Development of N-Acetylated Dipalmitoyl-S-Glyceryl Cysteine Analogs as Efficient TLR2/TLR6 Agonists
Abstract
1. Introduction
2. Results
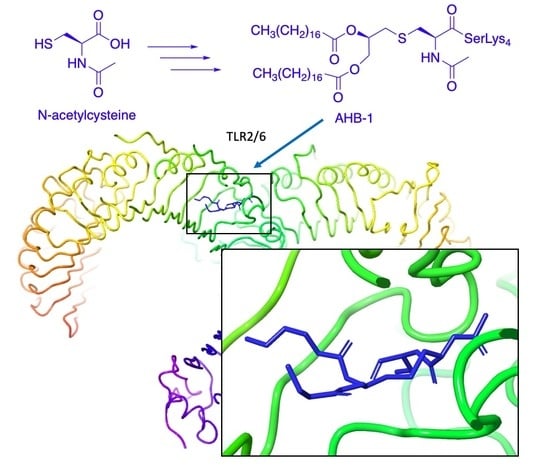
2.1. Synthesis
2.2. In Vitro TLR2/6 Activation
2.3. Docking Studies
3. Discussion
4. Materials and Methods
4.1. Synthesis of (1,4-Dioxa-spiro[4,5]dec-2-yl)-methanol (3)

4.2. Synthesis of Toluene-4-sulfonic acid 1,4-dioxaspiro[4,5]dec-2-yl-methyl ester (5)

4.3. Synthesis of 2-Acetyl amino-3-(1,4-dioxa-spiro[4,5]dec-2-ylmethyl sulfanyl)-propionic acid (7)

4.4. Synthesis of 2-Acetyl amino-3-(2,3-dihydroxy-propyl sulfanyl)-propionic acid (8)

4.5. Synthesis of N-Acetyl Pam2Cys and Analogs (AHB-1–4)




4.6. Cell Culture
4.7. NF-kB Activation Assay
4.8. Molecular Docking
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kingwell, K. CAR T therapies drive into new terrain. Nat. Rev. Drug Discov. 2017, 16, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Temizoz, B.; Kuroda, E.; Ishii, K.J. Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol. 2016, 28, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, G. Unmet needs in modern vaccinology: Adjuvants to improve the immune response. Vaccine 2010, 28 (Suppl. 3), C25–C36. [Google Scholar] [CrossRef]
- Gavin, A.L.; Hoebe, K.; Duong, B.; Ota, T.; Martin, C.; Beutler, B.; Nemazee, D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science 2006, 314, 1936–1938. [Google Scholar] [CrossRef] [PubMed]
- Schijns, V.E. Immunological concepts of vaccine adjuvant activity. Curr. Opin. Immunol. 2000, 12, 456–463. [Google Scholar] [CrossRef]
- Jackson, D.C.; Lau, Y.F.; Le, T.; Suhrbier, A.; Deliyannis, G.; Cheers, C.; Smith, C.; Zeng, W.; Brown, L.E. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc. Natl. Acad. Sci. USA 2004, 101, 15440–15445. [Google Scholar] [CrossRef] [PubMed]
- Aliprantis, A.O.; Yang, R.B.; Mark, M.R.; Suggett, S.; Devaux, B.; Radolf, J.D.; Klimpel, G.R.; Godowski, P.; Zychlinsky, A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 1999, 285, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Moyle, P.M.; Toth, I. Self-adjuvanting lipopeptide vaccines. Curr. Med. Chem. 2008, 15, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Nan, X.; Jin, M.S.; Youn, S.J.; Ryu, Y.H.; Mah, S.; Han, S.H.; Lee, H.; Paik, S.G.; Lee, J.O. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 2009, 31, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Metzger, J.W.; Wiesmuller, K.H.; Jung, G. Synthesis of N alpha-Fmoc protected derivatives of S-(2,3-dihydroxypropyl)-cysteine and their application in peptide synthesis. Int. J. Pept. Protein Res. 1991, 38, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, R.; Malladi, S.S.; Warshakoon, H.J.; Kimbrell, M.R.; Amolins, M.W.; Ukani, R.; Datta, A.; David, S.A. Structure-activity relationships in toll-like receptor-2 agonistic diacylthioglycerol lipopeptides. J. Med. Chem. 2010, 53, 3198–3213. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, G.; Crall, B.M.; Lewis, T.C.; Day, T.P.; Balakrishna, R.; Warshakoon, H.J.; Malladi, S.S.; David, S.A. Structure-activity relationships in toll-like receptor 2-agonists leading to simplified monoacyl lipopeptides. J. Med. Chem. 2011, 54, 8148–8160. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Eriksson, E.; Chua, B.; Grollo, L.; Jackson, D.C. Structural requirement for the agonist activity of the TLR2 ligand Pam2Cys. Amino Acids 2010, 39, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.; Sawahata, R.; Akao, Y.; Ebihara, T.; Yamazaki, S.; Matsumoto, M.; Hashimoto, M.; Fukase, K.; Fujimoto, Y.; Seya, T. The peptide sequence of diacyl lipopeptides determines dendritic cell TLR2-mediated NK activation. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.H.; Brooks, A.E.S.; Didsbury, A.J.; McIntosh, J.D.; Burkert, K.; Yeung, H.; Williams, G.M.; Dunbar, P.R.; Brimble, M.A. An Improved Method for the Synthesis of Lipopeptide TLR2-Agonists Using Click Chemistry. Synlett 2013, 24, 1835–1841. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.S.; Kim, S.E.; Heo, J.Y.; Lee, M.E.; Kim, H.M.; Paik, S.G.; Lee, H.; Lee, J.O. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 2007, 130, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





| Entry | Nature of R | Nature of R′ | Yield (%) |
|---|---|---|---|
| AHB1 | CH3-(CH2)16- | -H | 87 |
| AHB2 | CH3-(CH2)14- | -H | 83 |
| AHB3 | CH3-(CH2)12- | -H | 78 |
| AHB4 | CH3-(CH2)10- | -H | 79 |
| AHB1-SK4 | CH3-(CH2)14- | -SK4 | 54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Banday, A.H.; Hruby, V.J.; Cai, M. Development of N-Acetylated Dipalmitoyl-S-Glyceryl Cysteine Analogs as Efficient TLR2/TLR6 Agonists. Molecules 2019, 24, 3512. https://doi.org/10.3390/molecules24193512
Zhou Y, Banday AH, Hruby VJ, Cai M. Development of N-Acetylated Dipalmitoyl-S-Glyceryl Cysteine Analogs as Efficient TLR2/TLR6 Agonists. Molecules. 2019; 24(19):3512. https://doi.org/10.3390/molecules24193512
Chicago/Turabian StyleZhou, Yang, Abid H. Banday, Victor J. Hruby, and Minying Cai. 2019. "Development of N-Acetylated Dipalmitoyl-S-Glyceryl Cysteine Analogs as Efficient TLR2/TLR6 Agonists" Molecules 24, no. 19: 3512. https://doi.org/10.3390/molecules24193512
APA StyleZhou, Y., Banday, A. H., Hruby, V. J., & Cai, M. (2019). Development of N-Acetylated Dipalmitoyl-S-Glyceryl Cysteine Analogs as Efficient TLR2/TLR6 Agonists. Molecules, 24(19), 3512. https://doi.org/10.3390/molecules24193512