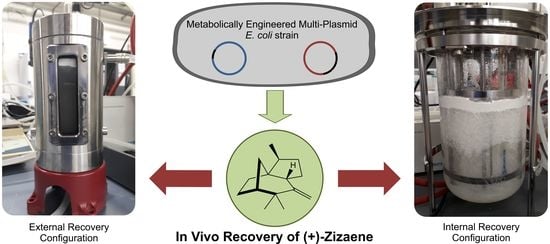
Improved Production and In Situ Recovery of Sesquiterpene (+)-Zizaene from Metabolically-Engineered E. coli
Abstract
:1. Introduction
2. Results
2.1. Product Volatilization Measurements and (+)-Zizaene Recovery by LLPPC
2.2. Screening of Polymeric Adsorbers for SLPPC
2.3. Assessment of Organic Solvents for the Desorption of (+)-Zizaene
2.4. Integration of In Situ Recovery of (+)-Zizaene to Fermentation at Bioreactor Scale
2.5. Bioreactor Cultivation with an Integrated ERC
2.6. Bioreactor Cultivation with an Integrated IRC
2.7. Bioreactor Cultivation with an Integrated IRC+GS
2.8. Accumulative (+)-Zizaene Production from the Bioreactor Configurations
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Strain and Pre-Cultures
4.3. Product Volatilization and LLPPC Experiments
4.4. Screening of Polymeric Adsorbers
4.5. Evaluation of Organic Solvents for the (+)-Zizaene Desorption
4.6. Bioreactor Cultivations with In Situ Recovery of (+)-Zizaene
4.6.1. IRC Bioreactor
4.6.2. ERC Bioreactor
4.7. Analytical procedures
4.7.1. Growth Kinetics Analysis
4.7.2. Soluble ZS Protein Fraction Analysis
4.7.3. Sample extraction
4.7.4. GC-MS analysis
4.7.5. GC-FID analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T.S. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2011, 2, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 2013, 496, 528–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westfall, P.J.; Gardner, T.S. Industrial fermentation of renewable diesel fuels. Curr. Opin. Biotechnol. 2011, 22, 344–350. [Google Scholar] [CrossRef]
- Leavell, M.D.; McPhee, D.J.; Paddon, C.J. Developing fermentative terpenoid production for commercial usage. Curr. Opin. Biotechnol. 2016, 37, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Lavania, A.C. Other uses, and utilization of vetiver: Vetiver Oil. In Proceedings of the Third International Conference on Vetiver; The Vetiver Network International: Guangzhou, China, 2003; p. 486. [Google Scholar]
- Sanganeria, P. Essential Oils Market Report Summer 2018; Report summer 2018; Ultra International B.V: Spijkenisse, The Netherlands, 2018; pp. 36–39. [Google Scholar]
- Belhassen, E.; Filippi, J.J.; Brévard, H.; Joulain, D.; Baldovini, N. Volatile constituents of vetiver: A review. Flavour Fragr. J. 2015, 30, 26–82. [Google Scholar] [CrossRef]
- Pripdeevech, P.; Wongpornchai, S.; Marriott, P.J. Comprehensive two-dimensional gas chromatography-mass spectrometry analysis of volatile constituents in Thai vetiver root oils obtained by using different extraction methods. Phytochem. Anal. 2010, 163–173. [Google Scholar] [CrossRef]
- Aguilar, F.; Scheper, T.; Beutel, S. Modulating the precursor and terpene synthase supply for the whole-cell biocatalytic production of the sesquiterpene (+)-zizaene in a pathway engineered E. coli. Genes 2019, 10, 478. [Google Scholar] [CrossRef]
- Aguilar, F.; Hartwig, S.; Scheper, T.; Beutel, S. Catalytical specificity, reaction mechanisms, and conformational changes during catalysis of the recombinant SUMO (+)-zizaene synthase from Chrysopogon zizanioides. ACS Omega 2019, 4, 6199–6209. [Google Scholar] [CrossRef]
- Halka, L.; Wichmann, R. Enhanced production and in situ product recovery of fusicocca-2,10(14)-diene from yeast. Fermentation 2018, 4, 65. [Google Scholar] [CrossRef]
- Tippmann, S.; Scalcinati, G.; Siewers, V.; Nielsen, J. Production of farnesene and santalene by Saccharomyces cerevisiae using fed-batch cultivations with RQ-controlled feed. Biotechnol. Bioeng. 2016, 113, 72–81. [Google Scholar] [CrossRef]
- Qiao, J.; Luo, Z.; Cui, S.; Zhao, H.; Tang, Q.; Mo, C.; Ma, X.; Ding, Z. Modification of isoprene synthesis to enable production of curcurbitadienol synthesis in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2019, 46, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Kim, S.K.; Yoon, P.K.S.; Kang, Y.; Kim, B.S.; Fu, Y.; Sung, B.H.; Jung, H.C.; Lee, D.H.; Kim, S.W.; et al. Fermentative production and direct extraction of (−)-α-bisabolol in metabolically engineered Escherichia coli. Microb. Cell Fact. 2016, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schewe, H.; Mirata, M.A.; Schrader, J. Bioprocess engineering for microbial synthesis and conversion of isoprenoids. In Biotechnology of Isoprenoids. Advances in Biochemical Engineering/Biotechnology; Schrader, J., Bohlmann, J., Eds.; Springer: Cham, Switzerland, 2015; pp. 251–286. [Google Scholar]
- Schügerl, K.; Hubbuch, J. Integrated bioprocesses. Curr. Opin. Microbiol. 2005, 8, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Buque-Taboada, E.M.; Straathof, A.J.J.; Heijnen, J.J.; Van Der Wielen, L.A.M. In situ product recovery (ISPR) by crystallization: Basic principles, design, and potential applications in whole-cell biocatalysis. Appl. Microbiol. Biotechnol. 2006, 71, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, J.; Bont, J.; Poolman, B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994, 269, 8022–8028. [Google Scholar] [PubMed]
- Bruce, L.J.; Daugulis, A.J. Solvent selection strategies for extractive biocatalysis. Biotechnol. Prog. 1991, 7, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Willrodt, C.; David, C.; Cornelissen, S.; Bühler, B.; Julsing, M.K.; Schmid, A. Engineering the productivity of recombinant Escherichia coli for limonene formation from glycerol in minimal media. Biotechnol. J. 2014, 9, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Boghigian, B.A.; Myint, M.; Wu, J.; Pfeifer, B.A. Simultaneous production and partitioning of heterologous polyketide and isoprenoid natural products in an Escherichia coli two-phase bioprocess. J. Ind. Microbiol. Biotechnol. 2011, 38, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Gionata, S.; Scalcinati, G.; Partow, S.; Siewers, V.; Schalk, M.; Daviet, L.; Nielsen, J. Combined metabolic engineering of precursor and co-factor supply to increase α-santalene production by Saccharomyces cerevisiae. Microb. Cell Fact. 2012, 11, 117. [Google Scholar] [CrossRef]
- Tsuruta, H.; Paddon, C.J.; Eng, D.; Lenihan, J.R.; Horning, T.; Anthony, L.C.; Regentin, R.; Keasling, J.D.; Renninger, N.S.; Newman, J.D. High-level production of amorpha-4,11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli. PLoS ONE 2009, 4, e4489. [Google Scholar] [CrossRef]
- Dafoe, J.T.; Daugulis, A.J. In situ product removal in fermentation systems: Improved process performance and rational extractant selection. Biotechnol. Lett. 2014, 36, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Gutierrez, J.; Chan, R.; Batt, T.S.; Adams, P.D.; Keasling, J.D.; Petzold, C.J.; Lee, T.S.; Adams, P.D. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng. 2013, 19, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.E.; Hart-Wells, E.A.; Matsuda, S.P.T. Metabolic engineering to produce sesquiterpenes in yeast. Org. Lett. 2003, 5, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Bormann, S.; Etschmann, M.M.W.; Mirata, M.A.; Schrader, J. Integrated bioprocess for the stereospecific production of linalool oxides from linalool with Corynespora cassiicola DSM 62475. J. Ind. Microbiol. Biotechnol. 2012, 39, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M.J.; Dossani, Z.Y.; Szmidt, H.L.; Chu, H.C.; Lee, T.S.; Keasling, J.D.; Hadi, M.Z.; Mukhopadhyay, A. Engineering microbial biofuel tolerance and export using efflux pumps. Mol. Syst. Biol. 2011, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Xiong, Z.Q.; Li, S.Y.; Wang, Y. Enhancing isoprenoid production through systematically assembling and modulating efflux pumps in Escherichia coli. Appl. Microbiol. Biotechnol. 2013, 97, 8057–8067. [Google Scholar] [CrossRef] [PubMed]
- Southall, N.T.; Dill, K.A.; Haymet, A.D.J. A view of the hydrophobic effect. J. Phys. Chem. B 2002, 106, 521–533. [Google Scholar] [CrossRef]
- Butt, H.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; Wiley-VCH Verlag GmbH & Co. KGaA: Heidelberg, Germany, 2003; pp. 177–205. [Google Scholar] [CrossRef]
- Grozdev, L.; Kaiser, J.; Berensmeier, S. One-step purification of microbially produced hydrophobic terpenes via process chromatography. Front. Bioeng. Biotechnol. 2019, 7, 1–12. [Google Scholar] [CrossRef]
- Freeman, A.; Woodley, J.M.; Lilly, M.D. In situ product removal as a tool for bioprocessing. Nat. Biotechnol. 1993, 11, 1007–1012. [Google Scholar] [CrossRef]
- Schempp, F.M.; Drummond, L.; Buchhaupt, M.; Schrader, J. Microbial cell factories for the production of terpenoid flavor and fragrance compounds. J. Agric. Food Chem. 2018, 66, 2247–2258. [Google Scholar] [CrossRef]
- Stark, D.; von Stockar, U. In situ product removal (ISPR) in whole cell biotechnology during the last twenty years. In Process Integration in Biochemical Engineering; Springer: Berlin/Heidelberg, Germany, 2007; Volume 80, pp. 149–175. [Google Scholar] [CrossRef]
- Moser, S.; Pichler, H. Identifying and engineering the ideal microbial terpenoid production host. Appl. Microbiol. Biotechnol. 2019, 103, 5501–5516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrish, J.L.E.; Daugulis, A.J. Improved reactor performance and operability in the biotransformation of carveol to carvone using a solid-liquid two-phase partitioning bioreactor. Biotechnol. Bioeng. 2008, 101, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, M.C.; Sraathof, A.J.J. Improving fermentation by product removal. In Intensification of Biobased Processes; Gorak, A., Stankiewicz, A., Eds.; Royal Society of Chemistry: London, UK, 2018; pp. 86–108. [Google Scholar]
- Krings, U.; Berger, R.G. In situ recovery of the aroma compound perillene from stirred-tank cultured Pleurotus ostreatus using gas stripping and adsorption on polystyrene. Biotechnol. Lett. 2008, 30, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Ling, T.C.; Mustafa, S.; Tam, Y.J.; Ramanan, R.N.; Ariff, A.B. An integrated bioreactor-expanded bed adsorption system for the removal of acetate to enhance the production of alpha-interferon-2b by Escherichia coli. Process Biochem. 2013, 48, 551–558. [Google Scholar] [CrossRef]
- Alemdar, S.; König, J.C.; Hartwig, S.; Frister, T.; Scheper, T.; Beutel, S. Bioproduction of α-humulene in metabolically engineered Escherichia coli and application in zerumbone synthesis. Eng. Life Sci. 2017, 17, 900–907. [Google Scholar] [CrossRef]
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/episys/ep1563221.html (accessed on 20 June 2019).
- Newman, J.D.; Marshall, J.; Chang, M.; Nowroozi, F.; Paradise, E.; Pitera, D.; Newman, K.L.; Keasling, J.D. High-level production of amorpha-4,11-diene in a two-phase partitioning bioreactor of metabolically engineered Escherichia coli. Biotechnol. Bioeng. 2006, 95, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Morrish, J.L.E.; Brennan, E.T.; Dry, H.C.; Daugulis, A.J. Enhanced bioproduction of carvone in a two-liquid-phase partitioning bioreactor with a highly hydrophobic biocatalyst. Biotechnol. Bioeng. 2008, 101, 768–775. [Google Scholar] [CrossRef]
- Westfall, P.J.; Pitera, D.J.; Lenihan, J.R.; Eng, D.; Woolard, F.X.; Regentin, R.; Horning, T.; Tsuruta, H.; Melis, D.J.; Owens, A.; et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc. Natl. Acad. Sci. USA 2012, 109, E111–E118. [Google Scholar] [CrossRef]
- Lomascolo, A.; Lesage-Meessen, L.; Labat, M.; Navarro, D.; Delattre, M.; Asther, M. Enhanced benzaldehyde formation by a monokaryotic strain of Pycnoporus cinnabarinus using a selective solid adsorbent in the culture medium. Can. J. Microbiol. 1999, 45, 653–657. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, S.W.; Hong, S.I. An integrated fermentation-separation process for the production of red pigment by Serratia sp. KH-95. Process Biochem. 1999, 35, 485–490. [Google Scholar] [CrossRef]
- Amsden, B.G.; Bochanysz, J.; Daugulis, A.J. Degradation of xenobiotics in a partitioning bioreactor in which the partitioning phase is a polymer. Biotechnol. Bioeng. 2003, 84, 399–405. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.R.; Van Keulen, F.; Da Fonseca, M.M.R. Production and recovery of limonene-1,2-diol and simultaneous resolution of a diastereomeric mixture of limonene-1,2-epoxide with whole cells of Rhodococcus erythropolis DCL14. Biocatal. Biotransform. 2000, 18, 223–235. [Google Scholar] [CrossRef]
- Mischko, W.; Hirte, M.; Roehrer, S.; Engelhardt, H.; Mehlmer, N.; Minceva, M.; Brück, T. Modular biomanufacturing for a sustainable production of terpenoid-based insect deterrents. Green Chem. 2018, 20, 2637–2650. [Google Scholar] [CrossRef]
- Bae, J.; Moon, H.; Oh, K.K.; Kim, C.H.; Sil Lee, D.; Kim, S.W.; Hong, S.I. A novel bioreactor with an internal adsorbent for integrated fermentation and recovery of prodigiosin-like pigment produced from Serratia sp. KH-95. Biotechnol. Lett. 2001, 23, 1315–1319. [Google Scholar] [CrossRef]
- Henke, N.A.; Wichmann, J.; Baier, T.; Frohwitter, J.; Lauersen, K.J.; Risse, J.M.; Peters-Wendisch, P.; Kruse, O.; Wendisch, V.F. Patchoulol production with metabolically engineered Corynebacterium glutamicum. Genes 2018, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Nybo, S.E.; Saunder, J.; McCormick, S. Metabolic engineering of Escherichia coli for production of valerenadiene. J. Biotechnol. 2017, 262, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yoon, S.H.; Shah, A.A.; Chung, Y.R.; Kim, J.Y.; Choi, E.S.; Keasling, J.D.; Kim, S.W. Farnesol production from Escherichia coli by harnessing the exogenous mevalonate pathway. Biotechnol. Bioeng. 2010, 107, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kessler, W.; Van Den Heuvel, J.; Rinas, U. Simple defined autoinduction medium for high-level recombinant protein production using T7-based Escherichia coli expression systems. Appl. Microbiol. Biotechnol. 2011, 91, 1203–1213. [Google Scholar] [CrossRef]
- Ude, C.; Ben-Dov, N.; Jochums, A.; Li, Z.; Segal, E.; Scheper, T.; Beutel, S. Online analysis of protein inclusion bodies produced in E. coli by monitoring alterations in scattered and reflected light. Appl. Microbiol. Biotechnol. 2016, 100, 4147–4159. [Google Scholar] [CrossRef]
- Lamy, E.; Rodrigues, L.; Guerreiro, O.; Soldado, D.; Francisco, A.; Lima, M.; Silva, F.C.E.; Lopes, O.; Santos-Silva, J.; Jerónimo, E. Changes in salivary protein composition of lambs supplemented with aerial parts and condensed tannins: Extract from Cistus ladanifer L.—A preliminary study. Agrofor. Syst. 2019, 4, 1–9. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |










| Variable | ERC | IRC | IRC+GS |
|---|---|---|---|
| Accumulative titer (mg L−1) | 98.78 ± 3.87 | 211.13 ± 1.97 | 208.41 ± 7.29 |
| Titer from adsorbers (mg L−1) | 93.42 ± 3.30 | 207.84 ± 1.68 | 203.44 ± 6.89 |
| Productivity (mg L−1 h−1) | 1.50 ± 0.06 | 3.20 ± 0.03 | 3.16 ± 0.11 |
| YP/X 1 (mgzizaene gDCW−1) | 12.32 ± 0.56 | 20.45 ± 6.98 | 21.41 ± 2.29 |
| YX/S 2 (gDCW gglucose−1) | 0.22 ± 0.003 | 0.28 ± 0.11 | 0.30 ± 0.02 |
| YP/S 3 (mgzizaene gglucose−1) | 2.69 ± 0.08 | 5.82 ± 0.09 | 6.52 ± 0.18 |
| Soluble ZS protein (mg L−1) | 35.42 ± 3.20 | 143.20 ± 2.95 | 129.80 ± 6.51 |
| Adsorber recovery ratio (%) | 94.60 ± 0.14 | 98.40 ± 0.21 | 97.60 ± 0.12 |
| Properties | Amberlite IRA400 Cl | Lewatit 1064 MD | Amberlite XAD16N | Amberlite XAD4 | Diaion HP20 |
|---|---|---|---|---|---|
| Particle size (µm) | 600–750 | 440–540 | 560–710 | 490–690 | 250–800 |
| Mean pore size (radius) (Å) | 100 | 50 | 200 | 100 | 290 |
| Surface area (m2 g−1) | ‒ | 800 | 800 | 750 | 590 |
| Pore volume (mL g−1) | ‒ | 1.2 | 0.55 | 0.5 | 1.3 |
| Particle density (mg L−1) | 1.06–1.09 | 1.02 | 1.02 | 1.02 | 1.01 |
| Functional groups | Dimethyl ethanol ammonium | ‒ | ‒ | ‒ | ‒ |
| Ionic form | Basic anion exchange | Non-ionic | Non-ionic | Non-ionic | Non-ionic |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, F.; Scheper, T.; Beutel, S. Improved Production and In Situ Recovery of Sesquiterpene (+)-Zizaene from Metabolically-Engineered E. coli. Molecules 2019, 24, 3356. https://doi.org/10.3390/molecules24183356
Aguilar F, Scheper T, Beutel S. Improved Production and In Situ Recovery of Sesquiterpene (+)-Zizaene from Metabolically-Engineered E. coli. Molecules. 2019; 24(18):3356. https://doi.org/10.3390/molecules24183356
Chicago/Turabian StyleAguilar, Francisco, Thomas Scheper, and Sascha Beutel. 2019. "Improved Production and In Situ Recovery of Sesquiterpene (+)-Zizaene from Metabolically-Engineered E. coli" Molecules 24, no. 18: 3356. https://doi.org/10.3390/molecules24183356
APA StyleAguilar, F., Scheper, T., & Beutel, S. (2019). Improved Production and In Situ Recovery of Sesquiterpene (+)-Zizaene from Metabolically-Engineered E. coli. Molecules, 24(18), 3356. https://doi.org/10.3390/molecules24183356