Vasorelaxant Effect of Prunus mume (Siebold) Siebold & Zucc. Branch through the Endothelium-Dependent Pathway
Abstract
1. Introduction
2. Results
2.1. Vasorelaxant Effects of PMB in Rat Aortic Rings with Intact or Denuded Endothelium
2.2. Vasorelaxant Effect of PMB on Endothelium-Intact Aortic Rings Pre-Incubated with NG-Nitro-l-Arginine Methyl Ester (l-NAME), Indomethacin, or Combination of l-NAME and Indomethacin
2.3. Vasorelaxant Effect of PMB on Endothelium-Intact Aortic Rings Pre-Incubated with 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-One (ODQ) or Methylene Blue (MB)
2.4. Vasorelaxant Effect of PMB on Endothelium-Intact Aortic Rings Pre-Incubated with Atropine
2.5. Vasorelaxant Effect of PMB on Endothelium-Intact Aortic Rings Pre-Incubated with Various Potassium Channel Blockers
2.6. Quantitative HPLC Analysis of Compounds in PMB
3. Discussion
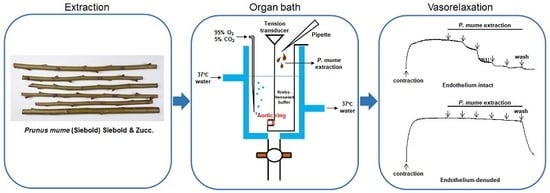
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material and Extraction
4.3. Animals
4.4. Preparation of Rat Aortic Rings
4.5. Experimental Protocols
4.6. Quantitative HPLC Analysis of Compounds in PMB
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/cardiovascular_diseases/about_cvd/en/ (accessed on 9 August 2019).
- World Health Organization. Available online: https://www.who.int/nmh/publications/ncd-profiles-2018/en/ (accessed on 10 August 2019).
- Al Disi, S.S.; Anwar, M.A.; Eid, A.H. Anti-hypertensive herbs and their mechanisms of action: Part I. Front Pharmacol 2016, 6, 323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Bentham, J.; Di Cesare, M.; Bixby, H.; Danaei, G.; Cowan, M.J.; Paciorek, C.J.; Singh, G.; Hajifathalian, K.; Bennett, J.E. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19· 1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef] [PubMed]
- Likuo, F.; Tao, H. Higher Plants of China; Qingdao Publishing House: Shandong, China, 2003; Volume 6, p. 761. [Google Scholar]
- Choi, H.; Bu, Y.; Kwon, D.; Lee, J.; Oh, M.; Seo, B. Bonchohak; Younglim-sa: Seoul, Korea, 2002; pp. 958–960. [Google Scholar]
- Zhonghua Bencao Edit Committee. Zhonghua Bencao; Shanghai Science and Technology Press: Shanghai, China, 1999; Volume 4, pp. 86–93. [Google Scholar]
- Shi, J.; Gong, J.; Liu, J.E.; Wu, X.; Zhang, Y. Antioxidant capacity of extract from edible flowers of Prunus mume in China and its active components. Lebensm. Wiss. Technol. 2009, 42, 477–482. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Ishiwada, T.; Morikawa, T.; Kagawa, M.; Higashi, Y.; Matsuda, H. New flavonol oligoglycosides and polyacylated sucroses with inhibitory effects on aldose reductase and platelet aggregation from the flowers of Prunus mume. J. Nat. Prod. 2002, 65, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.T.; Moon, J.-H.; Park, K.-H.; Shin, C.S. Isolation and characterization of a new compound from Prunus mume fruit that inhibits cancer cells. J. Agric. Food Chem. 2006, 54, 2123–2128. [Google Scholar] [CrossRef]
- Ina, H.; Yamada, K.; Matsumoto, K.; Miyazaki, T. Effects of benzyl glucoside and chlorogenic acid from Prunus mume on adrenocorticotropic hormone (ACTH) and catecholamine levels in plasma of experimental menopausal model rats. Biol. Pharm. Bull. 2004, 27, 136–137. [Google Scholar] [CrossRef][Green Version]
- Chuda, Y.; Ono, H.; Ohnishi-Kameyama, M.; Matsumoto, K.; Nagata, T.; Kikuchi, Y. Mumefural, citric acid derivative improving blood fluidity from fruit-juice concentrate of Japanese apricot (Prunus mume Sieb. et Zucc). J. Agric. Food Chem. 1999, 47, 828–831. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Takekoshi, S.; Gato, N.; Utatsu, H.; Motley, E.D.; Eguchi, K.; Fitzgerald, T.G.; Mifune, M.; Frank, G.D.; Eguchi, S. Fruit-juice concentrate of Asian plum inhibits growth signals of vascular smooth muscle cells induced by angiotensin II. Life Sci. 2002, 72, 659–667. [Google Scholar] [CrossRef]
- Pi, K.; Lee, K. Prunus mume extract exerts antioxidant activities and suppressive effect of melanogenesis under the stimulation by alpha-melanocyte stimulating hormone in B16-F10 melanoma cells. Biosci. Biotech. Biochem. 2017, 81, 1883–1890. [Google Scholar] [CrossRef]
- Khan, A.; Pan, J.H.; Cho, S.; Lee, S.; Kim, Y.J.; Park, Y.H. Investigation of the Hepatoprotective Effect of Prunus mume Sieb. et Zucc Extract in a Mouse Model of Alcoholic Liver Injury Through High-Resolution Metabolomics. J. Med. Food 2017, 20, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Takemura, S.; Yoshimasu, K.; Fukumoto, J.; Mure, K.; Nishio, N.; Kishida, K.; Yano, F.; Mitani, T.; Takeshita, T.; Miyashita, K. Safety and adherence of Umezu polyphenols in the Japanese plum (Prunus mume) in a 12-week double-blind randomized placebo-controlled pilot trial to evaluate antihypertensive effects. Environ. Health Prev. Med. 2014, 19, 444. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ham, I.; Yang, G.; Lee, M.; Bu, Y.; Kim, H.; Choi, H.-Y. Vasorelaxant effect of Prunus yedoensis bark. BMC Complement Altern. Med. 2013, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, K.-W.; Heo, H.; Ham, I.; Lee, M.-H.; Kim, B.; Bu, Y.; Kim, H.; Choi, H.-Y. Vasorelaxant Effect of Prunus yedoensis leaf on Rat Aortic Rings. Kor. J. Herbol. 2013, 28, 63–69. [Google Scholar] [CrossRef]
- Ibarra-Alvarado, C.; Rojas, A.; Luna, F.; Rojas, J.I.; Rivero-Cruz, B.; Rivero-Cruz, J.F. Vasorelaxant constituents of the leaves of Prunus serotina “capulín”. Rev. Latinoam. Quim 2009, 37, 164–173. [Google Scholar]
- Luna-Vázquez, F.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Romo-Mancillas, A.; López-Vallejo, F.; Solís-Gutiérrez, M.; Rojas-Molina, J.; Rivero-Cruz, F. Role of nitric oxide and hydrogen sulfide in the vasodilator effect of ursolic acid and uvaol from black cherry Prunus serotina fruits. Molecules 2016, 21, 78. [Google Scholar] [CrossRef]
- Babaei, H.; Sadeghpour, O.; Nahar, L.; Delazar, A.; Nazemiyeh, H.; Mansouri, M.R.; Poursaeid, N.; Asnaashari, S.; Moghadam, S.B.; Sarker, S.D. Antioxidant and vasorelaxant activities of flavonoids from Amygdalus lycioides var. horrida. Turk. J. Biol. 2008, 32, 203–208. [Google Scholar]
- Kim, B.; Jo, C.; Choi, H.-Y.; Lee, K. Prunetin Relaxed Isolated Rat Aortic Rings by Blocking Calcium Channels. Molecules 2018, 23, 2372. [Google Scholar] [CrossRef]
- Lu, B.; Wu, X.; Dong, Y.; Gong, J.; Zhang, Y. Mutagenicity and safety evaluation of ethanolic extract of Prunus mume. J. Food Sci. 2009, 74, T82–T88. [Google Scholar] [CrossRef]
- Tom, E.N.L.; Girard-Thernier, C.; Demougeot, C. The Janus face of chlorogenic acid on vascular reactivity: A study on rat isolated vessels. Phytomedicine 2016, 23, 1037–1042. [Google Scholar] [CrossRef]
- Zhou, X.; Yao, H.; Xia, M.; Cao, C.; Jiang, H.; Xia, Q. Comparison of vasodilatation effect between quercetin and rutin in the isolated rat thoracic aorta. Zhejiang Da Xue Xue Bao. Yi Xue Ban 2006, 35, 29–33. [Google Scholar] [PubMed]
- Andriambeloson, E.; Magnier, C.L.; Haan-Archipoff, G.; Lobstein, A.; Anton, R.; Beretz, A.; Stoclet, J.C.; Andriantsitohaina, R. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J. Nutr. 1998, 128, 2324–2333. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xia, Q.; Wang, X.; Song, J.; Bruce, I. Luteolin induces vasorelaxion in rat thoracic aorta via calcium and potassium channels. Pharmazie 2005, 60, 444–447. [Google Scholar] [PubMed]
- Stankevičius, E.; Kėvelaitis, E.; Vainorius, E.; Simonsen, U. Role of nitric oxide and other endothelium-derived factors. Medicina (Kaunas) 2003, 39, 333–341. [Google Scholar] [PubMed]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, M.P.; Birdsall, N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998, 50, 279–290. [Google Scholar] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J.; Barakeh, J.; Laskey, R.; Van Breemen, C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989, 3, 2389–2400. [Google Scholar] [CrossRef]
- Nelson, M.T.; Quayle, J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. Cell Physiol. 1995, 268, C799–C822. [Google Scholar] [CrossRef]
- Tykocki, N.R.; Boerman, E.M.; Jackson, W.F. Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles. Compr. Physiol. 2017, 7, 485–581. [Google Scholar]
- Lee, J.; Yang, G.; Lee, K.; Lee, M.-H.; Eom, J.-W.; Ham, I.; Choi, H.-Y. Anti-inflammatory effect of Prunus yedoensis through inhibition of nuclear factor-κB in macrophages. BMC Complement Altern. Med. 2013, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Geibel, M.; Geiger, H.; Treutter, D. Tectochrysin 5-and genistein 5-glucosides from the bark of Prunus cerasus. Phytochemistry 1990, 29, 1351–1353. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, C.; Kim, B.; Lee, S.; Ham, I.; Lee, K.; Choi, H.-Y. Vasorelaxant Effect of Prunus mume (Siebold) Siebold & Zucc. Branch through the Endothelium-Dependent Pathway. Molecules 2019, 24, 3340. https://doi.org/10.3390/molecules24183340
Jo C, Kim B, Lee S, Ham I, Lee K, Choi H-Y. Vasorelaxant Effect of Prunus mume (Siebold) Siebold & Zucc. Branch through the Endothelium-Dependent Pathway. Molecules. 2019; 24(18):3340. https://doi.org/10.3390/molecules24183340
Chicago/Turabian StyleJo, Cheolmin, Bumjung Kim, Somin Lee, Inhye Ham, Kyungjin Lee, and Ho-Young Choi. 2019. "Vasorelaxant Effect of Prunus mume (Siebold) Siebold & Zucc. Branch through the Endothelium-Dependent Pathway" Molecules 24, no. 18: 3340. https://doi.org/10.3390/molecules24183340
APA StyleJo, C., Kim, B., Lee, S., Ham, I., Lee, K., & Choi, H.-Y. (2019). Vasorelaxant Effect of Prunus mume (Siebold) Siebold & Zucc. Branch through the Endothelium-Dependent Pathway. Molecules, 24(18), 3340. https://doi.org/10.3390/molecules24183340