A Facile Strategy to Prepare Shaped ZSM-5 Catalysts with Enhanced Para-Xylene Selectivity and Stability for Toluene Methylation: The Effect of In Situ Modification by Attapulgite
Abstract
:1. Introduction
2. Results and Discussion
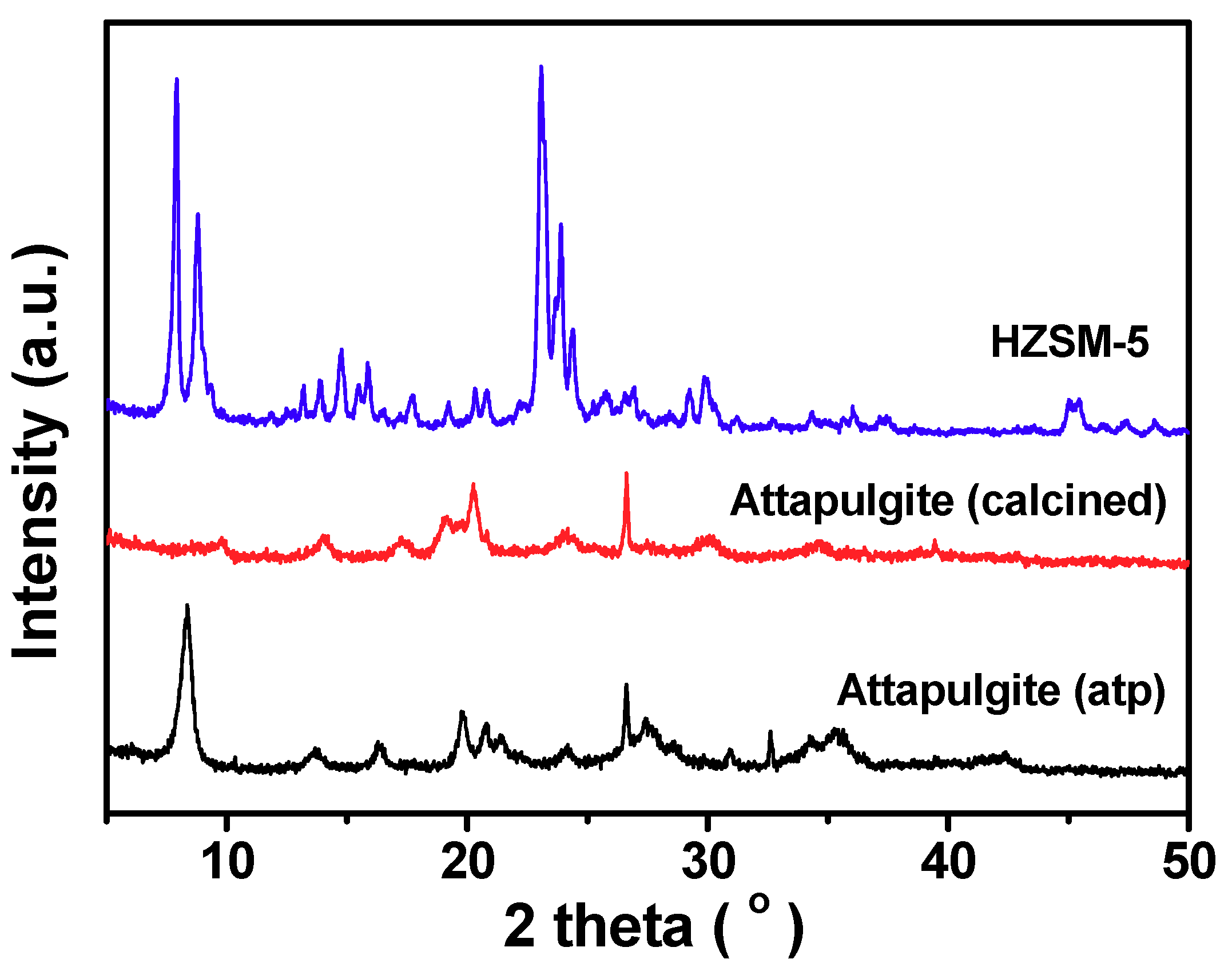
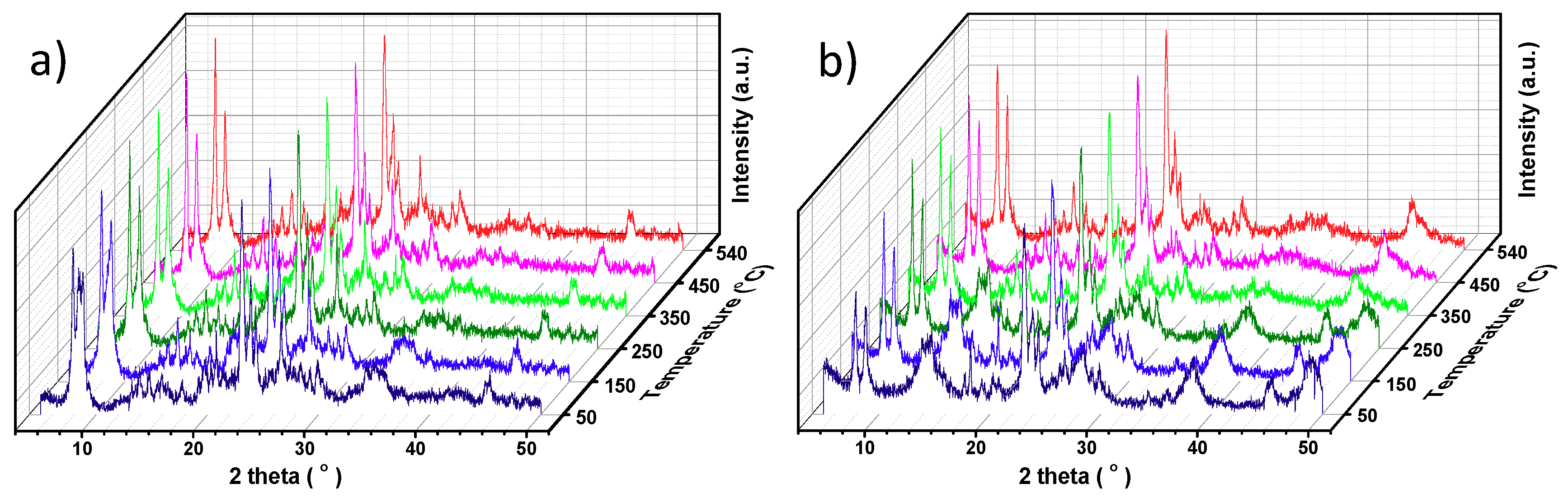
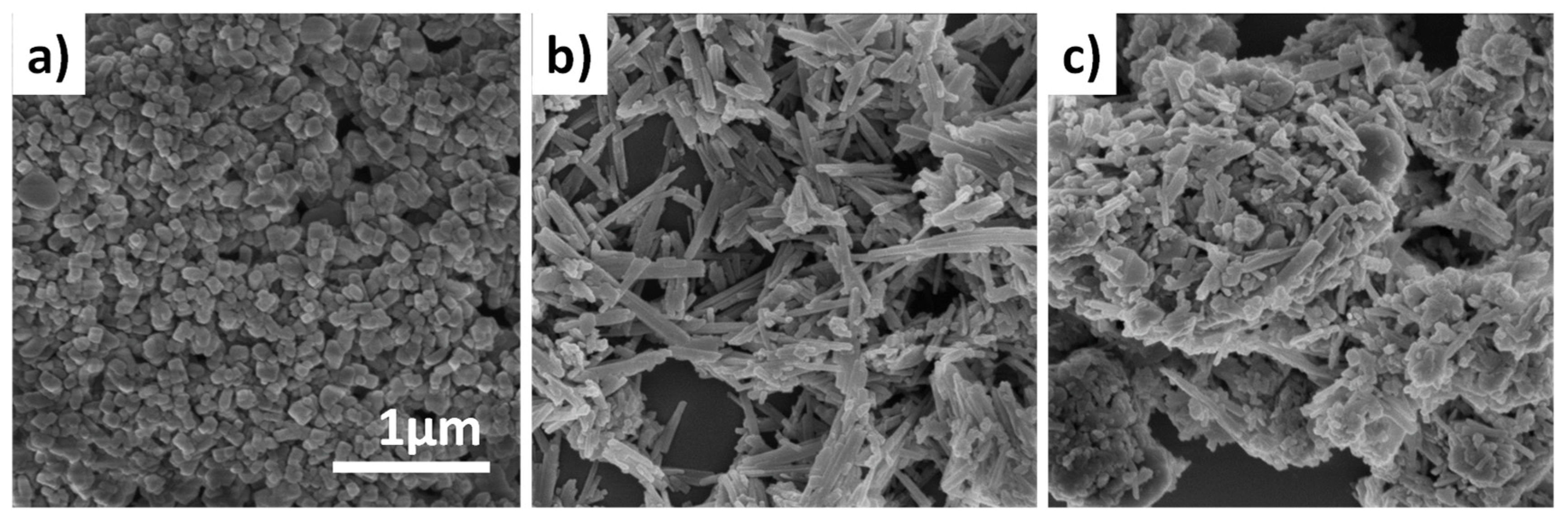
2.1. Preparation and Characteristics of ZSM-5 Extrudates
2.2. Catalytic Performance in Toluene Methylation Process
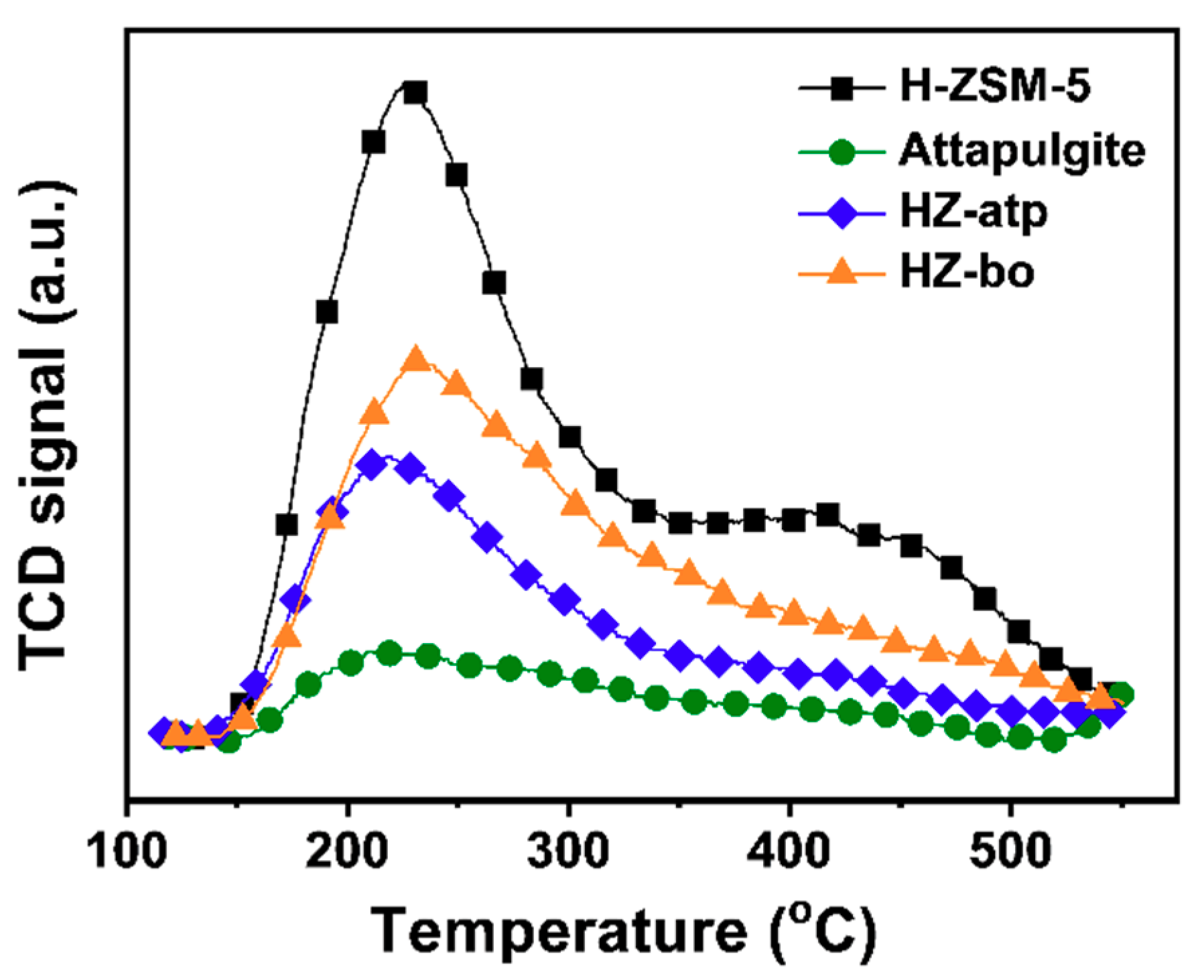
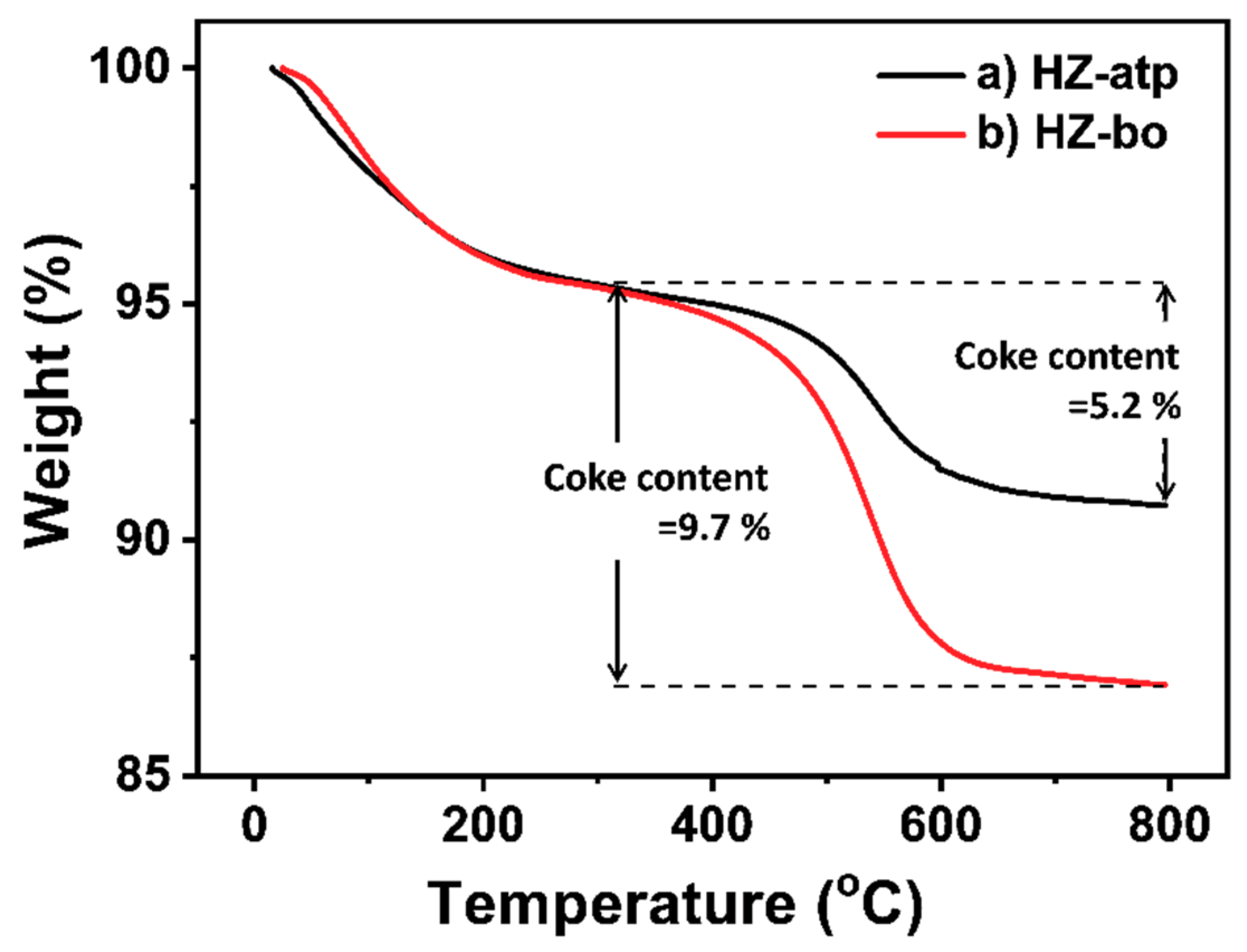
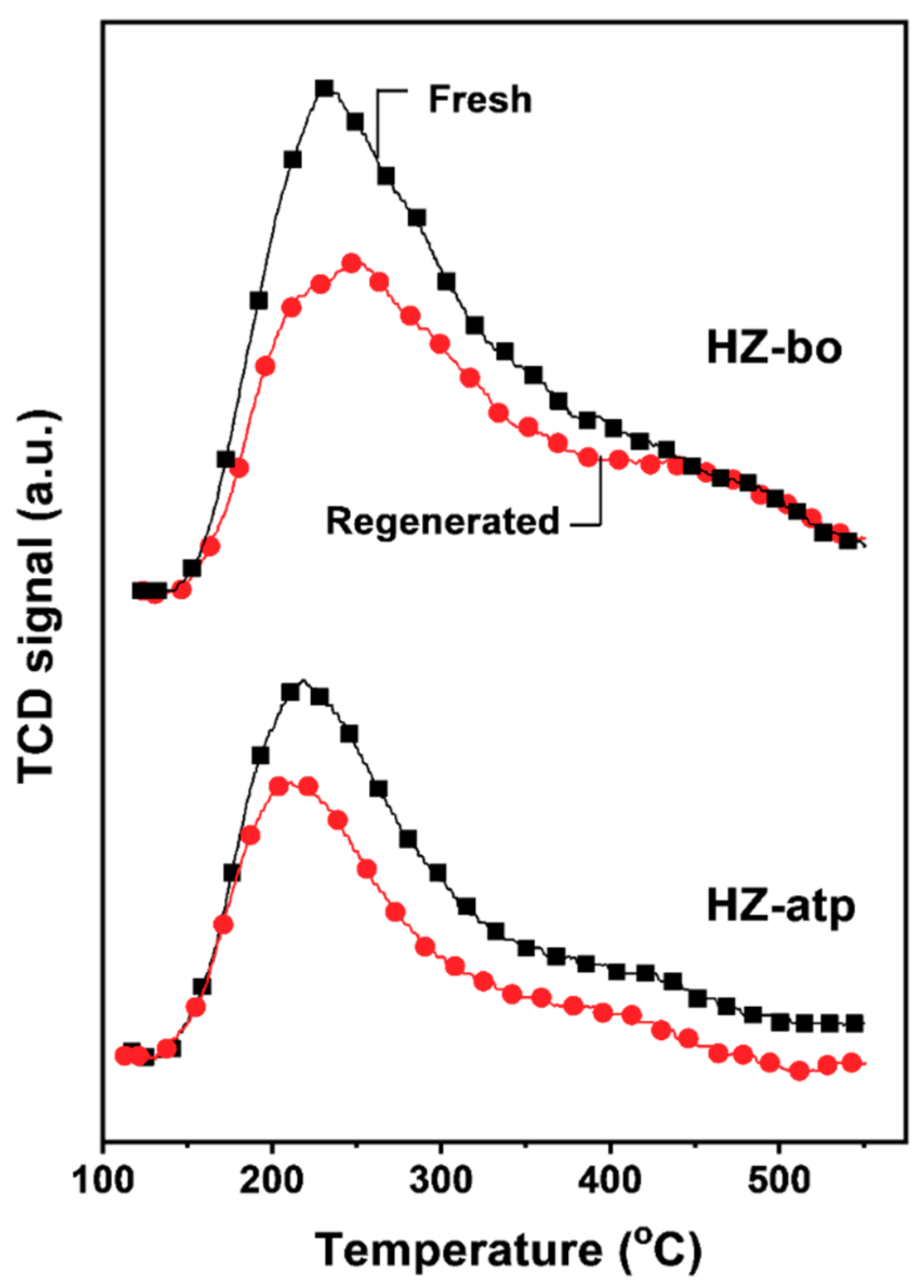
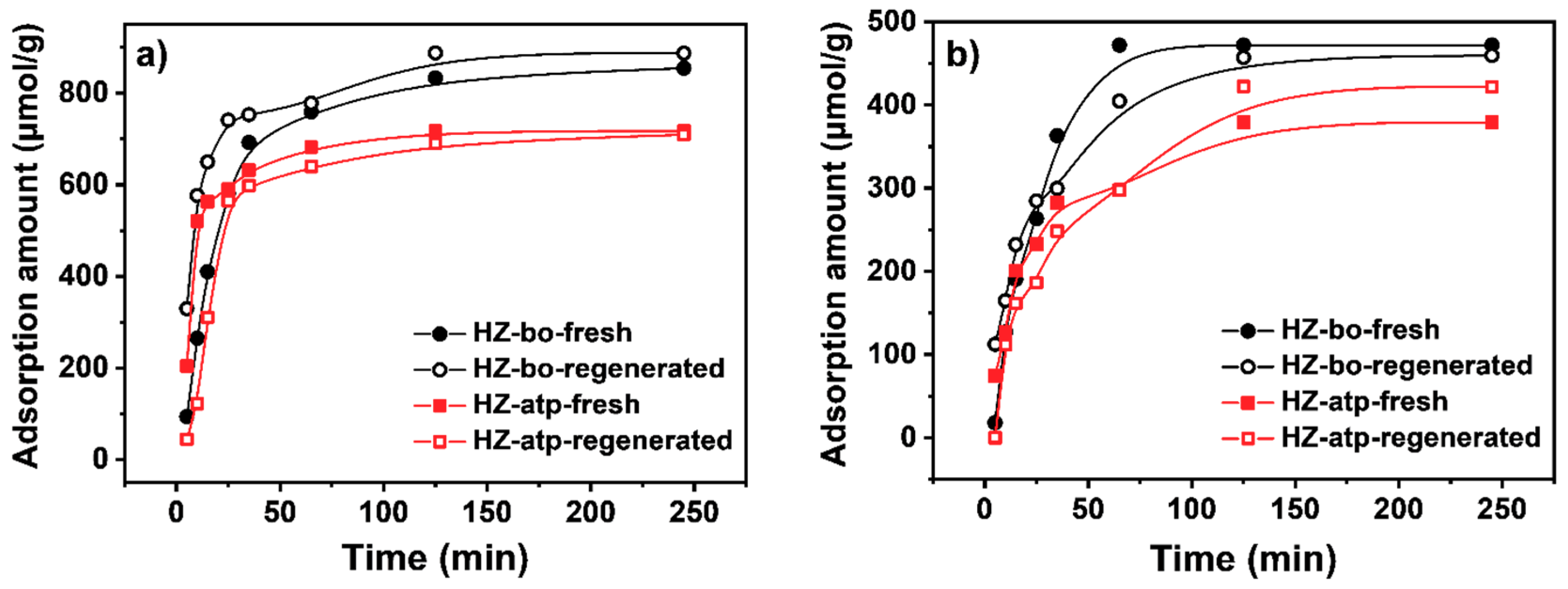
2.3. In-situ Ion Exchange Modification Effect of Attapulgite
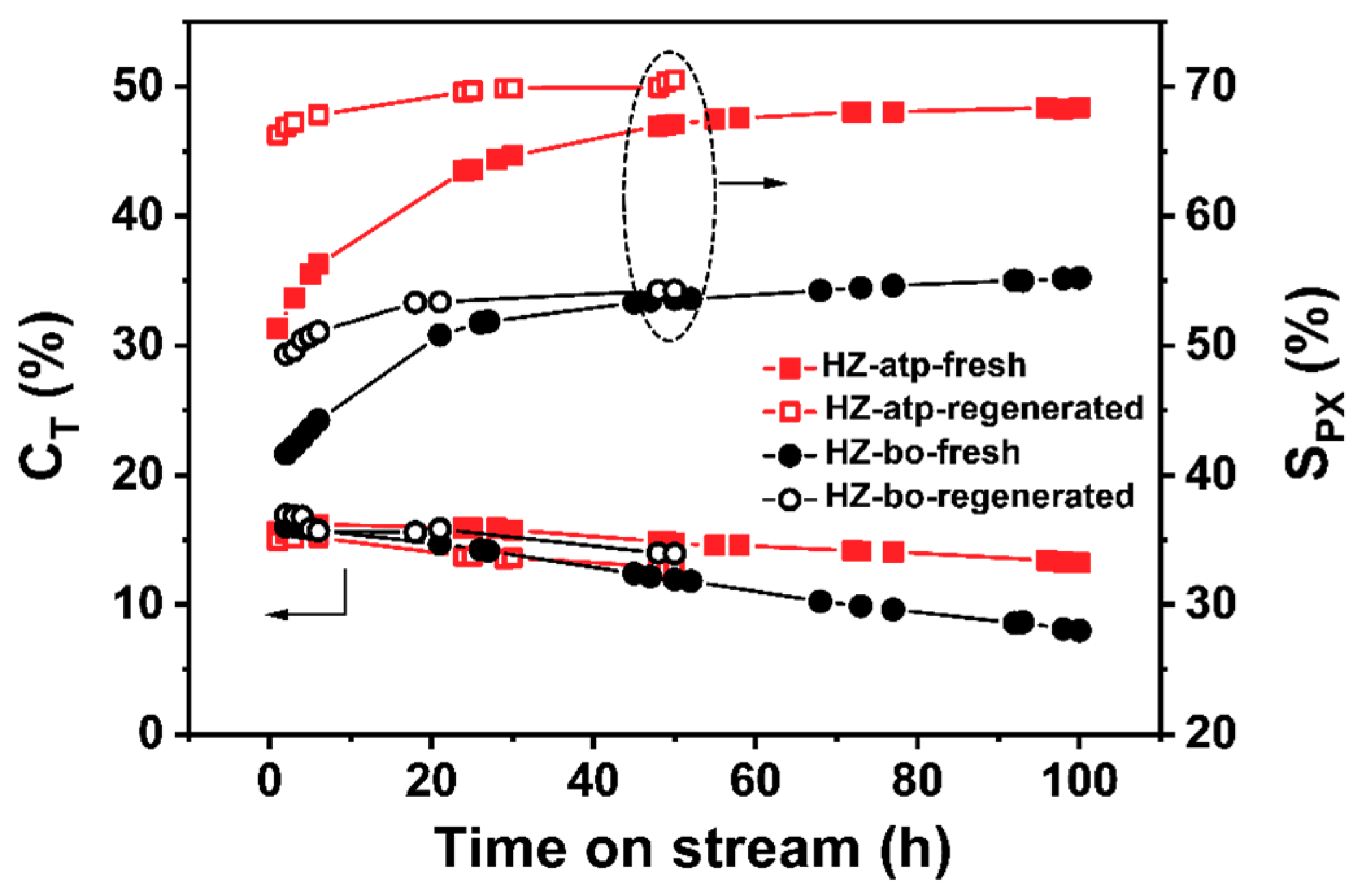
2.4. Catalytic Performance of Attapulgite-Bound Modified ZSM-5 Extrudates
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Song, C.; Garcés, J.M.; Sugi, Y. Shape-Selective Catalysis; American Chemical Society: WA, USA, 1999; Volume 738, p. 428. [Google Scholar]
- Vermeiren, W.; Gilson, J.P. Impact of Zeolites on the Petroleum and Petrochemical Industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- Kim, J.H.; Kunieda, T.; Niwa, M. Generation of Shape-Selectivity of p-Xylene Formation in the Synthesized ZSM-5 Zeolites. J. Catal. 1998, 173, 433–439. [Google Scholar] [CrossRef]
- Niziolek, A.M.; Onel, O.; Floudas, C.A. Production of benzene, toluene, and xylenes from natural gas via methanol: Process synthesis and global optimization. AIChE J. 2016, 62, 1531–1556. [Google Scholar] [CrossRef]
- Kaeding, W.W.; Chu, C.; Young, L.B.; Weinstein, B.; Butter, S.A. Selective alkylation of toluene with methanol to produce para-Xylene. J. Catal. 1981, 67, 159–174. [Google Scholar] [CrossRef]
- Wender, I. Reactions of synthesis gas. Fuel Process. Technol. 1996, 48, 189–297. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Y.; Guo, J. High Selectively Catalytic Conversion of Lignin-Based Phenols into para-/m-Xylene over Pt/HZSM-5. Catalysts 2016, 6, 19. [Google Scholar] [CrossRef]
- Chen, N.Y. Reactions of mixtures of toluene and methanol over ZSM-5. J. Catal. 1988, 114, 17–22. [Google Scholar] [CrossRef]
- Tan, W.; Liu, M.; Zhao, Y.; Hou, K.; Wu, H.; Zhang, A.; Liu, H.; Wang, Y.; Song, C.; Guo, X. Para-selective methylation of toluene with methanol over nano-sized ZSM-5 catalysts: Synergistic effects of surface modifications with SiO2, P2O5 and MgO. Micropor. Mesopor. Mat. 2014, 196, 18–30. [Google Scholar] [CrossRef]
- Ashraf, M.T.; Chebbi, R.; Darwish, N.A. Process of p-Xylene Production by Highly Selective Methylation of Toluene. Ind. Eng. Chem. Res. 2013, 52, 13730–13737. [Google Scholar] [CrossRef]
- Song, A.; Ma, J.; Xu, D.; Li, R. Adsorption and Diffusion of Xylene Isomers on Mesoporous Beta Zeolite. Catalysts 2015, 5, 2098. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, Y.; Wei, Y.; He, Y.; Yu, Z.; Liu, Z.; Xu, L. Improved Selectivity toward Light Olefins in the Reaction of Toluene with Methanol Over the Modified HZSM-5 Catalyst. ChemCatChem 2014, 6, 713–718. [Google Scholar] [CrossRef]
- Breen, J.P.; Burch, R.; Kulkarni, M.; McLaughlin, D.; Collier, P.J.; Golunski, S.E. Improved selectivity in the toluene alkylation reaction through understanding and optimising the process variables. Appl. Catal. A-Gen. 2007, 316, 53–60. [Google Scholar] [CrossRef]
- Hu, H.; Lyu, J.; Cen, J.; Zhang, Q.; Wang, Q.; Han, W.; Rui, J.; Li, X. Promoting effects of MgO and Pd modification on the catalytic performance of hierarchical porous ZSM-5 for catalyzing benzene alkylation with methanol. RSC Adv. 2015, 5, 63044–63049. [Google Scholar] [CrossRef]
- Mitchell, S.; Michels, N.L.; Perez-Ramirez, J. From powder to technical body: The undervalued science of catalyst scale up. Chem. Soc. Rev. 2013, 42, 6094–6112. [Google Scholar] [CrossRef] [PubMed]
- Freiding, J.; Kraushaar-Czarnetzki, B. Novel extruded fixed-bed MTO catalysts with high olefin selectivity and high resistance against coke deactivation. Appl. Catal. A-Gen. 2011, 391, 254–260. [Google Scholar] [CrossRef]
- Pérez-Uriarte, P.; Gamero, M.; Ateka, A.; Díaz, M.; Aguayo, A.T.; Bilbao, J. Effect of the Acidity of HZSM-5 Zeolite and the Binder in the DME Transformation to Olefins. Ind. Eng. Chem. Res. 2016, 55, 1513–1521. [Google Scholar] [CrossRef]
- Hargreaves, J.S.J.; Munnoch, A.L. A survey of the influence of binders in zeolite catalysis. Catal. Sci. Tech. 2013, 3, 1165–1171. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.H.; Park, D.-W.; Cho, S.J. Effect of base binder, flash calcined hydrotalcite, in MFI zeolite granule: Catalytic activity over 1-butene isomerization and MTO reaction. Appl. Catal. A-Gen. 2015, 502, 42–47. [Google Scholar] [CrossRef]
- Whiting, G.T.; Chowdhury, A.D.; Oord, R.; Paalanen, P.; Weckhuysen, B.M. The curious case of zeolite-clay/binder interactions and their consequences for catalyst preparation. Faraday Discuss. 2016, 188, 369–386. [Google Scholar] [CrossRef]
- Hernando, H.; Ochoa-Hernández, C.; Shamzhy, M.; Moreno, I.; Fermoso, J.; Pizarro, P.; Coronado, J.M.; Čejka, J.; Serrano, D.P. The crucial role of clay binders in the performance of ZSM-5 based materials for biomass catalytic pyrolysis. Catal. Sci. Tech. 2019, 9, 789–802. [Google Scholar] [CrossRef]
- Yuan, B.; Yin, X.Q.; Liu, X.Q.; Li, X.Y.; Sun, L.B. Enhanced Hydrothermal Stability and Catalytic Performance of HKUST-1 by Incorporating Carboxyl-Functionalized Attapulgite. ACS Appl. Mater. Interfaces 2016, 8, 16457–16464. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.C.V.; Santos, P.D. Special clays: What they are, characterization and properties. Quim. Nova 2007, 30, 146–152. [Google Scholar] [CrossRef]
- Fan, Q.H.; Tan, X.L.; Li, J.X.; Wang, X.K.; Wu, W.S.; Montavon, G. Sorption of Eu(III) on Attapulgite Studied by Batch, XPS, and EXAFS Techniques. Environ. Sci. Technol. 2009, 43, 5776–5782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.Y.; Zhang, D.Y.; Liu, X.Q.; Shi, L.Y.; Sun, L.B. A tandem demetalization-desilication strategy to enhance the porosity of attapulgite for adsorption and catalysis. Chem. Eng. Sci. 2016, 141, 184–194. [Google Scholar] [CrossRef]
- Wang, Y.S.; Chen, M.Q.; Liang, T.; Yang, Z.L.; Yang, J.; Liu, S.M. Hydrogen Generation from Catalytic Steam Reforming of Acetic Acid by Ni/Attapulgite Catalysts. Catalysts 2016, 6, 16. [Google Scholar] [CrossRef]
- An, L.; Pan, Y.; Shen, X.; Lu, H.; Yang, Y. Rod-like attapulgite/polyimide nanocomposites with simultaneously improved strength, toughness, thermal stability and related mechanisms. J. Mater. Chem. 2008, 18, 4928–4941. [Google Scholar] [CrossRef]
- Lu, L.; Li, X.Y.; Liu, X.Q.; Wang, Z.M.; Sun, L.B. Enhancing the hydrostability and catalytic performance of metal-organic frameworks by hybridizing with attapulgite, a natural clay. J. Mater. Chem. A 2015, 3, 6998–7005. [Google Scholar] [CrossRef]
- Michels, N.-L.; Mitchell, S.; Pérez-Ramírez, J. Effects of Binders on the Performance of Shaped Hierarchical MFI Zeolites in Methanol-to-Hydrocarbons. ACS Catal. 2014, 4, 2409–2417. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Whiting, G.T.; Meirer, F.; Mertens, M.M.; Bons, A.-J.; Weiss, B.M.; Stevens, P.A.; de Smit, E.; Weckhuysen, B.M. Binder Effects in SiO2- and Al2O3-Bound Zeolite ZSM-5-Based Extrudates as Studied by Microspectroscopy. ChemCatChem 2015, 7, 1312–1321. [Google Scholar] [CrossRef]
- Cañizares, P.; Durán, A.; Dorado, F.; Carmona, M. The role of sodium montmorillonite on bounded zeolite-type catalysts. Appl. Clay Sci. 2000, 16, 273–287. [Google Scholar] [CrossRef]
- Pradhan, A.R.; Wu, J.F.; Jong, S.J.; Tsai, T.C.; Liu, S.B. An ex situ methodology for characterization of coke by TGA and 13C CP-MAS NMR spectroscopy. Appl. Catal. A-Gen. 1997, 165, 489–497. [Google Scholar] [CrossRef]
- Chmelik, C.; Karger, J. In situ study on molecular diffusion phenomena in nanoporous catalytic solids. Chem. Soc. Rev. 2010, 39, 4864–4884. [Google Scholar] [CrossRef] [PubMed]
- Szostak, R. Molecular Sieves: Principles of Synthesis and Identification; Blackie Academic & Professional: London, UK, 1989. [Google Scholar]
- Whiting, G.T.; Chung, S.-H.; Stosic, D.; Chowdhury, A.D.; van der Wal, L.I.; Fu, D.; Zecevic, J.; Travert, A.; Houben, K.; Baldus, M.; et al. Multiscale Mechanistic Insights of Shaped Catalyst Body Formulations and Their Impact on Catalytic Properties. ACS Catal. 2019, 9, 4792–4803. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, W.; Wu, H.; Zhang, A.; Liu, M.; Li, G.; Wang, X.; Song, C.; Guo, X. Effect of Pt on stability of nano-scale ZSM-5 catalyst for toluene alkylation with methanol into p-xylene. Catal Today 2011, 160, 179–183. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Zhang, A.; Zuo, Y.; Ding, F.; Chang, Y.; Song, C.; Guo, X. Methanol Usage in Toluene Methylation over Pt Modified ZSM-5 Catalyst: Effects of Total Pressure and Carrier Gas. Ind. Eng. Chem. Res. 2017, 56, 4709–4717. [Google Scholar] [CrossRef]
- Zhang, W.; Bao, X.; Guo, X.; Wang, X. A high-resolution solid-state NMR study on nano-structured HZSM-5 zeolite. Catal. Lett. 1999, 60, 89–94. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Catalyst | SBET a | Vtotal b | Vmicro c | Cacid d | Cacid d | CT e | SPX e | Activity Loss f |
|---|---|---|---|---|---|---|---|---|
| (m2/g) | (cm3/g) | (cm3/g) | (μmol/g) | [μmol/g(ZSM-5)] | (%) | (%) | (%) | |
| H-ZSM-5 | 376 | 0.21 | 0.13 | 385 | 385 | 16.4 | 30.0 | -- |
| Attapulgite | 107 | 0.23 | 0 | -- | -- | 0.2 | 58.2 | -- |
| Boehmite | 255 | 0.28 | 0 | -- | -- | 0.2 | 54.5 | -- |
| HZ-atp | 227 | 0.22 | 0.07 | 153 | 306 | 16.1 | 55.9 | 17.5 |
| HZ-bo | 283 | 0.28 | 0.04 | 235 | 470 | 15.9 | 43.5 | 50.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chang, Y.; Liu, M.; Zhang, A.; Guo, X. A Facile Strategy to Prepare Shaped ZSM-5 Catalysts with Enhanced Para-Xylene Selectivity and Stability for Toluene Methylation: The Effect of In Situ Modification by Attapulgite. Molecules 2019, 24, 3462. https://doi.org/10.3390/molecules24193462
Wang Y, Chang Y, Liu M, Zhang A, Guo X. A Facile Strategy to Prepare Shaped ZSM-5 Catalysts with Enhanced Para-Xylene Selectivity and Stability for Toluene Methylation: The Effect of In Situ Modification by Attapulgite. Molecules. 2019; 24(19):3462. https://doi.org/10.3390/molecules24193462
Chicago/Turabian StyleWang, Yiren, Yang Chang, Min Liu, Anfeng Zhang, and Xinwen Guo. 2019. "A Facile Strategy to Prepare Shaped ZSM-5 Catalysts with Enhanced Para-Xylene Selectivity and Stability for Toluene Methylation: The Effect of In Situ Modification by Attapulgite" Molecules 24, no. 19: 3462. https://doi.org/10.3390/molecules24193462





