Steam Explosion Conditions Highly Influence the Biogas Yield of Rice Straw
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Steam Explosion Pretreatment
2.3. Chemical Analysis of Untreated Rice Straw and Steam-Exploded Residue
2.4. Specific Biogas Yield
2.5. Theoretical Methane and Biogas Yield
3. Results and Discussion
3.1. Characterization of the Solid Residue after Steam Explosion
3.1.1. Chemical Composition
3.1.2. Water-Extractable Components
3.1.3. Thermogravimetric Analysis
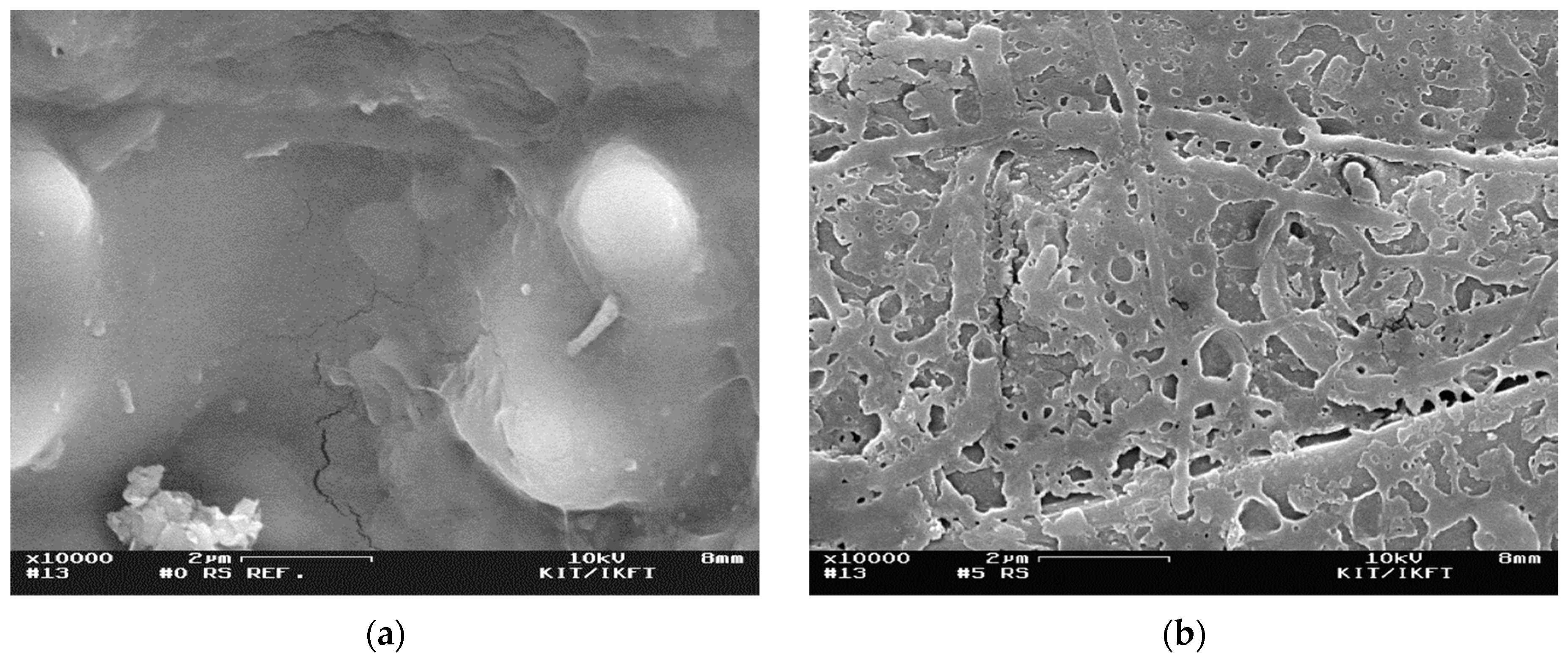
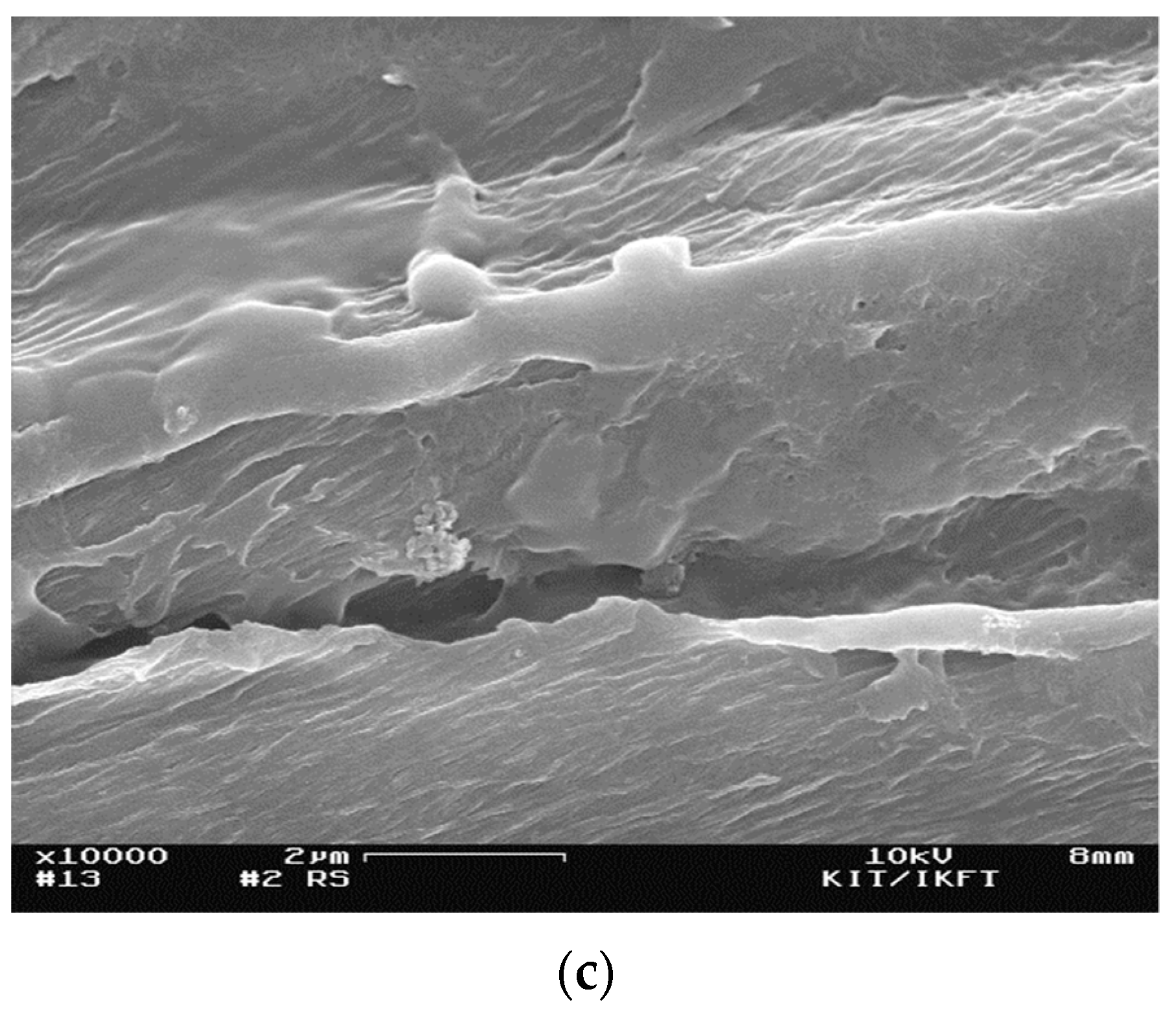
3.1.4. Particle Morphology
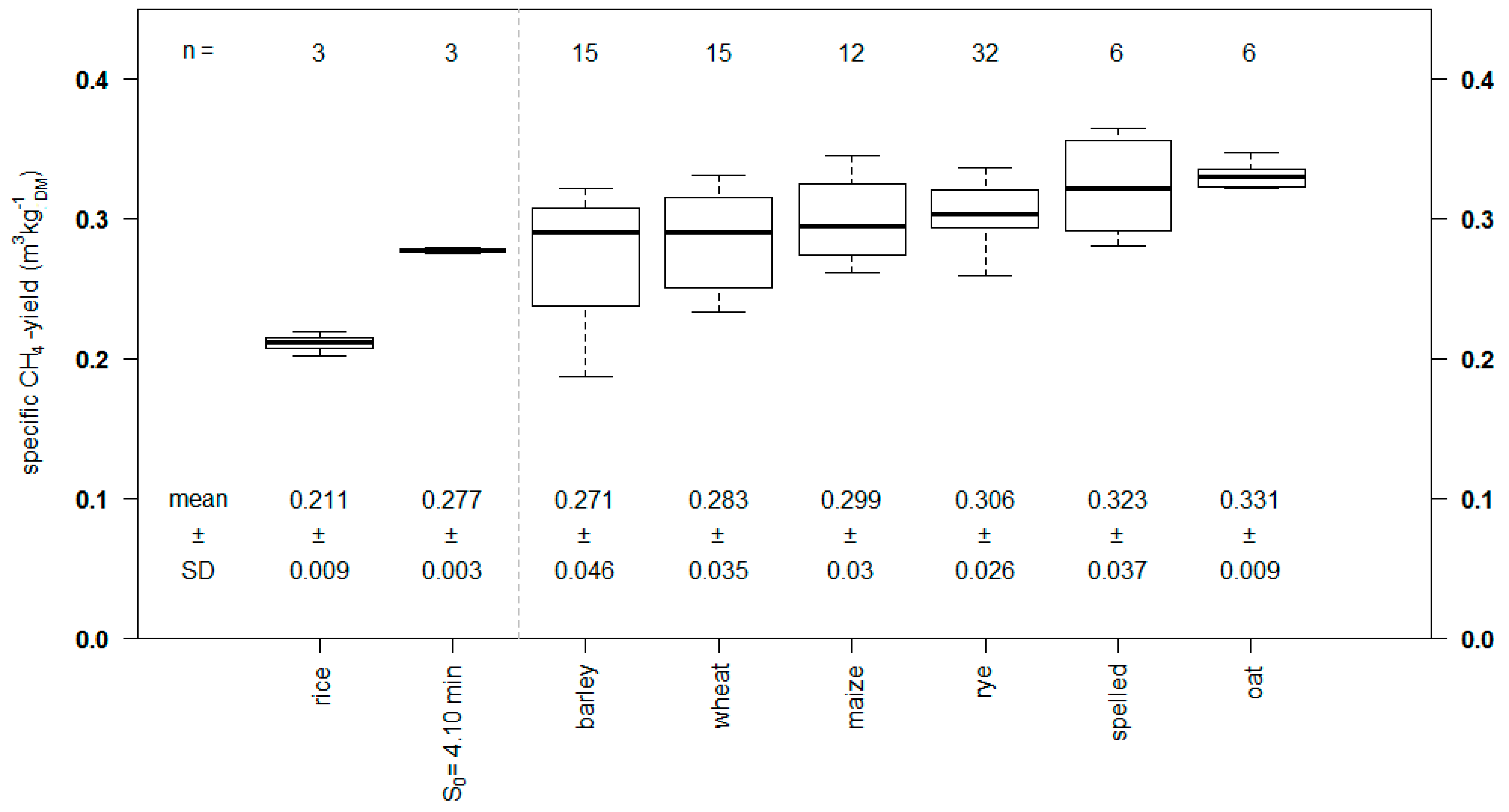
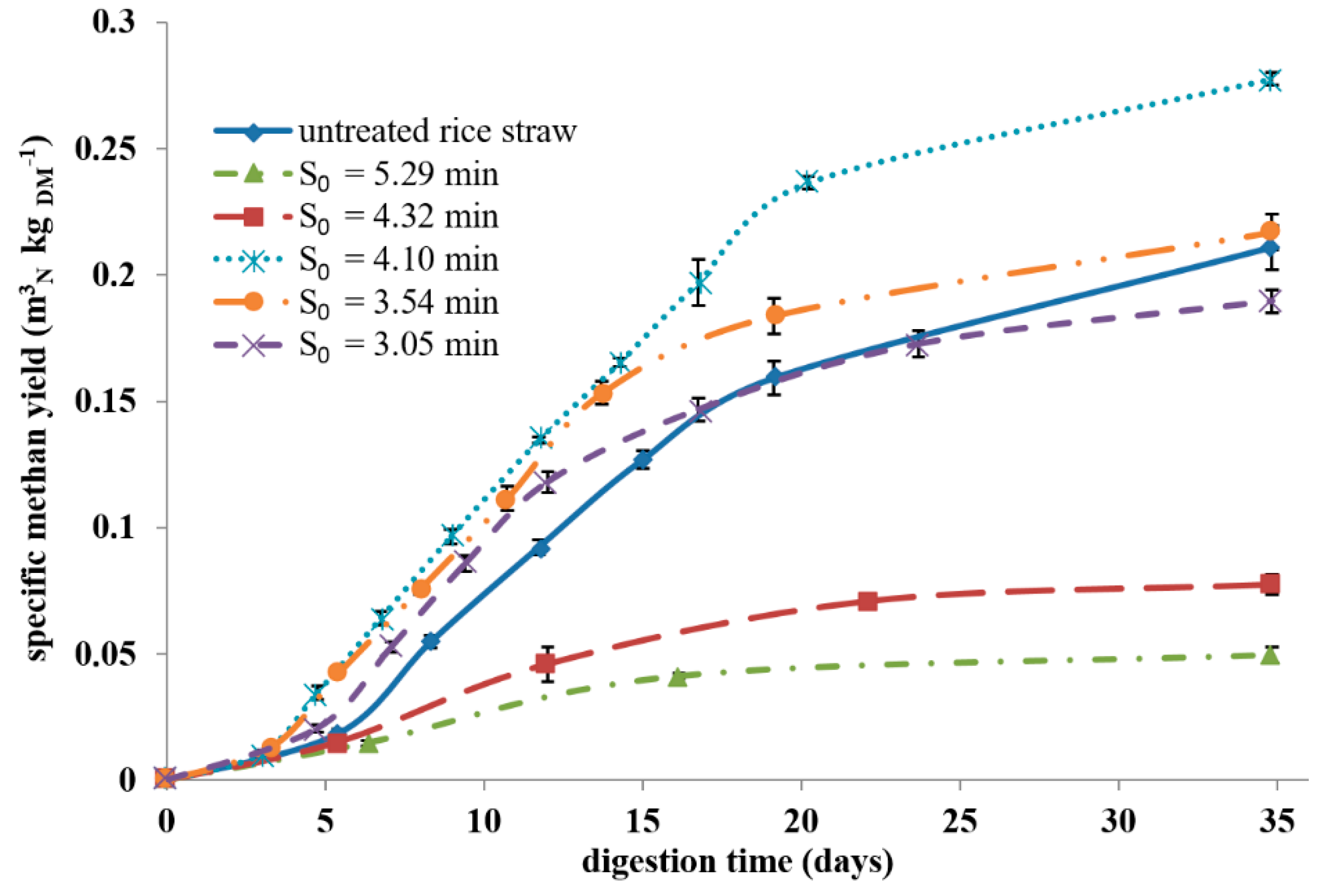
3.2. Specific Biogas Yields
4. Conclusions
- If the conditions of the steam explosion are too mild, the methane yield remains constant compared to untreated rice straw.
- If conditions are too severe, the methane yield drops dramatically. At these conditions, hemicelluloses are largely destroyed, and repolymerization leads to a more inert material.
- If conditions are moderate, the methane yield is increased, caused by a very porous structure and altered hemicellulose.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization. FAO Statistical Pocketbook 2015; Food and Agriculture Organization: Rome, Italy, 2015; p. 236. [Google Scholar]
- Lal, R. World crop residues production and implications of its use as a biofuel. Environ. Int. 2005, 31, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Binod, P.; Sindhu, R.; Singhania, R.R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R.K.; Pandey, A. Bioethanol production from rice straw: An overview. Bioresour. Technol. 2010, 101, 4767–4774. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Zhang, X.; Wang, Y.; Zheng, F. Estimation of emissions from field burning of crop straw in China. Chin. Sci. Bull. 2008, 53, 784–790. [Google Scholar] [CrossRef]
- Engling, G.; Lee, J.J.; Tsai, Y.-W.; Lung, S.-C.C.; Chou, C.C.K.; Chan, C.-Y. Size-resolved anhydrosugar composition in smoke aerosol from controlled field burning of rice straw. Aerosol Sci. Technol. 2009, 43, 662–672. [Google Scholar] [CrossRef]
- Gadde, B.; Bonnet, S.; Menke, C.; Garivait, S. Air pollutant emissions from rice straw open field burning in India, Thailand and the Philippines. Environ. Pollut. 2009, 157, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Frigon, J.C.; Guiot, S.R. Biomethane production from starch and lignocellulosic crops: a comparative review. Biofuel Bioprod. Bior. 2010, 4, 447–458. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Bobleter, O. Hydrothermal degradation of polymers derived from plants. Prog. Polym. Sci. 1994, 19, 797–841. [Google Scholar] [CrossRef]
- Steinbach, D.; Kruse, A.; Sauer, J. Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production—A review. Biomass Convers. Biorefinery 2017, 1–28. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, J.M.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int. J. Mol. Sci. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Schwald, W.; Breuil, C.; Brownell, H.H.; Chan, M.; Saddler, J.N. Assessment of pretreatment conditions to obtain fast complete hydrolysis on high substrate concentrations. Appl. Biochem. Biotech. 1989, 20–21, 29–44. [Google Scholar] [CrossRef]
- Zimbardi, F.; Viola, E.; Nanna, F.; Larocca, E.; Cardinale, M.; Barisano, D. Acid impregnation and steam explosion of corn stover in batch processes. Ind. Crop. Prod. 2007, 26, 195–206. [Google Scholar] [CrossRef]
- Ramos, L.P. The chemistry involved in the steam treatment of lignocellulosic materials. Quim. Nova 2003, 26, 863–871. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Nilsen, P.J.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Biomethane potential of wheat straw: Influence of particle size, water impregnation and thermal hydrolysis. Chem. Eng. J. 2014, 242, 254–259. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. T. R. Soc. A 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Schumacher, B.; Oechsner, H.; Senn, T.; Jungbluth, T. Thermo-mechanical pre-treatment of ripe triticale for biogas production. Landtechnik 2007, 62, 162–163, 190. [Google Scholar]
- Schumacher, B. Untersuchungen zur Aufbereitung und Umwandlung von Energiepflanzen in Biogas und Bioethanol; Universität Hohenheim: Stuttgart, Germany, 2008. [Google Scholar]
- Bauer, A.; Bösch, P.; Friedl, A.; Amon, T. Analysis of methane potentials of steam-exploded wheat straw and estimation of energy yields of combined ethanol and methane production. J. Biotechnol. 2009, 142, 50–55. [Google Scholar] [CrossRef]
- Theuretzbacher, F.; Lizasoain, J.; Lefever, C.; Saylor, M.K.; Enguidanos, R.; Weran, N.; Gronauer, A.; Bauer, A. Steam explosion pretreatment of wheat straw to improve methane yields: Investigation of the degradation kinetics of structural compounds during anaerobic digestion. Bioresour. Technol. 2015, 179, 299–305. [Google Scholar] [CrossRef]
- Risberg, K.; Sun, L.; Levén, L.; Horn, S.J.; Schnürer, A. Biogas production from wheat straw and manure— Impact of pretreatment and process operating parameters. Bioresour. Technol. 2013, 149, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Sapci, Z.; Morken, J.; Linjordet, R. An investigation of the enhancement of biogas yields from lignocellulosic material using two pretreatment methods: microwave irradiation and steam explosion. Bioresources 2013, 8, 1976–1985. [Google Scholar] [CrossRef]
- Lindorfer, J.; Steinmuller, H.; Jager, A.; Eder, A.; Hofer, B.; Nidetzky, B.; Loncar, E.; Auer, W. Research into the production of bioethanol and biogas from Lignocellulose raw materials after pretreatment with steam explosion and cellulases. Chem.-Ing.-Tech. 2010, 82, 1197–1202. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Donoso-Bravo, A.; Nilsen, P.J.; Fdz-Polanco, F.; Perez-Elvira, S.I. Influence of thermal pretreatment on the biochemical methane potential of wheat straw. Bioresour. Technol. 2013, 143, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Lizasoain, J.; Theuretzbacher, F.; Agger, J.W.; Rincón, M.; Menardo, S.; Saylor, M.K.; Enguídanos, R.; Nielsen, P.J.; Potthast, A.; et al. Steam explosion pretreatment for enhancing biogas production of late harvested hay. Bioresour. Technol. 2014, 166, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Vivekanand, V.; Olsen, E.F.; Eijsink, V.G.H.; Horn, S.J. Methane potential and enzymatic saccharification of steam-exploded bagasse. Bioresources 2014, 9, 1311–1324. [Google Scholar] [CrossRef]
- De Paoli, F.; Bauer, A.; Leonhartsberger, C.; Amon, B.; Amon, T. Utilization of by-products from ethanol production as substrate for biogas production. Bioresour. Technol. 2011, 102, 6621–6624. [Google Scholar] [CrossRef] [PubMed]
- Vivekanand, V.; Ryden, P.; Horn, S.J.; Tapp, H.S.; Wellner, N.; Eijsink, V.G.H.; Waldron, K.W. Impact of steam explosion on biogas production from rape straw in relation to changes in chemical composition. Bioresour. Technol. 2012, 123, 608–615. [Google Scholar] [CrossRef]
- Yue, Z.B.; Liu, R.H.; Yu, H.Q.; Chen, H.Z.; Yu, B.; Harada, H.; Li, Y.Y. Enhanced anaerobic ruminal degradation of bulrush through steam explosion pretreatment. Ind. Eng. Chem. Res. 2008, 47, 5899–5905. [Google Scholar] [CrossRef]
- Wang, J.; Yue, Z.-B.; Chen, T.-H.; Peng, S.-C.; Yu, H.-Q.; Chen, H.-Z. Anaerobic digestibility and fiber composition of bulrush in response to steam explosion. Bioresour. Technol. 2010, 101, 6610–6614. [Google Scholar] [CrossRef]
- Menardo, S.; Bauer, A.; Theuretzbacher, F.; Piringer, G.; Nilsen, P.J.; Balsari, P.; Pavliska, O.; Amon, T. Biogas production from steam-exploded miscanthus and utilization of biogas energy and CO2 in greenhouses. Bioenerg. Res. 2013, 6, 620–630. [Google Scholar] [CrossRef]
- Vivekanand, V.; Olsen, E.F.; Eijsink, V.G.H.; Horn, S.J. Effect of different steam explosion conditions on methane potential and enzymatic saccharification of birch. Bioresour. Technol. 2013, 127, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.J.; Estevez, M.M.; Nielsen, H.K.; Linjordet, R.; Eijsink, V.G.H. Biogas production and saccharification of Salix pretreated at different steam explosion conditions. Bioresour. Technol. 2011, 102, 7932–7936. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.M.; Linjordet, R.; Morken, J. Effects of steam explosion and co-digestion in the methane production from Salix by mesophilic batch assays. Bioresour. Technol. 2012, 104, 749–756. [Google Scholar] [CrossRef]
- Take, H.; Andou, Y.; Nakamura, Y.; Kobayashi, H.; Kurimoto, Y.; Kuwahara, M. Production of methane gas from Japanese cedar chips pretreated by various delignification methods. Biochem. Eng. J. 2006, 28, 30–35. [Google Scholar] [CrossRef]
- Zhou, J.; Yan, B.H.; Wang, Y.; Yong, X.Y.; Yang, Z.H.; Jia, H.H.; Jiang, M.; Wei, P. Effect of steam explosion pretreatment on the anaerobic digestion of rice straw. Rsc. Adv. 2016, 6, 88417–88425. [Google Scholar] [CrossRef]
- Wood, I.P.; Cao, H.-G.; Tran, L.; Cook, N.; Ryden, P.; Wilson, D.R.; Moates, G.K.; Collins, S.R.A.; Elliston, A.; Waldron, K.W. Comparison of saccharification and fermentation of steam exploded rice straw and rice husk. Biotechnol. Biofuels 2016, 9, 193. [Google Scholar] [CrossRef]
- Moniruzzaman, M. Effect of steam explosion on the physicochemical properties and enzymatic saccharification of rice straw. Appl. Biochem. Biotech. 1996, 59, 283–297. [Google Scholar] [CrossRef]
- Taniguchi, M.; Takahashi, D.; Watanabe, D.; Sakai, K.; Hoshino, K.; Kouya, T.; Tanaka, T. Effect of steam explosion pretreatment on treatment with Pleurotus ostreatus for the enzymatic hydrolysis of rice straw. J. Biosci. Bioeng. 2010, 110, 449–452. [Google Scholar] [CrossRef]
- Montané, D.; Overend, R.P.; Chornet, E. Kinetic models for non-homogeneous complex systems with a time-dependent rate constant. Can. J. Chem. Eng. 1998, 76, 58–68. [Google Scholar] [CrossRef]
- ASTM Standard. Standard test method for acid-insoluble lignin in wood; ASTM Standard: West Conshohocken, PA, USA, 2013; Vol. D1106 −96. [Google Scholar]
- VDI. Fermentation of organic materials; VDI-Gesellschaft Energietechnik: Düsseldorf, Germany, 2006; Volume 4630. [Google Scholar]
- Helffrich, D.; Oechsner, H. Hohenheimer Biogasertragstest-Vergleich verschiedener Laborverfahren zur Vergärung von Biomasse. Agrartech. Forsch. 2003, 9, 27–30. [Google Scholar]
- Mittweg, G.; Oechsner, H.; Hahn, V.; Lemmer, A.; Reinhardt-Hanisch, A. Repeatability of a laboratory batch method to determine the specific biogas and methane yields. Eng. Life Sci. 2012, 12, 270–278. [Google Scholar] [CrossRef]
- Boyle, W.C. Energy recovers from sanitary landfills – a review. In Microbial Energy Conversion; Schlegel, H., Barnea, J., Eds.; Elsevier: Pergamon, Turkey, 1977; pp. 119–138. [Google Scholar]
- Park, J.Y.; Seyama, T.; Shiroma, R.; Ike, M.; Srichuwong, S.; Nagata, K.; Arai-Sanoh, Y.; Kondo, M.; Tokuyasu, K. Efficient recovery of glucose and fructose via enzymatic saccharification of rice straw with soft carbohydrates. Biosci. Biotech. Bioch. 2009, 73, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef] [PubMed]
- Levén, L.; Nyberg, K.; Schnürer, A. Conversion of phenols during anaerobic digestion of organic solid waste – A review of important microorganisms and impact of temperature. J. Environ. Manag. 2012, 95, S99–S103. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.-P.; Sun, Z.-J.; Shi, Z.-J.; Xu, F.; Sun, R.-C. Impact of hot compressed water pretreatment on the structural changes of woody biomass for bioethanol production. BioResources 2011, 6, 1576–1598. [Google Scholar]
- Chen, W.H.; Ye, S.C.; Sheen, H.K. Hydrolysis characteristics of sugarcane bagasse pretreated by dilute acid solution in a microwave irradiation environment. Appl. Energ. 2012, 93, 237–244. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]

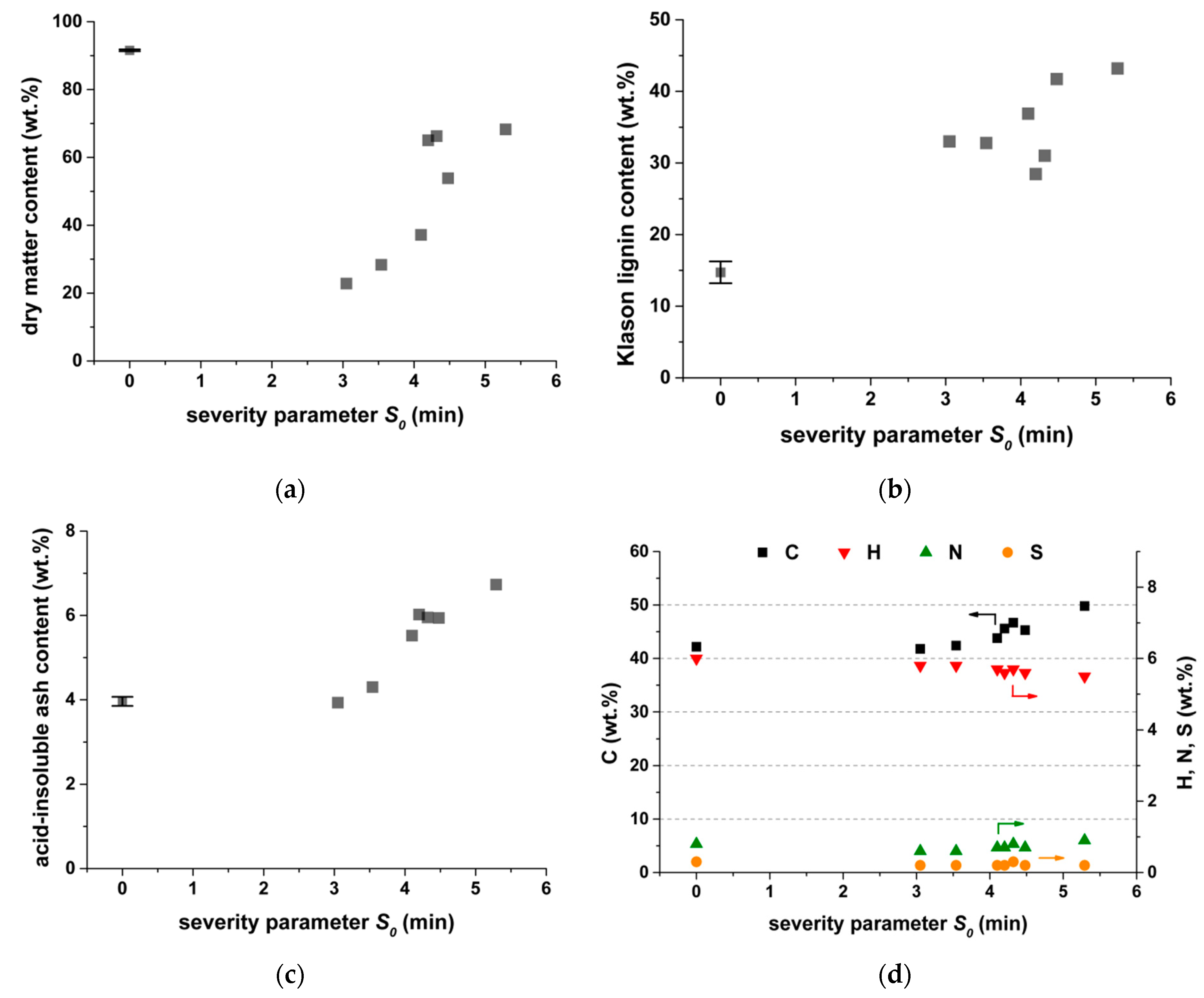
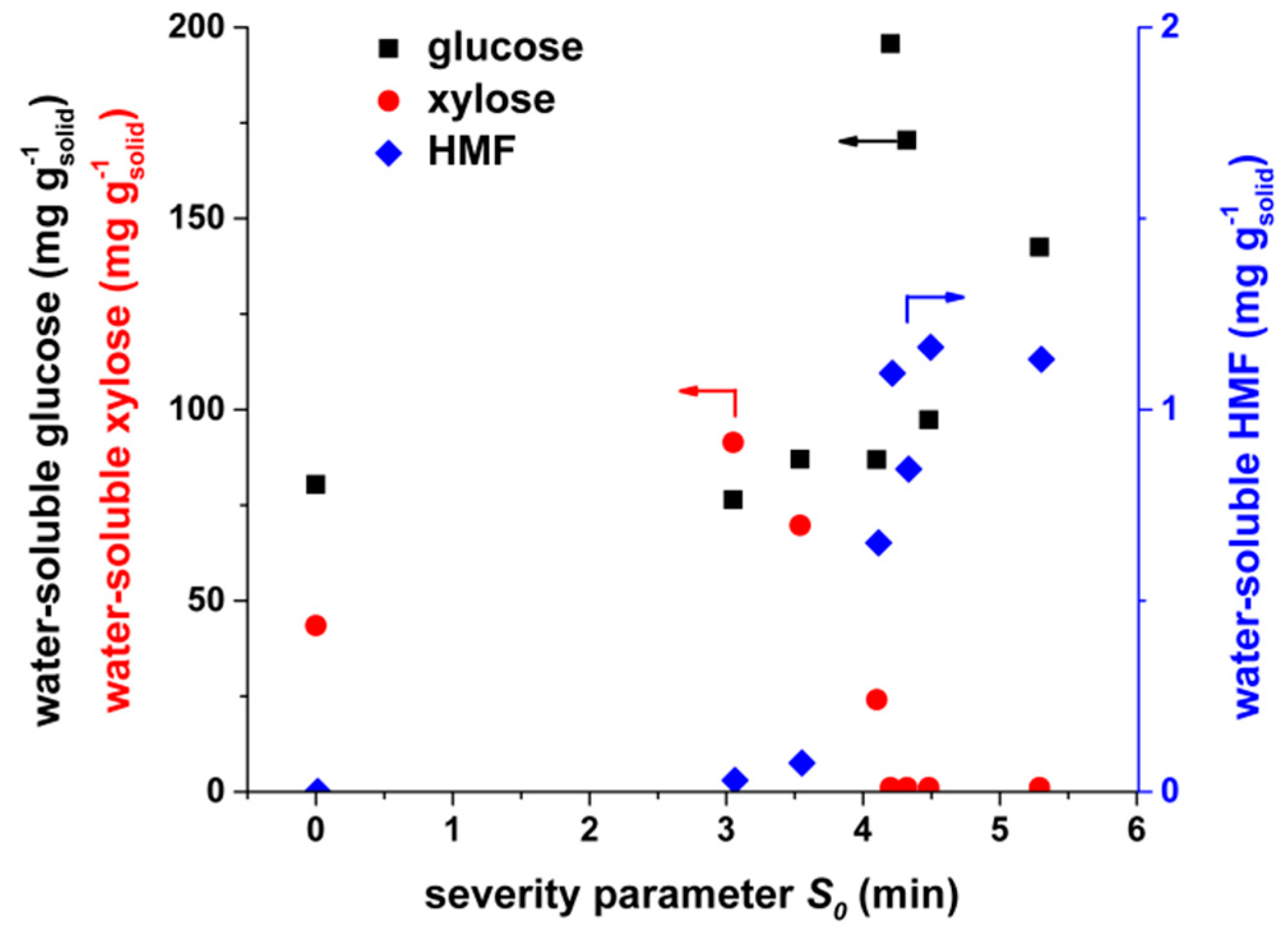

| Ash Content after 550 °C | Ash Content after 1000 °C | Neutral Detergent Fiber | Acid Detergent Fiber | C | H | N | S | O | Si | K | Ca | Mg | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (wt.%) | |||||||||||||
| 12.0 ± 0.1 | 10.7 ± 0.2 | 70.8 ± 2.8 | 46.2 ± 1.7 | 42.2 | 6.0 | 0.8 | 0.3 | 38.7 | 3.6 | 1.4 | 0.5 | 0.3 | 0.1 |
| Severity Parameter S0 (min) | 3.05 | 3.54 | 4.10 | 4.20 | 4.32 | 4.48 | 5.29 |
|---|---|---|---|---|---|---|---|
| Steam input time (min) | 30 | 30 | 30 | 16 | 12 | 30 | 32 |
| Steam input mass (g) | 150 | 150 | 150 | 78 | 60 | 150 | 60 |
| Maximum reactor temperature (°C) 1 | 162 | 174 | 206 | 222 | 229 | 222 | 240 |
| Maximum pressure (bar) 1 | 6.5 | 9.0 | 18.5 | 25.0 | 29.0 | 26.0 | 26.5 |
| Severity Parameter | Biogas Yield (m3N kg DM−1) | CH4 (vol.%) | Specific Methane Yield (m3N kg DM−1) |
|---|---|---|---|
| Untreated | 0.368 ± 0.024 | 57.4 ± 1.5 | 0.211 ± 0.009 |
| S0 = 3.05 min | 0.331 ± 0.009 | 57.3 ± 0.1 | 0.190 ± 0.005 |
| S0 = 3.54 min | 0.393 ± 0.007 | 55.2 ± 0.9 | 0.217 ± 0.007 |
| S0 = 4.10 min | 0.542 ± 0.011 | 51.3 ± 0.6 | 0.278 ± 0.003 |
| S0 = 4.32 min | 0.128 ± 0.008 | 60.6 ± 2.0 | 0.078 ± 0.004 |
| S0 = 5.29 min | 0.082 ± 0.002 | 60.8 ± 3.1 | 0.050 ± 0.003 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinbach, D.; Wüst, D.; Zielonka, S.; Krümpel, J.; Munder, S.; Pagel, M.; Kruse, A. Steam Explosion Conditions Highly Influence the Biogas Yield of Rice Straw. Molecules 2019, 24, 3492. https://doi.org/10.3390/molecules24193492
Steinbach D, Wüst D, Zielonka S, Krümpel J, Munder S, Pagel M, Kruse A. Steam Explosion Conditions Highly Influence the Biogas Yield of Rice Straw. Molecules. 2019; 24(19):3492. https://doi.org/10.3390/molecules24193492
Chicago/Turabian StyleSteinbach, David, Dominik Wüst, Simon Zielonka, Johannes Krümpel, Simon Munder, Matthias Pagel, and Andrea Kruse. 2019. "Steam Explosion Conditions Highly Influence the Biogas Yield of Rice Straw" Molecules 24, no. 19: 3492. https://doi.org/10.3390/molecules24193492
APA StyleSteinbach, D., Wüst, D., Zielonka, S., Krümpel, J., Munder, S., Pagel, M., & Kruse, A. (2019). Steam Explosion Conditions Highly Influence the Biogas Yield of Rice Straw. Molecules, 24(19), 3492. https://doi.org/10.3390/molecules24193492







