C.-H.; Shen, M.-Y. Sesamol reduces the atherogenicity of electronegative L5 LDL in vivo and in vitro. J. Nat. Prod. 2015, 78, 225–233. [Google Scholar] [CrossRef]
- Srisayam, M.; Weerapreeyakul, N.; Kanokmedhakul, K. Inhibition of two stages of melanin synthesis by sesamol, sesamin and sesamolin. Asian Pac. J. Trop. Biomed. 2017, 7, 886–895. [Google Scholar] [CrossRef]
- Wu, P.-Y.; You, Y.-J.; Liu, Y.-J.; Hou, C.-W.; Wu, C.-S.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. Sesamol inhibited melanogenesis by regulating melanin-related signal transduction in B16F10 cells. Int. J. Mol. Sci. 2018, 19, 1108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xiang, Q.; Du, L.; Song, G.; Wang, Y.; Liu, X. The interaction of sesamol with DNA and cytotoxicity, apoptosis, and localization in HepG2 cells. Food Chem. 2013, 141, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Jan, K.-C.; Ho, C.-T.; Hwang, L.S. Bioavailability and tissue distribution of sesamol in rat. J. Agric. Food Chem. 2008, 56, 7032–7037. [Google Scholar] [CrossRef] [PubMed]
- Geetha, T.; Kapila, M.; Prakash, O.; Deol, P.K.; Kakkar, V.; Kaur, I.P. Sesamol-loaded solid lipid nanoparticles for treatment of skin cancer. J. Drug Target. 2015, 23, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Wolk, O.; Agbaria, R. Provisional in-silico biopharmaceutics classification (BCS) to guide oral drug product development. Drug Des. Dev. Ther. 2014, 8, 1563. [Google Scholar] [CrossRef] [PubMed]
- Daumar, P.; Dufour, R.; Dubois, C.; Penault-Llorca, F.; Bamdad, M.; Mounetou, E. Development and validation of a high-performance liquid chromatography method for the quantitation of intracellular PARP inhibitor Olaparib in cancer cells. J. Pharm. Biomed. Anal. 2018, 152, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bourgne, C.; Bamdad, M.; Janel, A.; Libert, F.; Gagnieu, M.-C.; Rapatel, C.; Pigeon, P.; Pereira, S.; Hermet, E.; Guerci, A.; et al. Measurement of imatinib uptake by flow cytometry. Cytom. Part A 2012, 81, 996–1004. [Google Scholar] [CrossRef]
- Geetha, T.; Singh, N.; Deol, P.K.; Kaur, I.P. Biopharmaceutical profiling of sesamol: Physiochemical characterization, gastrointestinal permeability and pharmacokinetic evaluation. RSC Adv. 2015, 5, 4083–4091. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Tian, J.; Ge, H.; Liang, X.; Xiao, J.; Lin, H. Simultaneous determination of four sesame lignans and conversion in Monascus aged vinegar using HPLC method. Food Chem. 2018, 256, 133–139. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, R. Determination of sesamol in sesame oil by anion exchange solid phase extraction coupled with HPLC. Anal. Methods 2014, 6, 6432–6436. [Google Scholar] [CrossRef]
- Srisayam, M.; Weerapreeyakul, N.; Barusrux, S.; Tanthanuch, W.; Thumanu, K. Application of FTIR microspectroscopy for characterization of biomolecular changes in human melanoma cells treated by sesamol and kojic acid. J. Dermatol. Sci. 2014, 73, 241–250. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Department of Health and Human Services Guidance for Industry Bioanalytical Method Validation. Available online: https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf (accessed on 8 March 2018).
- Jan, K.-C.; Ho, C.-T.; Hwang, L.S. Elimination and metabolism of sesamol, a bioactive compound in sesame oil, in rats. Mol. Nutr. Food Res. 2009, 53, S36–S43. [Google Scholar] [CrossRef] [PubMed]
- Belouafa, S.; Habti, F.; Benhar, S.; Belafkih, B.; Tayane, S.; Hamdouch, S.; Bennamara, A.; Abourriche, A. Statistical tools and approaches to validate analytical methods: Methodology and practical examples. Int. J. Metrol. Qual. Eng. 2017, 8, 9. [Google Scholar] [CrossRef]
- Mateus, A.; Gordon, L.J.; Wayne, G.J.; Almqvist, H.; Axelsson, H.; Seashore-Ludlow, B.; Treyer, A.; Matsson, P.; Lundbäck, T.; West, A.; et al. Prediction of intracellular exposure bridges the gap between target-and cell-based drug discovery. Proc. Natl. Acad. Sci. USA 2017, 114, E6231–E6239. [Google Scholar] [CrossRef]
- Smith, D.; Artursson, P.; Avdeef, A.; Di, L.; Ecker, G.F.; Faller, B.; Houston, J.B.; Kansy, M.; Kerns, E.H.; Krämer, S.D.; et al. Passive lipoidal diffusion and carrier-mediated cell uptake are both important mechanisms of membrane permeation in drug disposition. Mol. Pharm. 2014, 11, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.; Van Der Graaf, P.H.; Arrowsmith, J.; Feltner, D.E.; Drummond, K.S.; Wegner, C.D.; Street, S.D.A. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov. Today 2012, 17, 419–424. [Google Scholar] [CrossRef]
- Gonçalves, J.; Alves, G.; Bicker, J.; Falcão, A.; Fortuna, A. Development and full validation of an innovative HPLC-diode array detection technique to simultaneously quantify lacosamide, levetiracetam and zonisamide in human plasma. Bioanalysis 2018, 10, 541–557. [Google Scholar] [CrossRef]
- Pocasap, P.; Weerapreeyakul, N.; Thumanu, K. Structures of isothiocyanates attributed to reactive oxygen species generation and microtubule depolymerization in HepG2 cells. Biomed. Pharmacother. 2018, 101, 698–709. [Google Scholar] [CrossRef]
Sample Availability: Not available. |





| Nominal Conc. (ng/mL) | a Percent Deviations (%) | ||
|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |
| b 10 | −4.41 | −7.87 | −8.78 |
| 20 | −12.75 | −9.10 | −6.47 |
| 50 | 5.67 | −12.57 | 2.67 |
| 100 | −12.69 | −14.14 | −11.55 |
| 200 | −8.48 | −0.66 | −4.28 |
| 400 | 10.44 | 9.17 | 9.67 |
| 900 | 0.20 | 0.34 | −5.66 |
| 1000 | −1.37 | −1.54 | 3.32 |
| Validation Parameters | Values |
|---|---|
| Retention time (min) | 4.152 ± 0.008 (0.19% RSD) |
| LOD (ng/mL) | 5 |
| LLOQ (ng/mL) | 10 (−8.78% to −4.41% deviation) |
| Precision of LLOQ (within-days and between-days; %RSD) | 3.57% and 7.87% RSD |
| Concentration (ng/mL) | Recovery (%) |
|---|---|
| QC-low (50 ng/mL) | 94.80 ± 4.69 |
| QC-mid (400 ng/mL) | 99.29 ± 3.58 |
| QC-high (900 ng/mL) | 99.09 ± 0.84 |
| Nominal Conc. (ng/mL) | Within-Days | Between-Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental Conc. (ng/mL) | Mean (ng/mL) | RSD (%) | Bias (%) | Experimental Conc. (ng/mL) | Mean (ng/mL) | RSD (%) | Bias (%) | |||
| QC-low (50 ng/mL) | 46.18 | 48.32 ± 2.21 | 4.57 | −3.36 | 52.17 | 46.18 | 52.03 | 49.11 ± 2.05 | 4.17 | −1.78 |
| 48.77 | 47.15 | 48.77 | 49.53 | |||||||
| 46.49 | 51.81 | 46.49 | 50.48 | |||||||
| 51.63 | 49.61 | 51.63 | 48.89 | |||||||
| 50.05 | 47.27 | 50.05 | 46.68 | |||||||
| 46.80 | 50.39 | 46.80 | 48.09 | |||||||
| QC-mid (400 ng/mL) | 399.27 | 392.02 ± 17.44 | 4.45 | −2.00 | 426.28 | 399.27 | 367.33 | 406.00 ± 21.42 | 5.28 | 1.50 |
| 393.54 | 425.87 | 393.54 | 401.74 | |||||||
| 372.18 | 431.66 | 372.18 | 426.13 | |||||||
| 420.87 | 418.56 | 420.87 | 430.98 | |||||||
| 376.70 | 413.16 | 376.70 | 414.39 | |||||||
| 389.54 | 419.26 | 389.54 | 380.61 | |||||||
| QC-high (900 ng/mL) | 901.66 | 896.33 ± 7.50 | 0.84 | −0.41 | 824.65 | 901.66 | 886.03 | 873.64 ± 34.58 | 3.96 | −2.93 |
| 888.34 | 825.93 | 888.34 | 877.35 | |||||||
| 893.68 | 832.01 | 893.68 | 929.97 | |||||||
| 897.18 | 831.50 | 897.18 | 917.07 | |||||||
| 889.32 | 835.72 | 889.32 | 878.83 | |||||||
| 907.81 | 828.47 | 907.81 | 879.92 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srisongkram, T.; Weerapreeyakul, N. Validation of Cell-Based Assay for Quantification of Sesamol Uptake and Its Application for Measuring Target Exposure. Molecules 2019, 24, 3522. https://doi.org/10.3390/molecules24193522
Srisongkram T, Weerapreeyakul N. Validation of Cell-Based Assay for Quantification of Sesamol Uptake and Its Application for Measuring Target Exposure. Molecules. 2019; 24(19):3522. https://doi.org/10.3390/molecules24193522
Chicago/Turabian StyleSrisongkram, Tarapong, and Natthida Weerapreeyakul. 2019. "Validation of Cell-Based Assay for Quantification of Sesamol Uptake and Its Application for Measuring Target Exposure" Molecules 24, no. 19: 3522. https://doi.org/10.3390/molecules24193522