Corilagin in Cancer: A Critical Evaluation of Anticancer Activities and Molecular Mechanisms
Abstract
:1. Introduction
2. Methodology for Literature Review
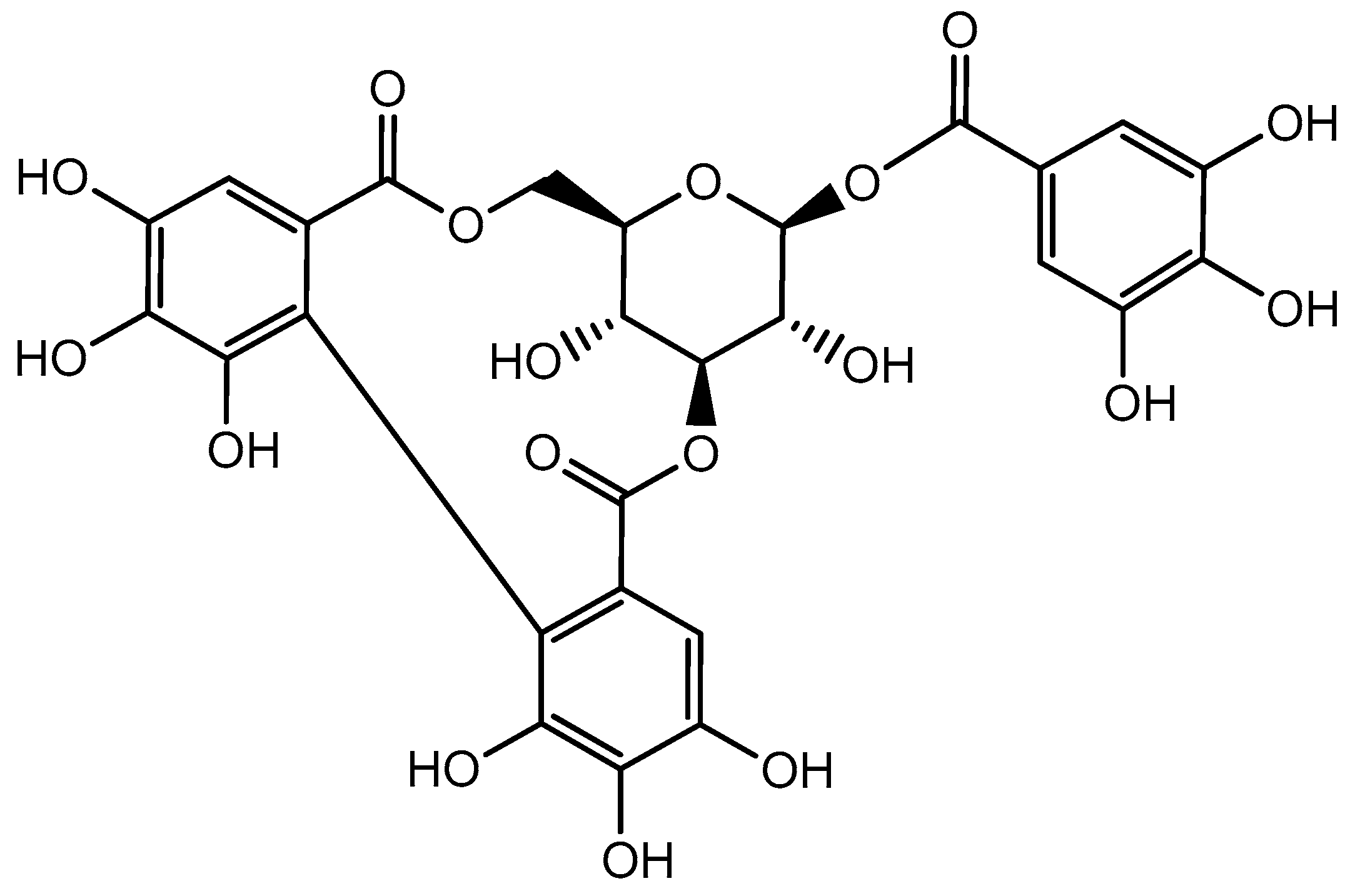
3. Distribution of Corilagin and Its Physicochemical Properties
4. Bioavailability of Corilagin
5. Anticancer Activity of Corilagin
5.1. Breast Cancer
5.2. Cholangiocarcinoma
5.3. Esophageal Cancer
5.4. Gastric Cancer
5.5. Hepatocellular Carcinoma
5.6. Lung Cancer
5.7. Neural Cancer
5.8. Ovarian Cancer
6. Effect of Corilagin on Various Signaling Pathways of Cancer Cells
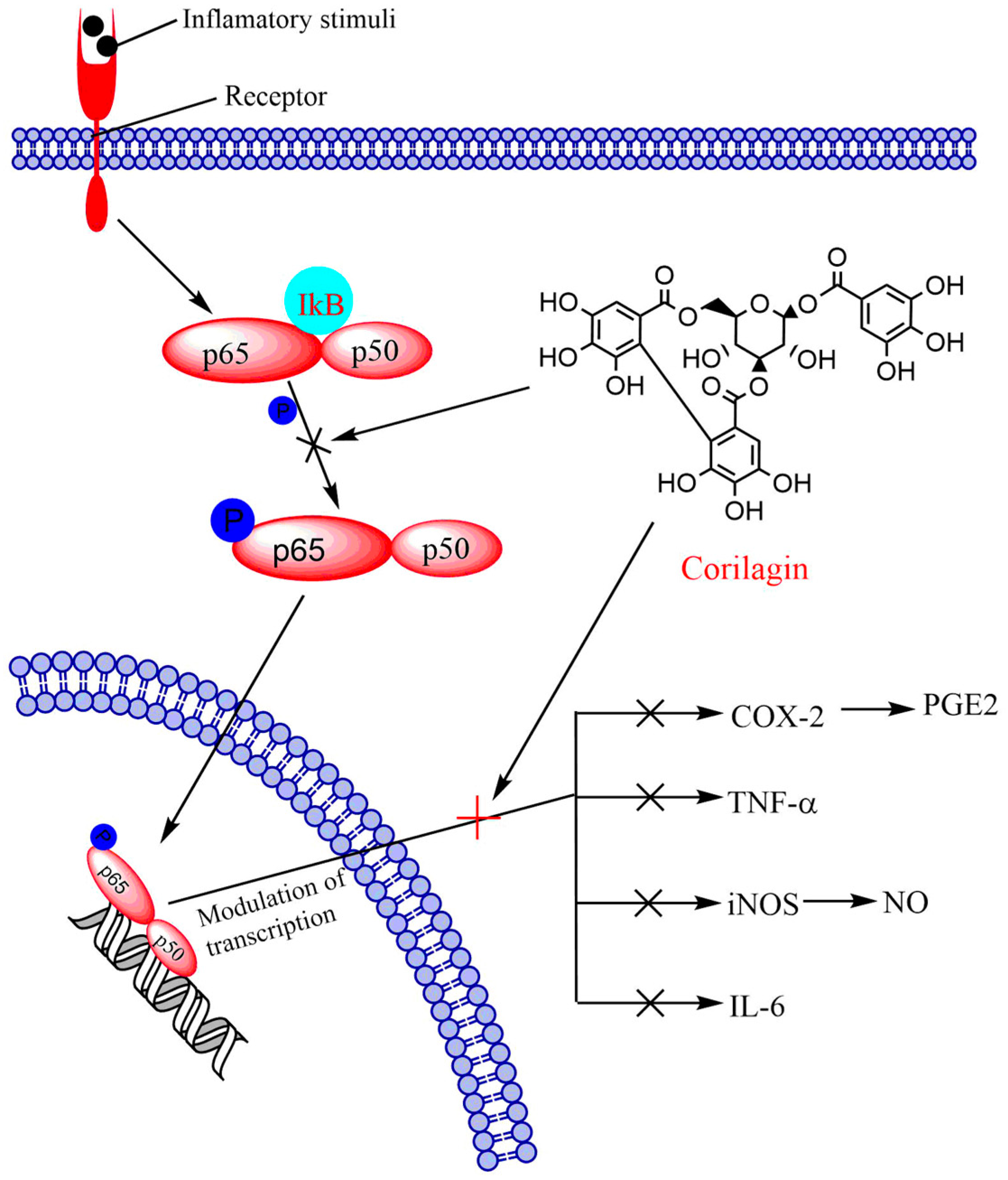
6.1. NF-κB Signaling Pathway
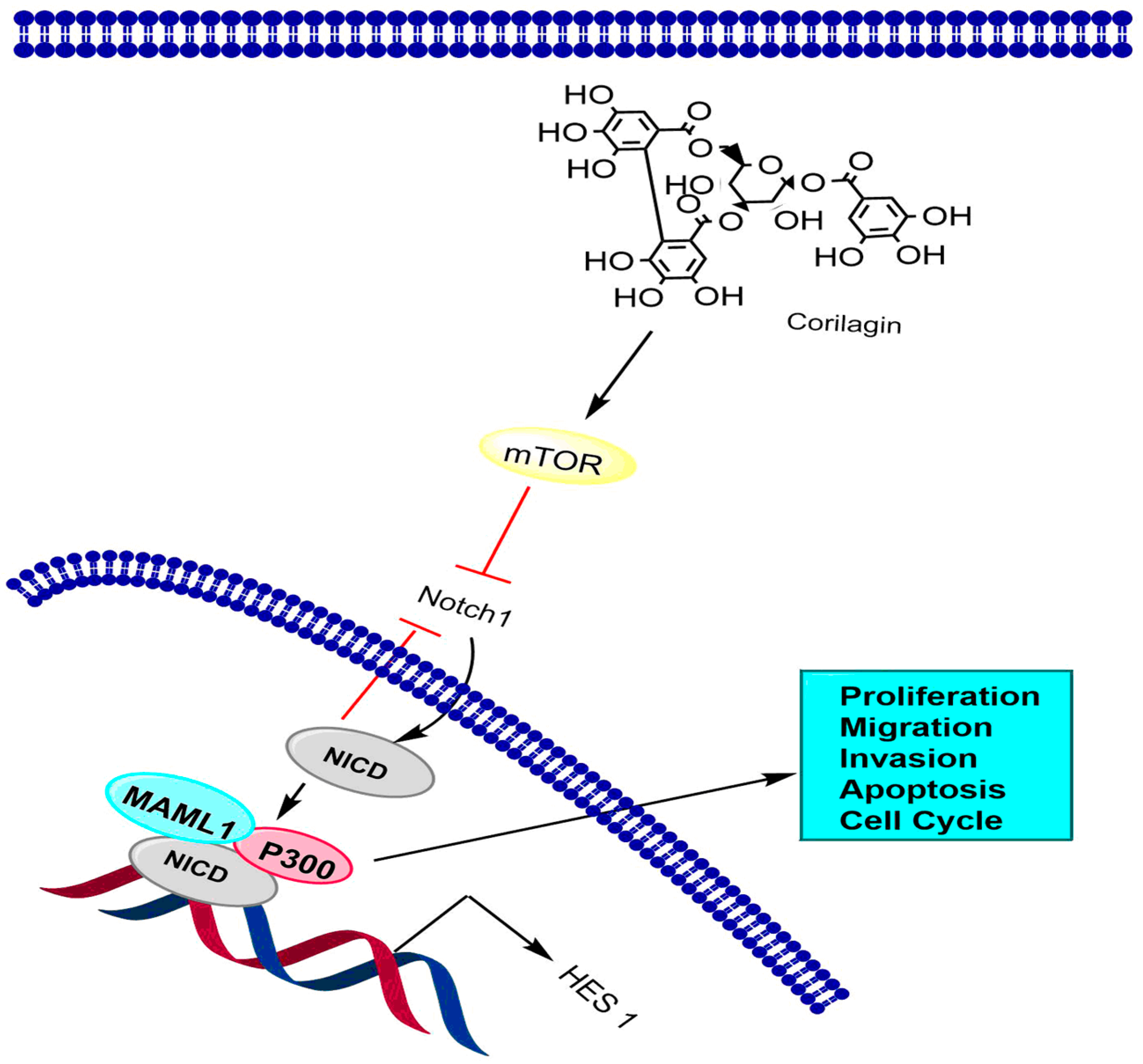
6.2. Notch-mTOR Signaling Pathway
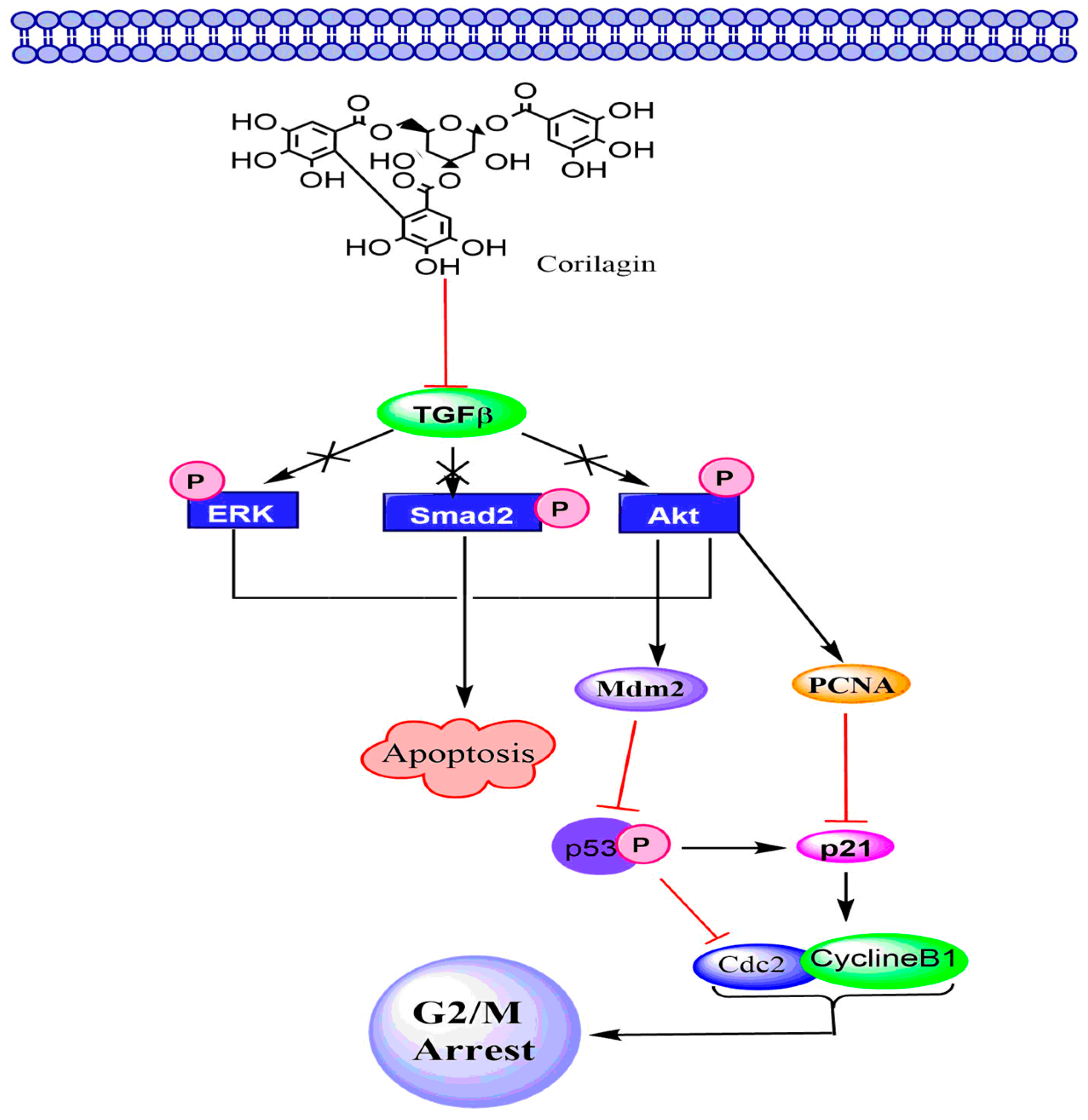
6.3. TGF-β Signaling Pathway
7. Safety Evaluation of Corilagin
8. Limitations and Future Prospects
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdullaev, F.I. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.). Exp. Biol. Med. 2002, 227, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Latest Global Cancer Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018; International Agency for Research on Cancer, World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/cancer/PRGlobocanFinal.pdf (accessed on 10 September 2019).
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pr. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Kumar, S.; Ahmad, M.K.; Waseem, M.; Pandey, A.K. Drug targets for cancer treatment: An overview. Med. Chem. 2015, 5, 115–123. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Sethi, G.; Um, J.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Singh, A.K.; Cabral, C.; Kumar, R.; Ganguly, R.; Rana, H.K.; Gupta, A.; Lauro, M.R.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Gupta, A.; Ganguly, R.; Pandey, A.K. Other secondary metabolites. In Secondary Metabolite and Functional Food Components: Role in Health and Disease; Kumar, S., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2018; pp. 99–122. [Google Scholar]
- Kumar, S.; Chashoo, G.; Saxena, A.K.; Pandey, A.K. Parthenium hysterophorus: A probable source of anticancer, antioxidant and anti-HIV agents. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, S.; Pandey, A.K. In vitro antibacterial, antioxidant, and cytotoxic activities of Parthenium hysterophorus and characterization of extracts by LC-MS analysis. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, U.K.; Pandey, A.K. Protective effect of Bauhinia variegata leaf extracts against oxidative damage, cell proliferation and bacterial growth. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 45–51. [Google Scholar] [CrossRef]
- Sharma, U.K.; Sharma, A.K.; Gupta, A.; Kumar, R.; Pandey, A.; Pandey, A.K. Pharmacological activities of cinnamaldehyde and eugenol: Antioxidant, cytotoxic and anti-leishmanial studies. Cell. Mol. Biol. 2017, 63, 73–78. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.K.; Pandey, A.K. Phenolics. In Secondary Metabolite and Functional Food Components: Role in Health and Disease; Kumar, S., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2018; pp. 13–22. [Google Scholar]
- Kashiwada, Y.; Nonaka, G.I.; Nishioka, I.; Chang, J.J.; Lee, K.H. Antitumor agents, 129. Tannins and related compounds as selective cytotoxic agents. J. Nat. Prod. 1992, 55, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Wakamori, S.; Hirokane, T.; Ikeuchi, K.; Matsumoto, S. Structural revisions in natural ellagitannins. Molecules 2018, 23, 1901. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chen, L.G.; Yang, L.L. Cuphiin D1, the macrocyclic hydrolyzable tannin induced apoptosis in HL-60 cell line. Cancer Lett. 2000, 149, 77–83. [Google Scholar] [CrossRef]
- Gonzalez-Sarria, A.; Yuan, T.; Seeram, N.P. Cytotoxicity and structure activity relationship studies of maplexins A-I, gallotannins from red maple (Acer rubrum). Food Chem. Toxicol. 2012, 50, 1369–1376. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Zhao, L.; Du, Y.; Feng, Y.; Li, Y. Hydrolysable tannins and related compound having cytotoxic activity of Geranium wilfordii maxim. Adv. J. Food Sci. Technol. 2013, 5, 255–257. [Google Scholar] [CrossRef]
- Tang, Y.Y.; He, X.M.; Sun, J.; Li, C.B.; Li, L.; Sheng, J.F.; Xin, M.; Li, Z.C.; Zheng, F.J.; Liu, G.M.; et al. Polyphenols and alkaloids in byproducts of Longan fruits (Dimocarpus Longan Lour.) and their bioactivities. Molecules 2019, 24, 1186. [Google Scholar] [CrossRef]
- Kolodziej, H.; Burmeister, A.; Trun, W.; Radtke, O.A.; Kiderlen, A.F.; Ito, H.; Hatano, T.; Yoshida, T.; Foo, L.Y. Tannins and related compounds induce nitric oxide synthase and cytokines gene expressions in Leishmania major-infected macrophage-like RAW 264.7 cells. Bioorg. Med. Chem. 2005, 13, 6470–6476. [Google Scholar] [CrossRef]
- Jin, F.; Cheng, D.; Tao, J.Y.; Zhang, S.L.; Pang, R.; Guo, Y.J.; Ye, P.; Dong, J.H.; Zhao, L. Anti-inflammatory and anti-oxidative effects of corilagin in a rat model of acute cholestasis. BMC Gastroenterol. 2013, 13, 79. [Google Scholar] [CrossRef]
- Miyasaki, Y.; Rabenstein, J.D.; Rhea, J.; Crouch, M.L.; Mocek, U.M.; Kittell, P.E.; Morgan, M.A.; Nichols, W.S.; Van Benschoten, M.M.; Hardy, W.D.; et al. Isolation and characterization of antimicrobial compounds in plant extracts against multidrug-resistant Acinetobacter Baumannii. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.T.; Lin, T.C.; Hsu, F.L. Antihypertensive effect of corilagin in the rat. Can. J. Physiol. Pharmacol. 1995, 73, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Vasquez, Y.; Ali, Z.; Khan, I.A.; Khan, S.I. Constituents from Terminalia species increase PPAR alpha and PPARgamma levels and stimulate glucose uptake without enhancing adipocyte differentiation. J. Ethnopharmacol. 2013, 149, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Lipinska, L.; Klewicka, E.; Sojka, M. Structure, occurrence and biological activity of ellagitannins: A general review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhang, G.; Li, Y.; Xu, J.; Yuan, J.; Zhang, B.; Hu, T.; Song, G. Corilagin inhibits breast cancer growth via reactive oxygen species-dependent apoptosis and autophagy. J. Cell. Mol. Med. 2018, 22, 3795–3807. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Z.; Chen, L.H.; Liu, S.S.; Deng, Y.; Zheng, G.H.; Ming, Y.L. Bioguided fraction and isolation of the antitumor component from Phylanthus niruri L. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Pan, R.; Li, M.; Li, X.; Zhang, H. HPLC profile of Longan (cv. Shixia) pericarp-sourced phenolics and their antioxidant and cytotoxic effects. Molecules 2019, 24, 619. [Google Scholar] [CrossRef]
- Guo, J.S.; Wang, S.X.; Li, X.; Zhu, T.R. Studies on the antibacterial constituents of Geranium sibiricum L. Acta Pharm. Sin. 1987, 22, 28–32. [Google Scholar]
- Ismail, T.; Calcabrini, C.; Diaz, A.R.; Fimognari, C.; Turrini, E.; Catanzaro, E.; Akhtar, S.; Sestili, P. Ellagitannins in cancer chemoprevention and therapy. Toxins 2016, 8, 151. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Ellagitannins in cancer chemoprevention and therapy. Toxins 2016, 8, 151. [Google Scholar]
- Li, X.; Deng, Y.; Zheng, Z.; Huang, W.; Chen, L.; Tong, Q.; Ming, Y. Corilagin, a promising medicinal herbal agent. Biomed. Pharmacother. 2018, 99, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 21. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zu, Y.; Fu, Y.; Kong, Y.; Zhao, J.; Li, X.; Li, J.; Wink, M.; Efferth, T. Antioxidant activities and xanthine oxidase inhibitory effects of extracts and main polyphenolic compounds obtained from Geranium sibiricum L. J. Agric. Food Chem. 2010, 58, 4737–4743. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Inoue, Y.; Nakama, S.; Ichiba, T.; Aniya, Y. Antioxidant and hepatoprotective actions of medicinal herb, Terminalia catappa L. from Okinawa Island and its tannin corilagin. Phytomedicine 2007, 14, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, S.L.; Tao, J.Y.; Pang, R.; Jin, F.; Guo, Y.J.; Dong, J.H.; Ye, P.; Zhao, H.Y.; Zheng, G.H. Preliminary exploration on anti-inflammatory mechanism of corilagin (beta-1-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-d-glucose) in vitro. Int. Immunopharmacol. 2008, 8, 1059–1064. [Google Scholar] [CrossRef]
- Moreira, J.; Klein-Junior, L.C.; Filho, V.C.; Buzzi, F.C. Anti-hyperalgesic activity of corilagin, a tannin isolated from Phyllanthus niruri L. (Euphorbiaceae). J. Ethanopharmacol. 2013, 146, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Jin, H.; Zhou, J.; Chen, L.; Lu, Y.; Ming, Y.; Yu, Y. A potential anti-tumor herbal medicine, corilagin, inhibits ovarian cancer cell growth through blocking the TGF-β signaling pathways. BMC Complement. Altern. Med. 2013, 13, 33. [Google Scholar] [CrossRef]
- Yisimayili, Z.; Guo, X.; Liu, H.; Xu, Z.; Abdulla, R.; Aisa, H.A.; Huang, C. Metabolic profiling analysis of corilagin in vivo and in vitro using high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2019, 165, 251–260. [Google Scholar] [CrossRef]
- Reddy, B.U.; Mullick, R.; Kumar, A.; Sharma, G.; Bag, P.; Roy, C.L.; Sudha, G.; Tandon, H.; Dave, P.; Shukla, A. A natural small molecule inhibitor corilagin blocks HCV replication and modulates oxidative stress to reduce liver damage. Antiviral Res. 2018, 150, 47–59. [Google Scholar] [CrossRef]
- Zheng, B.; Chen, D.; Yang, X.; PhembaIgo, L.; Li, Z.; Ye, X.; Xiang, Z. Development and validation of an UPLC-PDA method for the determination of corilagin in rat plasma and its application to pharmacokinetic study. J. Chromatogr. B. 2016, 1031, 76–79. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhao, L.; Li, H.; Xu, H.; Chen, W.W.; Tao, L. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Biol. Med. 2014, 11, 92–100. [Google Scholar] [PubMed]
- Subramanian, A.P.; John, A.A.; Vellayappan, M.V.; Balaji, A.; Jaganathan, S.K.; Supriyanto, E.; Yusof, M. Gallic acid: Prospects and molecular mechanisms of its anticancer activity. RSC Adv. 2015, 5, 35608–35621. [Google Scholar] [CrossRef]
- Adesina, S.K.; Idowu, O.; Ogundaini, A.O.; Oladimeji, H.; Olugbade, T.A.; Onawunmi, G.O.; Pais, M. Antimicrobial constituents of the leaves of Acalypha wilkesiana and Aacalypha hispida. Phytother. Res. 2000, 14, 467–472. [Google Scholar] [CrossRef]
- Conegero, L.d.S.; Ide, R.M.; Nazari, A.S.; Sarragiotto, M.H.; Filho, B.P.D.; Nakamura, C.V.; Carvalho, J.E.; Foglio, M.A. Chemical contituents of Alchornea glandulosa (Euphorbiaceae). Quim. Nova 2003, 26, 825–827. [Google Scholar] [CrossRef]
- Manpong, P.; Douglas, S.; Douglas, P.L.; Pongamphai, S.; Teppaitoon, W.; Kaewprakaisangkul, O. Response surface methodology applied to the extraction of phenolic compounds from Jatropha curcas linn. leaves using supercritical Co2 with a methanol co-solvent. J. Food Process Eng. 2011, 34, 1661–1681. [Google Scholar] [CrossRef]
- Gunawan-Puteri, M.D.; Kawabata, J. Novel α-glucosidase inhibitors from Macaranga tanarius leaves. Food Chem. 2010, 123, 384–389. [Google Scholar] [CrossRef]
- Tabata, H.; Katsube, T.; Moriya, K.; Utsumi, T.; Yamasaki, Y. Protective activity of components of an edible plant, Mallotus japonicus, against oxidative modification of proteins and lipids. Food Chem. 2010, 118, 548–553. [Google Scholar] [CrossRef]
- Agyare, C.; Lechtenberg, M.; Deters, A.; Petereit, F.; Hensel, A. Ellagitannins from Phyllanthus muellerianus (Kuntze) exell.: Geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine 2011, 18, 617–624. [Google Scholar] [CrossRef]
- Colombo, R.; de, L.B.A.N.; Teles, H.L.; Silva, G.H.; Bomfim, G.C.; Burgos, R.C.; Cavalheiro, A.J.; da Silva Bolzani, V.; Silva, D.H.; Pelicia, C.R.; et al. Validated HPLC method for the standardization of Phyllanthus niruri (herb and commercial extracts) using corilagin as a phytochemical marker. Biomed. Chromatogr. 2009, 23, 573–580. [Google Scholar] [CrossRef]
- Gunawan-Puteri, M.D.; Kato, E.; Kawabata, J. α-Amylase inhibitors from an Indonesian medicinal herb, Phyllanthus urinaria. J. Sci. Food Agric. 2012, 92, 606–609. [Google Scholar] [CrossRef]
- Devkota, H.P.; Basnet, P.; Yahara, S. Diterpene esters anphenolic compounds from Sapium insigne (ROYLE) BENTH. ex HOOK. Fil. Chem. Pharm. Bull. 2009, 57, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Notka, F.; Meier, G.; Wagner, R. Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo. Antivir. Res. 2004, 64, 93–102. [Google Scholar] [CrossRef]
- Okabe, S.; Suganuma, M.; Imayoshi, Y.; Taniguchi, S.; Yoshida, T.; Fujiki, H. New TNF-alpha releasing inhibitors, geraniin and corilagin, in leaves of Acer nikoense, Megusurino-ki. Biol. Pharm. Bull. 2001, 24, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Honma, A.; Koyama, T.; Yazawa, K. Antihyperglycemic effects of Japanese maple Acer amoenum leaf extract and its constituent corilagin. J. Wood Sci. 2010, 56, 507–512. [Google Scholar] [CrossRef]
- Pham, A.T.; Malterud, K.E.; Paulsen, B.S.; Diallo, D.; Wangensteen, H. DPPH radical scavenging and xanthine oxidase inhibitory activity of Terminalia macroptera leaves. Nat. Prod. Commun. 2011, 6, 1125–1128. [Google Scholar] [CrossRef]
- Lin, T.C.; Hsu, F.L.; Cheng, J.T. Antihypertensive activity of corilagin and chebulinic acid, tannins from Lumnitzera racemose. J. Nat. Prod. 1993, 56, 629–632. [Google Scholar] [CrossRef]
- Fogliani, B.; Raharivelomanana, P.; Bianchini, J.P.; Bouraima-Madjebi, S.; Hnawia, E. Bioactive ellagitannins from Cunonia macrophylla, an endemic Cunoniaceae from new Caledonia. Phytochemistry 2005, 66, 241–247. [Google Scholar] [CrossRef]
- Olennikov, D.; Chekhirova, G. “6”-Galloylpicein and other phenolic compounds from Arctostaphylos uva-ursi. Chem. Nat. Compd. 2013, 49, 1–7. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bao, Y.; Zhao, M.; Wei, X.; Jiang, Y.; Feng, C. High pressure extraction of corilagin from longan (Dimocarpus longan Lour.) fruit pericarp. Sep. Purif. Technol. 2009, 70, 41–45. [Google Scholar] [CrossRef]
- Burapadaja, S.; Bunchoo, A. Antimicrobial activity of tannins from Terminalia citrine. Planta Med. 1995, 61, 365–366. [Google Scholar] [CrossRef]
- Thitilertdecha, N.; Rakariyatham, N. Phenolic content and free radical scavenging activities in rambutan during fruit maturation. Sci. Hortic. 2011, 129, 247–252. [Google Scholar] [CrossRef]
- Avula, B.; Wang, Y.H.; Wang, M.; Shen, Y.H.; Khan, I.A. Simultaneous determination and characterization of tannins and triterpene saponins from the fruits of various species of Terminalia and Phyllantus emblica using a UHPLC-UV-MS method: Application to triphala. Planta Med. 2013, 79, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Rangkadilok, N.; Worasuttayangkurn, L.; Bennett, R.N.; Satayavivad, J. Identification and quantification of polyphenolic compounds in longan (Euphoria longana Lam.) fruit. J. Agric. Food Chem. 2005, 53, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.N.; Zhao, Y.L.; Gao, X.L.; Zhao, Z.F.; Jing, Z.; Zeng, W.C.; Yang, R.; Peng, R.; Tong, T.; Wang, L.F. Intestinal α-glucosidase inhibitory activity and toxicological evaluation of Nymphaea stellata flowers extract. J. Ethnopharmacol. 2010, 131, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.I.; Ahn, B.T.; Lee, H.B.; Kim, Y.K.; Lee, K.S.; Bok, S.H.; Kim, Y.T.; Kim, S.U. Inhibitory activity for chitin synthase II from Saccharomyces cerevisiae by tannins and related compounds. Planta Med. 2001, 67, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.K.; Nam, J.A.; Jeon, S.Y.; Kim, S.I.; Lee, H.J.; Chung, T.H.; Song, K.S. A prolyl endopeptidase-inhibiting antioxidant from Phyllanthus Ussurensis. Arch. Pharm. Res. 2003, 26, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Y.; Zhou, Y.; Jin, X.; Guan, Y.; Xu, M.; Liu, L.F. Chromatographic fingerprint and the simultaneous determination of five bioactive components of Geranium carolinianum L. water extract by high performance liquid chromatography. Int. J. Mol. Sci. 2011, 12, 8740–8749. [Google Scholar] [CrossRef]
- Ercıl, D.; Kaloga, M.; Radtke, O.A.; Sakar, M.K.; Kiderlen, A.F.; Kolodziej, H. O-galloyl flavonoids from Geranium pyrenaicum and their in vitro antileishmanial activity. Turk. J. Chem. 2005, 29, 437–443. [Google Scholar]
- Gayosso-De-Lucio, J.A.; Torres-Valencia, J.M.; Cerda-Garcia-Rojas, C.M.; Joseph Nathan, P. Ellagitannins from Geranium potentillaefolium and G. Bell. Nat. Prod. Commun. 2010, 5, 531–534. [Google Scholar]
- Zhao, X.; Yin, H. Simultaneous determination of four acids active compounds in Erodium stephanianum by RP-HPLC. Eur. PMC 2011, 36, 3137–3140. [Google Scholar]
- Latte, K.P.; Kolodziej, H. Pelargoniins, new ellagitannins from Pelargonium reniforme. Phytochemistry 2000, 54, 701–708. [Google Scholar] [CrossRef]
- Fecka, I.; Cisowski, W. Tannins and flavonoids from the Erodium cicutarium herb. Z. Nat. B 2005, 60, 555–560. [Google Scholar] [CrossRef]
- Xiao, H.T.; Tsang, S.W.; Qin, H.Y.; Choi, F.; Yang, Z.J.; Han, Q.B.; Chen, H.B.; Xu, H.X.; Shen, H.; Lu, A.P. A bioactivity-guided study on the anti-diarrheal activity of Polygonum chinense Linn. J. Ethnopharmacol. 2013, 149, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ren, L.; Chen, Y. Chemical constituents of Saururus chinensis (Lour.) Bail. Eur. PMC 1999, 24, 479–481. [Google Scholar]
- Chen, L.; Chen, R.; Wei, K. Constituents of tannins from Euphorbia prostrata ait. Eur. PMC 1992, 17, 225–226. [Google Scholar]
- Li, X.F.; Guo, Y.J.; Zhang, D.M.; Chen, Z.; Wei, X.; Li, Y.H.; Zhang, S.L.; Tao, J.Y.; Dong, J.H.; Mei, Y.W.; et al. Protective activity of the ethanol extract of Cynanchum paniculatum (BUNGE) Kitagawa on treating herpes simplex encephalitis. Int. J. Immunopathol. Pharm. 2012, 25, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Chen, C.C.; Hsu, F.L.; Chen, C.F. Two tannins from Phyllanthus tenellus. J. Nat. Prod. 1998, 61, 523–524. [Google Scholar] [CrossRef]
- Priya, O.S.; Viswanathan, M.B.; Balakrishna, K.; Venkatesan, M. Chemical constituents and in vitro antioxidant activity of Phyllanthus wightianus. Nat. Prod. Res. 2011, 25, 949–958. [Google Scholar] [CrossRef]
- Youn, K.; Jun, M. In vitro BACE1 inhibitory activity of Geraniin and corilagin from Geranium thunbergii. Planta Med. 2013, 79, 1038–1042. [Google Scholar] [CrossRef]
- Schmidt, O.T.; Lademann, R. Corilagin, ein weiterer kristallisierter Gerbstoff aus Dividivi. X. Mitteilung über natürliche Gerbstoffe, Justus Liebigs. Ann. Chem. 1951, 571, 232–237. [Google Scholar] [CrossRef]
- Liu, D.; Su, Z.; Wang, C.; Gu, M.; Xing, S. Separation and purification of hydrolysable tannin from Geranium wilfordii maxim by reversed-phase and normal-phase high speed counter-current chromatography. J. Sep. Sci. 2010, 33, 2266–2271. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xia, W. Preparative separation and purification of phenolic compounds from Canarium album L. by macroporous resins. J. Sci. Food Agric. 2008, 88, 493–498. [Google Scholar] [CrossRef]
- Da Silveira, C.V.; Trevisan, M.T.; Rios, J.B.; Erben, G.; Haubner, R.; Pfundstein, B.; Owen, R.W. Secondary plant substances in various extracts of the leaves, fruits, stem and bark of Caraipa densifolia mart. Food Chem. Toxicol. 2010, 48, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Kusuda, M.; Inada, K.; Ogawa, T.-O.; Yoshida, T.; Shiota, S.; Tsuchiya, T.; Hatano, T. Polyphenolic constituent structures of Zanthoxylum piperitum fruit and the antibacterial effects of its polymeric procyanidin on methicillin-resistant Staphylococcus aureus. Biosci. Biotechnol. Biochem. 2006, 70, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nonaka, G.I.; Nishioka, I. Punicafolin, an ellagitannin from the leaves of Punica. Granatum. Phytochem. 1985, 24, 2075–2078. [Google Scholar] [CrossRef]
- Kakiuchi, N.; Hattori, M.; Namba, T.; Nishizawa, M.; Yamagishi, T.; Okuda, T. Inhibitory effect of tannins on reverse transcriptase from RNA tumor virus. J. Nat. Prod. 1985, 48, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Kishi, N.; Koshiura, R.; Yoshida, T.; Hatano, T.; Okuda, T. Relationship between the structures and the antitumor activities of tannins. Chem. Pharm. Bull. 1987, 35, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Medicinal attributes of Solanum xanthocarpum fruit consumed by several tribal communities as food: An in vitro antioxidant, anticancer and anti HIV perspective. BMC Complement. Altern. Med. 2014, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.E.; MacKenzie, L.; Shultis, E.A.; Chan, J.A.; Hecht, S.M. Naturally occurring inhibitors of topoisomerase I mediated DNA relaxation. J. Org. Chem. 1992, 57, 420–422. [Google Scholar] [CrossRef]
- Hecht, S.M.; Berry, D.E.; MacKenzie, L.J.; Busby, R.W.; Nasuti, C.A. A strategy for identifying novel, mechanistically unique inhibitors of topoisomerase-I. J. Nat. Prod. 1992, 55, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Komori, A.; Yatsunami, J.; Suganuma, M.; Okabe, S.; Abe, S.; Sakai, A.; Sasaki, K.; Fujiki, H. Tumor necrosis factor acts as a tumor promoter in BALB/3T3 cell transformation. Cancer Res. 1993, 53, 1982–1985. [Google Scholar] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Takagi, A.; Kano, M.; Kaga, C. Possibility of breast cancer prevention: Use of soy isoflavones and fermented soy beverage produced using probiotics. Int. J. Mol. Sci. 2015, 16, 10907. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Bishayee, A.; Pandey, A.K. Targeting histone deacetylases with natural and synthetic agent: An emerging anticancer strategy. Nutrients 2018, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.J.; Lin, S.C.; Dong, M.Q.; Han, J. RIP3, an energy metabolism regulator that switches tnf-induced cell death from apoptosis to necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor Interacting Protein Kinase-3 Determines Cellular Necrotic Response to TNF-α. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef]
- Cho, Y.S.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K.M. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virusinduced inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef]
- Singal, A.K.; Vauthey, J.N.; Grady, J.J.; Stroehlein, J.R. Intrahepatic cholangiocarcinoma—Frequency and demographic patterns: Thirty-year data from the M.D. Anderson Cancer Center. J. Cancer Res. Clin. Oncol. 2011, 137, 1071–1078. [Google Scholar] [CrossRef]
- Al-Bahrani, R.; Abuetabh, Y.; Zeitouni, N.; Sergi, C. Cholangiocarcinoma: Risk factors, environmental influences and oncogenesis. Ann. Clin. Lab. Sci. 2013, 43, 195–210. [Google Scholar]
- Poggi, G.; Quaretti, P.; Minoia, C.; Palumbo, I.; Villani, L.; Amatu, A.; Teragni, C.; Scelsi, M.; Zappoli, F.; Bernardo, G. Oxaliplatin-eluting microspheres for the treatment of intrahepatic cholangiocarcinoma: A case report. Anticancer Res. 2008, 28, 2987–2990. [Google Scholar] [PubMed]
- Shitara, K.; Ikami, I.; Munakata, M.; Muto, O.; Sakata, Y. Hepatic arterial infusion of mitomycin C with degradable starch microspheres for unresectable intrahepatic cholangiocarcinoma. Clin. Oncol. 2008, 20, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Huppert, S.S.; Jacobsen, T.L.; Muskavitch, M.A. Feedback regulation is central to Delta-Notch signalling required for Drosophila wing vein morphogenesis. Development 1997, 124, 3283–3291. [Google Scholar] [PubMed]
- Gu, Y.; Xiao, L.; Ming, Y.; Zheng, Z.; Li, W. Corilagin suppresses cholangiocarcinoma progression through Notch signaling pathway in vitro and in vivo. Int. J. Oncol. 2016, 48, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Liu, L.; Lin, Y.; Yang, Z.; Qiu, F. Corilagin inhibits esophageal squamous cell carcinoma by inducing DNA damage and down-regulation of RNF8. Anticancer Agent Med. Chem. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, J.; Cai, M.; Zhu, Z.; Gu, W.; Yu, Y.; Zhang, X. DBGC: A database of human gastric cancer. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.B.; Qu, Z.Y.; Zou, X. Juglone-induced apoptosis in human gastric cancer SGC-7901 cells via the mitochondrial pathway. Exp. Toxicol. Pathol. 2011, 63, 69–78. [Google Scholar] [CrossRef]
- Dai, Z.J.; Gao, J.; Ji, Z.Z.; Wang, X.J.; Ren, H.T.; Liu, X.X.; Wu, W.Y.; Kang, H.F.; Guan, H.T. Matrine induces apoptosis in gastric carcinoma cells via alteration of Fas/FasL and activation of caspase-3. J. Ethnopharmacol. 2009, 123, 91–96. [Google Scholar] [CrossRef]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, G.; Tong, Y.; Yuan, J.; Li, Y.; Song, G. Corilagin induces apoptosis, autophagy and ROS generation in gastric cancer cells in vitro. Int. J. Mol. Med. 2019, 43, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Alves, D.; Guz, B.; Matos, C.; Viana, M.; Harriz, M.; Terrabuio, D.; Kondo, M.; Gampel, O.; Polletti, P. Advanced hepatocellular carcinoma. Review of targeted molecular drugs. Ann. Hepatol. 2011, 10, 21–27. [Google Scholar] [PubMed]
- Singh, A.K.; Kumar, R.; Pandey, A.K. Hepatocellular carcinoma: Causes, mechanism of progression and biomarkers. Curr. Chem. Genom. Transl. Med. 2018, 12, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Thorgeirsson, S.S.; Gail, M.H.; Lu, P.; Harris, C.C.; Wang, N.; Shao, Y.; Wu, Z.; Liu, G.; Wang, X.; et al. Dominant role of hepatitis B virus and cofactor role of aflatoxin in hepatocarcinogenesis in Qidong, China. Hepatology 2002, 36, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J. Non-surgical treatment of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2005, 7, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.T.; Mau, L.C.; Poon, R.T.; Yeung, C.; Leung, L.C.; Yuen, W.K.; Ming, L.C.; Ng, K.K.; Ching, C.S. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: A 20-year experience. Ann. Surg. 2011, 253, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.L.; Zheng, Z.Z.; Chen, L.H.; Zheng, G.H.; Liu, S.S.; Yu, Y.; Tong, Q.X. Corilagin inhibits hepatocellular carcinoma cell proliferation by inducing G2/M phase arrest. Cell Biol. Int. 2013, 37, 1046–1054. [Google Scholar] [CrossRef]
- Abbas, T.; Sivaprasad, U.; Terai, K.; Amador, V.; Pagano, M.; Dutta, A. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev. 2008, 22, 2496–2506. [Google Scholar] [CrossRef]
- Coutts, A.S.; Adams, C.J.; La Thangue, N.B. p53 Ubiquitination by Mdm2: A never ending tail? DNA Repair 2009, 8, 483–490. [Google Scholar] [CrossRef]
- Deng, Y.; Li, X.; Li, X.; Zheng, Z.; Huang, W.; Chen, L.; Tong, Q.; Ming, Y. Corilagin induces the apoptosis of hepatocellular carcinoma cells through the mitochondrial apoptotic and death receptor pathways. Oncol. Rep. 2018, 39, 2545–2552. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von-Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Chen, X.; Ma, X.; Wang, D.; Guo, Z. The efficacy and safety of various dosedense regimens of temozolomide for recurrent high-grade glioma: A systematic review with meta-analysis. J. Neurooncol. 2015, 125, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Seystahl, K.; Wick, W.; Weller, M. Therapeutic options in recurrent glioblastoma-An update. Crit. Rev. Oncol. Hematol. 2016, 99, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, K.; Clavreul, A.; Lagarce, F. Toward an effective strategy in glioblastoma treatment. Part I: Resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov. Today 2015, 20, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Milani, R.; Brognara, E.; Fabbri, E.; Finotti, A.; Borgatti, M.; Lampronti, I.; Marzaro, G.; Chilin, A.; Lee, K.K.H.; Kok, S.H.L.; et al. Corilagin induces high levels of apoptosis in the temozolomide-resistant T98G glioma cell line. Oncol. Res. 2018, 26, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Li, G.H.; Li, Z.Y.; Feng, S.; Liu, X.Q.; Han, G.K.; Zhang, H.; Qin, X.Y.; Zhang, R.; Nie, Q.M.; et al. Effect of corilagin on the proliferation and NF-κB in U251 glioblastoma cells and U251 glioblastoma stem-like cells. Evid. Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Doufekas, K.; Olaitan, A. Clinical epidemiology of epithelial ovarian cancer in the UK. Int. J. Womens Health 2014, 6, 537–545. [Google Scholar] [PubMed] [Green Version]
- Cheng, J.C.; Auersperg, N.; Leung, P.C. TGF-beta induces serous borderline ovarian tumor cell invasion by activating EMT but triggers apoptosis in low-grade serous ovarian carcinoma cells. PLoS ONE. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.D.; Bellone, S.; Ravaggi, A.; Roman, J.; Smith, C.V.; Pecorelli, S.; Cannon, M.J.; Parham, G.P. Increased levels of interleukin-10 and transforming growth factor-beta in the plasma and ascitic fluid of patients with advanced ovarian cancer. Brit. J. Obstet. Gynaec. 2001, 108, 804–808. [Google Scholar] [CrossRef] [Green Version]
- Do, T.V.; Kubba, L.A.; Du, H.; Sturgis, C.D.; Woodruff, T.K. Transforming growth factor-beta1, transforming growth factor-beta2, and transforming growth factor-beta3 enhance ovarian cancer metastatic potential by inducing a Smad3-dependent epithelial-to-mesenchymal transition. Mol. Cancer Res. 2008, 6, 695–705. [Google Scholar] [CrossRef]
- Ten Dijke, P.; Hill, C.S. New insights into TGF-β-Smad signalling. Trends Biochem. Sci. 2004, 29, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Nuclear factor-kappaB: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, R.Z.; Baldwin, A.S., Jr. NF-kappaB as a therapeutic target in cancer. Trends Mol. Med. 2002, 8, 385–389. [Google Scholar] [CrossRef]
- Lee, D.W.; Ramakrishnan, D.; Valenta, J.; Parney, I.F.; Bayless, K.J.; Sitcheran, R. The NF-κB RelB protein is an oncogenic driver of mesenchymal glioma. PLoS ONE 2013, 8, e57489. [Google Scholar] [CrossRef]
- Jane, E.P.; Premkumar, D.R.; Pollack, I.F. Bortezomib sensitizes malignant human glioma cells to TRAIL, mediated by inhibition of the NF-κB signaling pathway. Mol. Cancer 2011, 10, 198–208. [Google Scholar] [CrossRef]
- Baud, V.; Karin, M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef]
- Vanden Berghe, W.; Plaisance, S.; Boone, E.; De Bosscher, K.; Lienhard Schmitz, M.; Fiers, W.; Haegeman, G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for NF-jB p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 1998, 273, 3285–3290. [Google Scholar] [CrossRef]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. marine sponge natural products with anticancer potential: An updated review. Mar. Drugs 2017, 15, 310. [Google Scholar] [CrossRef]
- Angulo, P.; Kaushik, G.; Subramaniam, D.; Dandawate, P.; Neville, K.; Chastain, K.; Anant, S. Natural compounds targeting major cell signaling pathways: A novel paradigm for osteosarcoma therapy. J. Hematol. Oncol. 2017, 10. [Google Scholar] [CrossRef]
- Gridley, T. Notch signaling and inherited disease syndromes. Hum. Mol. Genet. 2003, 12, R9–R13. [Google Scholar] [CrossRef]
- Kopan, R.; Ilagan, M.X. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Sayed, V. TGF-β signaling in Cancer. J. Cell. Biochem. 2016, 117, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Hyytiainen, M.; Penttinen, C.; Keski-Oja, J. Latent TGF-beta binding proteins: Extracellular matrix association and roles in TGF-beta activation. Crit. Rev. Clin. Lab. Sci. 2004, 41, 233–264. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.C.; Haisley, C.; Hurteau, J.; Moser, T.L.; Whitaker, R.; Bast, R.C., Jr.; Stack, M.S. Regulation of invasion of epithelial ovarian cancer by transforming growth factor-β. Gynecol. Oncol. 2001, 80, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Siegel, P.M.; Massague, J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat. Rev. Cancer 2003, 3, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Vu, M.; Booker, T.; Santner, S.J.; Miller, F.R.; Anver, M.R.; Wakefield, L.M. TGF-β switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J. Clin. Investig. 2003, 112, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, Z.; Fu, X. The primary study on the inhibition effect of corilagin on human pancreatic cancer cell line Bxpc-3 cells. J. Jinzhou Med. Coll. 2005, 26, 25–27. [Google Scholar]
- Kang, J.; Zhang, Y.; Chen, D.; Ma, X.; Lu, Y. Study on apoptosis of laryngeal carcinoma Hep-2 induced by corilagin. Pharm. Clin. Chin. Mater. Med. 2012, 28, 24–27. [Google Scholar]
- Liu, Z.; Wang, D.; Chen, Y.; Ren, L.; Xu, J.; Li, K.; Wang, Q.; Zhang, W. Studies on antitumor activity, mutagenicity action by corilagin. China J. Cancer Prevent. Treat. 2003, 10, 469–472. [Google Scholar]
| Plant Part | Plant Species | Reference | Plant Part | Plant Species | Reference |
|---|---|---|---|---|---|
| Leaf | Terminalia catappa L. | [37] | Leaf | Acalypha wilkesiana Mull. Arg. | [46] |
| Acalypha hispida Burm. f. | [46] | Alchornea glandulosa Poepp. | [47] | ||
| Jatropha curcas L. | [48] | Macaranga tanarius Mull. Arg. | [49] | ||
| Mallotus japonicus Mull. Arg. | [50] | Phyllanthus muellerianus (Kuntze) Exell. | [51] | ||
| Phyllanthus niruri L. | [52] | Phyllanthus urinaria L. | [53] | ||
| Sapium insigne (Royle) Benth.ex Hook.fil | [54] | Phyllanthus amarus Schumach. and Thonn. | [55] | ||
| Acer nikoense (Miq.) Maxim. | [56] | Acer amoenum (Carriere) | [57] | ||
| Terminalia macroptera Guill. and Perr. | [58] | Lumnitzera racemosa Willd. | [59] | ||
| Cunonia macrophylla Brongn. and Gris | [60] | Arctostaphylos uva-ursi (L.) Spreng. | [61] | ||
| Fruit rind | Dimocarpus longan Lour. | [62] | Fruit rind | Terminalia citrina (Gaertn.) Roxb. | [63] |
| Nephelium lappaceum L. | [64] | Terminalia arjuna | [65] | ||
| Terminalia. chebula Retz. | [65] | ||||
| Seed, peel | Euphorbia longana Lam. | [66] | Flower | Nymphaea stellata Willd. | [67] |
| Aerial part | Geranium sibiricum L. | [37] | Aerial part | Euphorbia pekinensis Rupr. | [68] |
| Phyllanthus ussuriensis Rupr. et Maxim. | [69] | Geranium carolinianum L. | [70] | ||
| Geranium pyrenaicum Burm.f. | [71] | Geranium potentillifolium DC. | [72] | ||
| Geranium bellum Rose | [72] | Erodium stephanianum Willd. | [73] | ||
| Pelargonium reniforme Spreng. | [74] | Erodium cicutarium L’Hér. ex Aiton | [75] | ||
| Polygonum chinense L. | [76] | Saururus chinensis (Lour.) Bail. | [77] | ||
| Whole plant | Euphorbia prostrata Aiton | [78] | Whole plant | Excoecaria agallocha L. | [79] |
| Cynanchum paniculatum (bunge) kitagawa | [79] | Phyllanthus tenellus Roxb | [80] | ||
| Phyllanthus wightianus Müll.Arg. | [81] | Geranium thunbergii Siebold ex Lindl. and Paxton | [82] | ||
| Caesalpinia coriaria (Jacq.) Willd. | [83] | Geranium wilfordii Maxim | [84] | ||
| Fruit | Phyllanthus emblica L. | [34] | Terminalia bellerica (Gaertn.) Roxb. | [65] | |
| Canarium album L. | [85] | Caraipa densifolia Mart. | [86] | ||
| Zanthoxylum piperitum (L.) DC. | [87] | ||||
| Cancer Type | Cell Line | Effect | Mechanism | Reference |
|---|---|---|---|---|
| Breast cancer | MCF-7, SK-BR3 | Apoptosis, autophagic cell death, necroptosis | ↓Procaspase-3, ↓procaspase-8, ↓procaspase-9, ↓PARP, ↓Bcl-2, ↑caspase-8, ↑caspase-9, ↑Bax, ↑cleaved PARP | [28] |
| Cholangiocarcinoma | QBC9939, MZ-Cha-1 | Antiproliferation, G2/M arrest | ↓Bcl-2, ↑caspase-3, ↑p-Akt, ↑pErk1/2 | [107] |
| Esophageal squamous cell carcinoma (ESCC) | ESCC cells | Antiproliferation, apoptosis | ↑DNA damage, ↓DNA repair, ↓E3 ubiquitin ligase RNF8 | [108] |
| Gastric carcinoma | SGC7901, BGC823 | Apoptosis, antiproliferation | ↑Caspase-3, ↑caspase-8, ↑caspase-9, ↑PARP | [114] |
| Glioblastoma multiforme | U251, T98G | Antiproliferation, apoptosis | ↑Caspase-3, ↑caspase-7 | [128] |
| Hepatocellular carcinoma | Bel7402, SMMC7721 | Antiproliferation, G2/M arrest | ↓p-Akt, ↓PCNA, ↑p-p53, ↑caspase-3, ↑caspase-9 | [120,123] |
| Lung cancer | A549 | Antiproliferation | ↑ DNA damage | [30] |
| Ovarian cancer | A2780, SKOv3ip, Hey | Apoptosis, G2/M arrest | ↓Cyclin B1, ↓Myt1, ↓phospho-cdc2, ↓phospho-Weel | [40] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, A.; Singh, A.K.; Kumar, R.; Ganguly, R.; Rana, H.K.; Pandey, P.K.; Sethi, G.; Bishayee, A.; Pandey, A.K. Corilagin in Cancer: A Critical Evaluation of Anticancer Activities and Molecular Mechanisms. Molecules 2019, 24, 3399. https://doi.org/10.3390/molecules24183399
Gupta A, Singh AK, Kumar R, Ganguly R, Rana HK, Pandey PK, Sethi G, Bishayee A, Pandey AK. Corilagin in Cancer: A Critical Evaluation of Anticancer Activities and Molecular Mechanisms. Molecules. 2019; 24(18):3399. https://doi.org/10.3390/molecules24183399
Chicago/Turabian StyleGupta, Ashutosh, Amit Kumar Singh, Ramesh Kumar, Risha Ganguly, Harvesh Kumar Rana, Prabhash Kumar Pandey, Gautam Sethi, Anupam Bishayee, and Abhay K. Pandey. 2019. "Corilagin in Cancer: A Critical Evaluation of Anticancer Activities and Molecular Mechanisms" Molecules 24, no. 18: 3399. https://doi.org/10.3390/molecules24183399







