Optimization of Production Parameters for Probiotic Lactobacillus Strains as Feed Additive
Abstract
1. Introduction
2. Results
2.1. Metabolic Fingerprints of the Lactobacillus Strains
2.2. Booster Effects of Selective Carbon Sources on Biomass Production
2.3. Effect of Aerobic or Anaerobic Incubation on Biomass Production
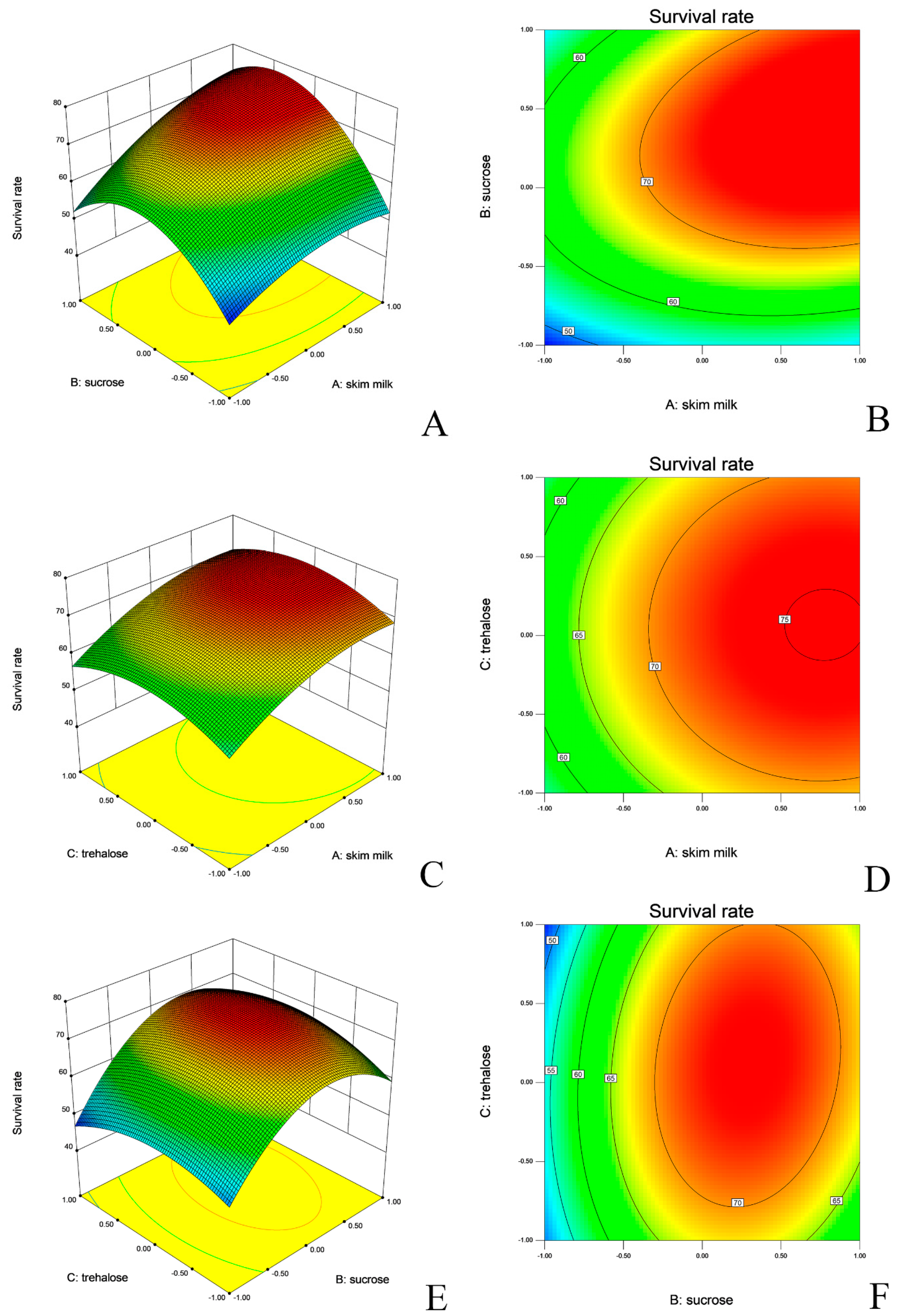
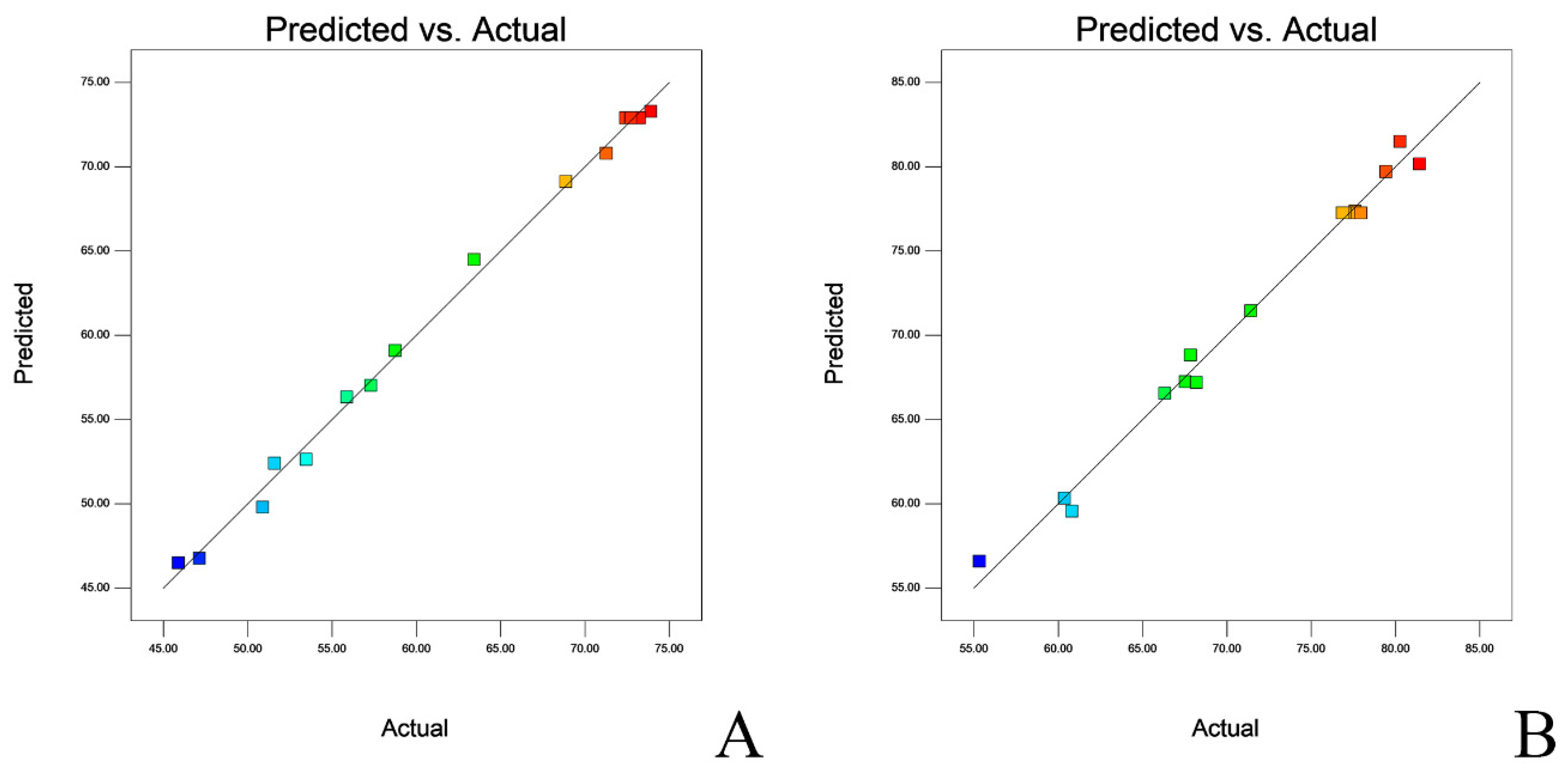
2.4. Lyophilization and Optimization of Lyo-Protectants
2.5. Stability during In-Feed Storage
3. Discussion
4. Materials and Methods
4.1. Strains and Medium
4.2. Metabolic Fingerprint of Probiotic Lactobacillus Strains
4.3. Booster Effects of Additional Carbohydrate Sources on Biomass Production
4.4. Determination of Bacterial Growth under Aerobic or Anaerobic Conditions
4.5. Lyophilization and Optimization of Cryoprotectants
4.6. In-Feed Stability of Probiotic Products
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grashorn, M.A. Use of phytobiotics in broiler nutrition – an alternative to infeed antibiotics? J. Anim. Feed Sci. 2010, 19, 338–347. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.W.; Harper, D.; et al. Alternatives to antibiotics—A pipeline portfolio review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef]
- Gao, P.; Ma, C.; Sun, Z.; Wang, L.; Huang, S.; Su, X.; Xu, J.; Zhang, H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome 2017, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ni, X.; Qing, X.; Zeng, D.; Luo, M.; Liu, L.; Li, G.; Pan, K.; Jing, B. Live Probiotic Lactobacillus johnsonii BS15 Promotes Growth Performance and Lowers Fat Deposition by Improving Lipid Metabolism, Intestinal Development, and Gut Microflora in Broilers. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Singh, P. Efficacy of species-specific probiotic Pediococcus acidilactici FT28 on blood biochemical profile, carcass traits and physicochemical properties of meat in fattening pigs. Res. Vet. Sci. 2018, 117, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.L.; Zamith-Miranda, D.; Martins, F.S.; Bozza, F.A.; Nimrichter, L.; Montero-Lomeli, M.; Marques, E.T.A.; Douradinha, B. Probiotic Saccharomyces cerevisiae strains as biotherapeutic tools: is there room for improvement? Appl. Microbiol. Biotechnol. 2015, 99, 6563–6570. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.M.; Hedia, R.H.; Omara, S.T.; Mahmoud, M.A.E.-F.; Kandil, M.M.; Bakry, M.A. Interspecies comparison of probiotics isolated from different animals. Vet. World 2018, 11, 227–230. [Google Scholar] [CrossRef]
- Ricci, A.; Chemaly, M.; Davies, R.; Salvador Fern Andez Esc Amez, P.; Gironés, R.; Herman, L.; Lindqvist, R.; Nørrung, B.; Robertson, L.; Ru, G.; et al. Hazard analysis approaches for certain small retail establishments in view of the application of their food safety management systems. Efsa J. 2017, 15, 4697. [Google Scholar]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef]
- Simon, O. Micro-organisms as feed additives–probiotics. In Advances in Pork Production; Zijlstra, R.O.B.R.T., Ed.; University of Alberta: Edmonton, AB, Canada, 2005; Volume 16. [Google Scholar]
- Hwang, C.-F.; Chang, J.-H.; Houng, J.-Y.; Tsai, C.-C.; Lin, C.-K.; Tsen, H.-Y. Optimization of medium composition for improving biomass production of Lactobacillus plantarum Pi06 using the Taguchi array design and the Box-Behnken method. Biotechnol. Bioprocess Eng. 2012, 17, 827–834. [Google Scholar] [CrossRef]
- Brinques, G.B.; do Carmo Peralba, M.; Ayub, M.A.Z. Optimization of probiotic and lactic acid production by Lactobacillus plantarum in submerged bioreactor systems. J. Ind. Microbiol. Biot. 2010, 37, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.; Qazi, J.I.; Haq, I.U.; Mukhtar, H.; Rasool, A. Significantly enhanced biomass production of a novel bio-therapeutic strain Lactobacillus plantarum (AS-14) by developing low cost media cultivation strategy. J. Biol. Eng. 2017, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.; Ariff, A.B.; Wasoh, H.; Kapri, M.R.; Halim, M. Strategies for improving production performance of probiotic Pediococcus acidilactici viable cell by overcoming lactic acid inhibition. AMB Express 2017, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An overview of beneficial effects. Antonie Van Leeuwenhoek 2002, 82, 279–289. [Google Scholar] [CrossRef]
- Zotta, T.; Guidone, A.; Ianniello, R.G.; Parente, E.; Ricciardi, A. Temperature and respiration affect the growth and stress resistance of Lactobacillus plantarum C17. J. Appl. Microbiol. 2013, 115, 848–858. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Effect of various growth media upon survival during storage of freeze-dried Enterococcus faecalis and Enterococcus durans. J. Appl. Microbiol. 2003, 94, 947–952. [Google Scholar] [CrossRef]
- Jalali, M.; Abedi, D.; Varshosaz, J.; Najjarzadeh, M.; Mirlohi, M.; Tavakoli, N. Stability evaluation of freeze-dried Lactobacillus paracasei subsp. tolerance and Lactobacillus delbrueckii subsp. bulgaricus in oral capsules. Res. Pharm. Sci. 2012, 7, 31–36. [Google Scholar]
- Saarela, M.; Virkajärvi, I.; Alakomi, H.-L.; Sigvart-Mattila, P.; Mättö, J. Stability and functionality of freeze-dried probiotic Bifidobacterium cells during storage in juice and milk. Int. Dairy J. 2006, 16, 1477–1482. [Google Scholar] [CrossRef]
- Schoug, Å.; Olsson, J.; Carlfors, J.; Schnürer, J.; Håkansson, S. Freeze-drying of Lactobacillus coryniformis Si3—effects of sucrose concentration, cell density, and freezing rate on cell survival and thermophysical properties. Cryobiology 2006, 53, 119–127. [Google Scholar] [CrossRef]
- Siaterlis, A.; Deepika, G.; Charalampopoulos, D. Effect of culture medium and cryoprotectants on the growth and survival of probiotic lactobacilli during freeze drying. Lett. Appl. Microbiol. 2009, 48, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Juárez Tomás, M.S.; Bru, E.; Martos, G.; Nader-Macías, M.E. Stability of freeze-dried vaginal Lactobacillus strains in the presence of different lyoprotectors. Can. J. Microbiol. 2009, 55, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.C.; Stanton, C.; Fitzgerald, G.F.; Daly, C.; Ross, R.P. Anhydrobiotics: The challenges of drying probiotic cultures. Food Chem. 2008, 106, 1406–1416. [Google Scholar] [CrossRef]
- Han, L.; Pu, T.; Wang, X.; Liu, B.; Wang, Y.; Feng, J.; Zhang, X. Optimization of a protective medium for enhancing the viability of freeze-dried Bacillus amyloliquefaciens B1408 based on response surface methodology. Cryobiology 2018, 81, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lu, Z.; Yuan, Y.; Lü, F.; Bie, X. Optimization of a protective medium for enhancing the viability of freeze-dried Lactobacillus delbrueckii subsp. bulgaricus based on response surface methodology. J. Ind. Microbiol. Biotechnol. 2006, 33, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Dewanjee, S. Chapter 3—Optimization of Extraction Using Mathematical Models and Computation. In Computational Phytochemistry; Sarker, S.D., Nahar, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 75–106. [Google Scholar] [CrossRef]
- Khoramnia, A.; Abdullah, N.; Liew, S.L.; Sieo, C.C.; Ramasamy, K.; Ho, Y.W. Enhancement of viability of a probiotic Lactobacillus strain for poultry during freeze-drying and storage using the response surface methodology. Anim. Sci. J. 2011, 82, 127–135. [Google Scholar] [CrossRef]
- Min, M.; Bunt, C.R.; Mason, S.L.; Bennett, G.N.; Hussain, M.A. Effect of Non-Dairy Food Matrices on the Survival of Probiotic Bacteria during Storage. Microorganisms 2017, 5, 43. [Google Scholar] [CrossRef]
- Vinderola, G.; Binetti, A.; Burns, P.; Reinheimer, J. Cell Viability and Functionality of Probiotic Bacteria in Dairy Products. Front. Microbiol. 2011, 2. [Google Scholar] [CrossRef]
- Govender, M.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; van Vuuren, S.; Pillay, V. A review of the advancements in probiotic delivery: Conventional vs. non-conventional formulations for intestinal flora supplementation. AAPS Pharmscitech 2013, 15, 29–43. [Google Scholar] [CrossRef]
- Del Piano, M.; Morelli, L.; Strozzi, G.P.; Allesina, S.; Barba, M.; Deidda, F.; Lorenzini, P.; Ballaré, M.; Montino, F.; Orsello, M.; et al. Probiotics: from research to consumer. Dig. Liver Dis. 2006, 38, S248–S255. [Google Scholar] [CrossRef]
- Hammes, W.P.; Hertel, C. The Genera Lactobacillus and Carnobacterium. In The Prokaryotes: Volume 4: Bacteria: Firmicutes, Cyanobacteria; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 320–403. [Google Scholar] [CrossRef]
- Söderberg, K.H.; Probanza, A.; Jumpponen, A.; Bååth, E. The microbial community in the rhizosphere determined by community-level physiological profiles (CLPP) and direct soil– and cfu–PLFA techniques. Appl. Soil Ecol. 2004, 25, 135–145. [Google Scholar] [CrossRef]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar]
- Stefanowicz, A. The Biolog Plates Technique as a Tool in Ecological Studies of Microbial Communities. Pol. J. Environ. Stud. 2006, 15, 669–676. [Google Scholar]
- Lee, H.M.; Lee, Y. A differential medium for lactic acid-producing bacteria in a mixed culture. Lett. Appl. Microbiol. 2008, 46, 676–681. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Nedovic, V.; Goyal, A.; Kourkoutas, Y. Immobilization technologies in probiotic food production. J. Nutr. Metab. 2013, 2013, 716861. [Google Scholar] [CrossRef]
- Maresca, D.; Zotta, T.; Mauriello, G. Adaptation to Aerobic Environment of Lactobacillus johnsonii/gasseri Strains. Front. Microbiol. 2018, 9, 157. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Pannella, G.; Lippolis, R.; Ricciardi, A.; Mazzeo, M.F.; Zotta, T. Impact of aerobic and respirative life-style on Lactobacillus casei N87 proteome. Int. J. Food Microbiol. 2019, 298, 51–62. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Growth of the bacteriocin-producing Lactobacillus sakei strain CTC 494 in MRS broth is strongly reduced due to nutrient exhaustion: a nutrient depletion model for the growth of lactic acid bacteria. Appl. Environ. Microbiol. 2001, 67, 4407–4413. [Google Scholar] [CrossRef]
- To, B.C.S.; Etzel, M.R. Spray Drying, Freeze Drying, or Freezing of Three Different Lactic Acid Bacteria Species. J. Food Sci. 1997, 62, 576–578. [Google Scholar] [CrossRef]
- Savini, M.; Cecchini, C.; Verdenelli, M.C.; Silvi, S.; Orpianesi, C.; Cresci, A. Pilot-scale production and viability analysis of freeze-dried probiotic bacteria using different protective agents. Nutrients 2010, 2, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Thammavongs, B.; Corroler, D.; Panoff, J.-M.; Auffray, Y.; Boutibonnes, P. Physiological response of Enterococcus faecalis JH2-2 to cold shock: growth at low temperatures and freezing/thawing challenge. Lett. Appl. Microbiol. 1996, 23, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Berny, J.F.; Hennebert, G.L. Viability and Stability of Yeast Cells and Filamentous Fungus Spores During Freeze-Drying: Effects of Protectants and Cooling Rates. Mycologia 1991, 83, 805–815. [Google Scholar] [CrossRef]
- Lapsiri, W.; Bhandari, B.; Wanchaitanawong, P. Viability of Lactobacillus plantarum TISTR 2075 in Different Protectants during Spray Drying and Storage. Dry. Technol. 2012, 30, 1407–1412. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Survival of freeze-dried Lactobacillus plantarum and Lactobacillus rhamnosus during storage in the presence of protectants. Biotechnol. Lett. 2002, 24, 1587–1591. [Google Scholar] [CrossRef]
- Zayed, G.; Roos, Y.H. Influence of trehalose and moisture content on survival of Lactobacillus salivarius subjected to freeze-drying and storage. Process Biochem. 2004, 39, 1081–1086. [Google Scholar] [CrossRef]
- Gwak, H.J.; Lee, J.-H.; Kim, T.-W.; Choi, H.-J.; Jang, J.-Y.; Lee, S.I.; Park, H.W. Protective effect of soy powder and microencapsulation on freeze-dried Lactobacillus brevis WK12 and Lactococcus lactis WK11 during storage. Food Sci. Biotechnol. 2015, 24, 2155–2160. [Google Scholar] [CrossRef]
- Ming, L.C.; Rahim, R.A.; Wan, H.Y.; Ariff, A.B. Formulation of Protective Agents for Improvement of Lactobacillus salivarius I 24 Survival Rate Subjected to Freeze Drying for Production of Live Cells in Powderized Form. Food Bioprocess Technol. 2009, 2, 431. [Google Scholar] [CrossRef]
- Hubálek, Z. Protectants used in the cryopreservation of microorganisms. Cryobiology 2003, 46, 205–229. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, L.; Yang, T.; Lv, F.; Lu, Z. Optimization of a cryoprotective medium to increase the viability of freeze-dried Streptococcus thermophilus by response surface methodology. LWT 2017, 80, 92–97. [Google Scholar] [CrossRef]
- Selmer-Olsen, E.; Birkeland, S.-E.; Sørhaug, T. Effect of protective solutes on leakage from and survival of immobilized Lactobacillus subjected to drying, storage and rehydration. J. Appl. Microbiol. 1999, 87, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Abadias, M.; Benabarre, A.; Teixidó, N.; Usall, J.; Viñas, I. Effect of freeze drying and protectants on viability of the biocontrol yeast Candida sake. Int. J. Food Microbiol. 2001, 65, 173–182. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Oliver, A.E.; Tsvetkova, N.; Wolkers, W.; Tablin, F. The Trehalose Myth Revisited: Introduction to a Symposium on Stabilization of Cells in the Dry State. Cryobiology 2001, 43, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Giulio, B.D.; Orlando, P.; Barba, G.; Coppola, R.; Rosa, M.D.; Sada, A.; Prisco, P.P.D.; Nazzaro, F. Use of alginate and cryo-protective sugars to improve the viability of lactic acid bacteria after freezing and freeze-drying. World J. Microbiol. Biotechnol. 2005, 21, 739–746. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Protective effect of sorbitol and monosodium glutamate during storage of freeze-dried lactic acid bacteria. Lait 2003, 83, 203–210. [Google Scholar] [CrossRef]
- Li, H.; Lu, M.; Guo, H.; Li, W.; Zhang, H. Protective Effect of Sucrose on the Membrane Properties of Lactobacillus casei Zhang Subjected to Freeze-Drying. J. Food Prot. 2010, 73, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-X.; Fang, X.-J.; Yu, Z.; Xin, Y.; Liu, X.-Y.; Shi, L.-E.; Tang, Z.-X. Encapsulation in alginate–skim milk microspheres improves viability of Lactobacillus bulgaricus in stimulated gastrointestinal conditions. Int. J. Food Sci. Nutr. 2013, 64, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Deeseenthum, S.; Leelavatcharamas, V.; D Brookes, J. Effect of Feeding Bacillus sp. As Probiotic Bacteria on Growth of Giant Freshwater Prawn (Macrobrachium rosenbergii de Man). Pak. J. Biol. Sci. Pjbs 2007, 10, 1481–1485. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sadguruprasad, L.; Basavaraj, M. Statistical modelling for optimized lyophilization of Lactobacillus acidophilus strains for improved viability and stability using response surface methodology. AMB Express 2018, 8, 129. [Google Scholar] [CrossRef]
- Baudoin, E.; Benizri, E.; Guckert, A. Metabolic fingerprint of microbial communities from distinct maize rhizosphere compartments. Eur. J. Soil Biol. 2001, 37, 85–93. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Latha, S.; Sivaranjani, G.; Dhanasekaran, D. Response surface methodology: A non-conventional statistical tool to maximize the throughput of Streptomyces species biomass and their bioactive metabolites. Crit. Rev. Microbiol. 2017, 43, 567–582. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| 12 h | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|
| L. salivarius | L. agilis | L. salivarius | L. agilis | L. salivarius | L. agilis | |
| Sucrose | 9.22 ± 0.02 * | 9.08 ± 0.02 * | 8.94 ± 0.05 * | 8.82 ± 0.06 * | 8.67 ± 0.12 | 8.11 ± 0.06 * |
| Maltose | 9.08 ± 0.05 * | 9.11 ± 0.07 | 8.74 ± 0.12 * | 8.74 ± 0.12 * | 8.26 ± 0.13 * | 8.1 ± 0.12 * |
| Mannitol | 9.04 ± 0.11 | 9.02 ± 0.09 | 8.75 ± 0.19 * | 8.54 ± 0.07 * | 8.45 ± 0.09 * | 8.21 ± 0.09 * |
| Mannose | 9.1 ± 0.04 * | 9.2 ± 0.06 * | 8.61 ± 0.03 * | 8.92 ± 0.05 * | 8.49 ± 0.04 * | 8.42 ± 0.1 * |
| Sorbitol | 9.18 ± 0.06 * | 9.17 ± 0.03 * | 8.88 ± 0.03 * | 8.88 ± 0.04 | 8.48 ± 0.11 * | 8.22 ± 0.06 * |
| Melibiose | 9 ± 0.06 | 9.03 ± 0.03 | 8.96 ± 0.05 | 8.52 ± 0.04 * | 8.63 ± 0.06 | 8.27 ± 0.1 * |
| MRS contol | 8.86 ± 0.1 | 8.98 ± 0.04 | 9.07 ± 0.07 | 9.13 ± 0.02 | 8.65 ± 0.04 | 8.65 ± 0.07 |
| Variables | Coefficient Estimates (± Standard Error) | F-Value | p Value | Model Significance | R2 | |
|---|---|---|---|---|---|---|
| L. salivarius | Intercept | 72.9 ± 0.4 | 233.22 | <0.0001 | <0.0001 ** | 0.9924 |
| Skim milk | 6.64 ± 0.32 | 430.95 | <0.0001 | |||
| X2 | 6.76 ± 0.32 | 446.4 | <0.0001 | |||
| X3 | 0.59 ± 0.32 | 3.41 | 0.1071 | |||
| Skim milk, sucrose | 3.69 ± 0.45 | 66.4 | <0.0001 | |||
| X1X3 | 0.24 ± 0.45 | 0.29 | 0.6063 | |||
| X2X3 | 2.11 ± 0.45 | 21.72 | 0.0023 | |||
| X12 | −4.2 ± 0.44 | 90.93 | <0.0001 | |||
| X22 | −12.49 ± 0.44 | 802.54 | <0.0001 | |||
| X32 | −5.37 ± 0.44 | 148.45 | <0.0001 | |||
| L. agilis | Intercept | 77.26 ± 0.52 | 82.44 | <0.0001 | <0.0001 ** | 0.9786 |
| X1 | 8.6 ± 0.41 | 440.12 | <0.0001 | |||
| X2 | 3.19 ± 0.41 | 60.54 | 0.0001 | |||
| X3 | 2.37 ± 0.41 | 33.46 | 0.0007 | |||
| X1X2 | −1.79 ± 0.58 | 9.58 | 0.0174 | |||
| X1X3 | −1.47 ± 0.58 | 6.45 | 0.0387 | |||
| X2X3 | −1.06 ± 0.58 | 3.36 | 0.1095 | |||
| X12 | −1.01 ± 0.56 | 3.23 | 0.1155 | |||
| X22 | −6.07 ± 0.56 | 115.44 | <0.0001 | |||
| X32 | −4.23 ± 0.56 | 56.11 | 0.0001 |
| Response Viability | Target | Predicted Results | Standard Deviation | 95% PI Low | 95% PI High |
|---|---|---|---|---|---|
| L. salivarius | Maximized | 76.19 | 3.91 | 65.54 | 86.83 |
| L. agilis | Maximized | 84.77 | 1.16 | 81.56 | 87.97 |
| L. salivarius | L. agilis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without Protectants | With Protectants | Without Protectants | With Protectants | |||||||||||||
| 20 °C | 4 °C | 20 °C | 4 °C | 20 °C | 4 °C | 20 °C | 4 °C | |||||||||
| BM | 9.01 ± 0.04 | 100.00% | 9.00 ± 0.02 | 100.00% | 9.01 ± 0.02 | 100.00% | 9.00 ± 0.04 | 100.00% | 9.02 ± 0.00 | 100.00% | 9.00 ± 0.03 | 100.00% | 9.01 ± 0.03 | 100.00% | 9.00 ± 0.01 | 100.00% |
| DPM0 | 8.97 ± 0.01 | 91.56% | 8.98 ± 0.01 | 95.33% | 9 ± 0.02 | 97.74% | 8.99 ± 0.03 | 98.00% | 9.00 ± 0.00 | 97.11% | 9.00 ± 0.01 | 100.67% | 9.01 ± 0.00 | 99.02% | 9.01 ± 0.01 | 101.00% |
| DPM1 | 8.97 ± 0.04 | 90.58% | 8.98 ± 0.02 | 95.00% | 8.99 ± 0.05 | 95.48% | 8.99 ± 0.03 | 98.00% | 9.00 ± 0.01 | 96.46% | 9.00 ± 0.03 | 100.33% | 9.01 ± 0.01 | 99.35% | 9.01 ± 0.02 | 101.00% |
| DPM2 | 8.96 ± 0.03 | 89.29% | 8.97 ± 0.04 | 93.33% | 8.99 ± 0 | 95.16% | 8.99 ± 0.01 | 98.67% | 8.99 ± 0.04 | 94.53% | 9.00 ±0.04 | 100.00% | 9.00 ± 0.02 | 97.07% | 9.01 ± 0.01 | 101.00% |
| DPM3 | 8.95 ± 0.03 | 87.34% | 8.96 ± 0.04 | 91.33% | 8.98 ± 0.02 | 93.55% | 8.99 ± 0.02 | 97.00% | 8.99 ± 0.02 | 93.89% | 8.99 ± 0.04 | 98.67% | 9.00 ± 0.00 | 97.07% | 9.00 ± 0.03 | 99.00% |
| DPM4 | 8.96 ± 0.02 | 88.31% | 8.96 ± 0.03 | 91.33% | 8.99 ± 0.01 | 93.55% | 8.99 ± 0.01 | 97.33% | 8.99 ± 0.01 | 94.86% | 8.99 ± 0.00 | 98.00% | 8.99 ± 0.03 | 96.74% | 9.00 ± 0.01 | 99.34% |
| DPM15 | 8.91 ± 0.01a | 78.90% | 8.92 ± 0.03ab | 83.67% | 8.96 ± 0.02b | 89.03% | 8.97 ± 0.01b | 94.33% | 8.95 ± 0.01a | 85.21% | 8.96 ± 0.02ab | 91.00% | 8.99 ± 0.01b | 95.44% | 8.99 ± 0.01b | 98.34% |
| DPM28 | 8.64 ± 0.05a | 42.86% | 8.83 ± 0.03b | 67.10% | 8.84 ± 0.02b | 67.74% | 8.93 ± 0.01c | 85.33% | 8.71 ± 0.04a | 49.84% | 8.85 ± 0.01a | 70.33% | 8.91 ± 0.02b | 79.48% | 8.95 ± 0.02b | 88.37% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Zentek, J.; Vahjen, W. Optimization of Production Parameters for Probiotic Lactobacillus Strains as Feed Additive. Molecules 2019, 24, 3286. https://doi.org/10.3390/molecules24183286
Ren H, Zentek J, Vahjen W. Optimization of Production Parameters for Probiotic Lactobacillus Strains as Feed Additive. Molecules. 2019; 24(18):3286. https://doi.org/10.3390/molecules24183286
Chicago/Turabian StyleRen, Hao, Jürgen Zentek, and Wilfried Vahjen. 2019. "Optimization of Production Parameters for Probiotic Lactobacillus Strains as Feed Additive" Molecules 24, no. 18: 3286. https://doi.org/10.3390/molecules24183286
APA StyleRen, H., Zentek, J., & Vahjen, W. (2019). Optimization of Production Parameters for Probiotic Lactobacillus Strains as Feed Additive. Molecules, 24(18), 3286. https://doi.org/10.3390/molecules24183286




