Optimization of Preparation and Preclinical Pharmacokinetics of Celastrol-Encapsulated Silk Fibroin Nanoparticles in the Rat
Abstract
:1. Introduction
2. Results
2.1. Preparation of Silk Fibroin Solution
2.2. Optimized Preparation of CL-Loaded Silk Fibroin Nanoparticles
2.2.1. Varying Storage Times at −20 °C
2.2.2. Varying Rotation Speeds
2.2.3. Inter-Day Evaluation of Optimized Formulation
2.3. Analytical Method Validation
2.3.1. Chromatographic Conditions
2.3.2. Selectivity and Specificity
2.3.3. Linearity and Sensitivity
2.3.4. Precision and Accuracy
2.3.5. Recovery
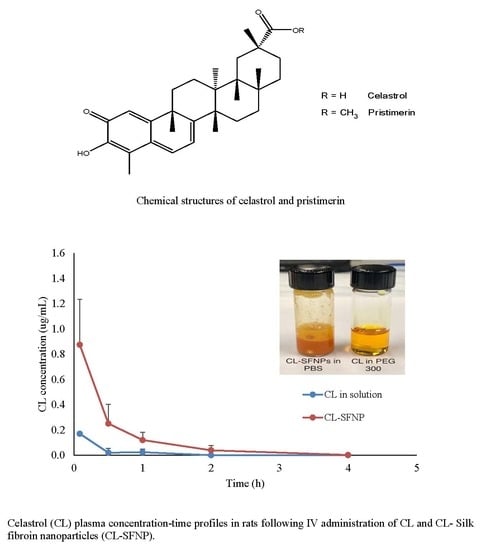
2.4. Pharmacokinetic Study
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Silk Fibroin Solution
4.3. Preparation of CL-Loaded Silk Fibroin Nanoparticles
4.4. Nanoparticle Characterization
4.4.1. Size and Zeta-potential
4.4.2. Encapsulation Efficiency and Drug Loading
4.5. Optimized Formulation of CL-Loaded Silk Fibroin Nanoparticles
4.5.1. Varying Storage Times at −20 °C
4.5.2. Varying Rotation Speeds
4.5.3. Inter-Day/Intra-Day Evaluation of the Finalized Formulation
4.6. Development and Validation of Method for Preclinical Studies
4.6.1. Chromatographic Conditions
4.6.2. Preparation of Standard and Quality Controls
4.6.3. Preparation of Plasma Samples
4.6.4. Specificity and Selectivity
4.6.5. Linearity and Sensitivity
4.6.6. Precision and Accuracy
4.6.7. Recovery
4.7. Pharmacokinetics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1388. [Google Scholar] [CrossRef]
- Efferth, T.; Li, P.C.H.; Badireenath Konkimalla, V.S.; Kaina, B. From traditional Chinese medicine to rational cancer therapy. Trends Mol. Med. 2007, 13, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, C.Y.; Xu, M.J.; Wu, T.; Chu, J.H.; Liu, S.J.; Ju, W.Z. Oral bioavailability and gender-related pharmacokinetics of celatrol following adminstration of pure celastrol and its related tablets in rats. J. Ethnopharmacol. 2012, 144, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, J.; Salazar Hernandez, M.-A.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef]
- Feng, X. 307-LB: IL1R1 Mediates Celastrol’s Leptin-Sensitization and Antiobesity Effects. Diabetes 2019, 68 (Suppl. S1), 307-LB. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, L.; Zhou, G.-B. The main anticancer bullets of the Chinese medicinal herb, thunder god vine. Molecules 2011, 16, 5283–5297. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.-W.; Cheng, K.-J.; Mei, X.-L.; Qiu, J.-G.; Zhang, W.-J.; Xue, Y.-Q.; Qin, W.-M.; Yang, Y.; Zheng, D.-W.; Chen, Y.; et al. Synergistic anticancer effects of triptolide and celastrol, two main compounds from thunder god vine. Oncotarget 2015, 6, 32790–32804. [Google Scholar] [CrossRef]
- Cascao, R.; Fonseca, J.E.; Moita, L.F. Celastrol: A spectrum of treatment opportunities in chronic diseases. Front. Med. (Lausanne) 2017, 4, 69. [Google Scholar] [CrossRef]
- Yang, H.; Chen, D.; Cui, Q.C.; Yuan, X.; Duo, Q.P. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006, 66, 4758–4765. [Google Scholar] [CrossRef]
- Sanna, V.; Chamcheu, J.C.; Pala, N.; Mukhtar, H.; Sechi, M.; Siddiqui, I.A. Nanoencapsulation of natural triterpenoid celastrol for prostate cancer treatment. Int. J. Nanomed. 2015, 10, 6835–6846. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed. Res. Int. 2014, 2014, 180549. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Kaplan, D.L. Silk-based delivery systems of bioactive molecules. Adv. Drug Deliv. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wani, S.U.D.; Veerabhadrappa, G.H. Silk Fibroin Based Drug Delivery Applications: Promises and Challenges. Curr. Drug Targets 2018, 19, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Wenk, E.; Merkle, H.P.; Meinel, L. Silk fibroin as a vehicle for drug delivery applications. J. Control Release 2011, 150, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Koh, L.-D.; Li, D.; Ji, B.; Han, M.-Y.; Zhang, Y.-W. On the strength of β-sheet crystallites of Bombyx mori silk fibroin. J. R. Soc. Interface 2014, 11, 20140305. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wahid, M.A.; Wang, Z.; Xie, C.; Thakkar, A.; Prabhu, S.; Wang, J. Triptolide and celastrol loaded silk fibroin nanoparticles show synergistic effect against human pancreatic cancer cells. Nanoscale 2017, 9, 11739–11753. [Google Scholar] [CrossRef]
- Chen, M.; Shao, Z.; Chen, X. Paclitaxel-loaded silk fibroin nanospheres. J. Biomed. Mater. Res. A 2012, 100, 203–210. [Google Scholar] [CrossRef]
- Nam, J.; Park, Y.H. Morphology of Regenerated Silk Fibroin: Effects of Freezing Temperature, Alcohol Addition, and Molecular Weight. J. Appl. Polym. Sci. 2001, 81, 3008–3021. [Google Scholar] [CrossRef]
- Wang, W.; Liu, K.; Dong, H.; Liu, W. High-performance liquid chromatography spectrometric analysis of tripterin in rat plasma. J. Chromatogr. Anal. Technol. Biomed. Life Sci. 2008, 863, 163–166. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation; Food and Drug Administration: Rockville, MD, USA, 2018.
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: an effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.M.; Siccardi, M. Optimizing nanomedicine pharmacokinetics using physiologically based pharmacokinetics modelling. Br. J. Pharmacol. 2014, 171, 3963–3979. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, T.; Ye, Y.; Zhang, X.; Wu, B. Enhanced bioavailability of tripterine through lipid nanoparticles using broccoli-derived lipids as a carrier material. Int. J. Pharm. 2015, 495, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, P.; Yin, Y.; Hou, Y.; Song, X. Optimization on biodistribution and antitumor activity of tripterine using polymeric nanoparticles through RES saturation. Drug Deliv. 2017, 24, 1891–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Qin, J.; Ma, N.; Chou, X.; Wu, Z. Solid self-microemulsifying dispersible tablets of celastrol: Formulation development, charaterization and bioavailability evaluation. Int. J. Pharm. 2014, 472, 40–47. [Google Scholar] [CrossRef]
- Song, J.; Shi, F.; Zhang, Z.; Zhu, F.; Xue, J.; Tan, X.; Zhang, L.; Jia, X. Formulation and evaluation of celastrol-loaded liposomes. Molecules 2011, 16, 7880–7892. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 12, 1612–1631. [Google Scholar] [CrossRef]
- Kundu, J.; Chung, Y.-I.; Kim, Y.H.; Tae, G.; Kundu, S.C. Silk fibroin nanoparticles for cellular uptake and control release. Int. J. Pharm. 2010, 388, 242–250. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; Mateus, J.A.; Pujadas, M.; Fonseca, F.; Torrens, M.; Olesti, E.; de la Torre, R.; Farré, M. Human Pharmacology of Mephedrone in Comparison with MDMA. Neuropsychopharmacology 2016, 41, 2704–2713. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds celastrol pure drug and celastrol- loaded silk fibroin nanoparticles are available from the authors. |




| Hours in −20 °C | Rotation Speed (rpm) | Yield (%) (n = 3) | Size (nm) (n = 3) |
|---|---|---|---|
| 1 | 750 | 1.52 ± 0.5 | 1390.2 ± 847.6 |
| 2 | 750 | 3.89 ± 1.3 | 424.1 ± 32.4 |
| 4 | 750 | 33.3 ± 6.9 | 266.1 ± 2.3 |
| 8 | 750 | 26.9 ± 8.1 | 321.2 ± 5.5 |
| 16 | 750 | 31.9 ± 3.1 | 277.1 ± 1.5 |
| 20 | 750 | 35.5 ± 7.5 | 333.5 ± 16.5 |
| 24 | 750 | 25.2 ± 2.9 | 312.1 ± 1.4 |
| 48 | 750 | 4.54 ± 2.1 | 4809.9 ± 7132.2 |
| 20 | 625 | 27.1 ± 4.7 | 256.2 ± 13.1 |
| 20 | 750 | 35.5 ± 7.5 | 333.5 ± 38.8 |
| 20 | 875 | 18.4 ± 7.2 | 292.7 ± 28.1 |
| Day | Yield (%) | Size (nm) | Zeta-Potential (mV) | Encapsulation Efficiency (%) | Drug Loading (µg/mg) |
|---|---|---|---|---|---|
| 1 | 26.5 ± 8.7 | 291.1 ± 22.1 | −25.2 ± 3.2 | 81.4 ± 8.2 | 97.2 ± 28.4 |
| 2 | 31.7 ± 16.9 | 298.9 ± 20.7 | −19.5 ± 5.4 | 83.2 ± 14.7 | 80.9 ± 20.0 |
| 3 | 34.3 ± 14.5 | 299.5 ± 20.3 | −22.3 ± 0.9 | 79.3 ± 11.3 | 80.9 ± 4.2 |
| Nominal Concentration (µg/mL) | Intra-Day (n = 5) | Inter-Day (n = 3) | ||
|---|---|---|---|---|
| Precision (% RSD) | Accuracy (%) | Precision (% RSD) | Accuracy (%) | |
| 0.05 (LLOQ) | 17.6 | 110.7 | 10.8 | 116.3 |
| 0.1 | 4.6 | 114.2 | 7.5 | 107.8 |
| 0.5 | 4.3 | 92.2 | 10.8 | 97.2 |
| 5.0 | 5.3 | 101.5 | 4.7 | 95.8 |
| Analyte | Concentration (µg/mL) | Recovery (%) |
|---|---|---|
| CL | 0.1 | 67.4 ± 5.3 |
| 0.5 | 42.0 ± 2.7 | |
| 5.0 | 41.0 ± 5.5 | |
| Pristimerin (IS) | 2.0 | 75.3 ± 8.8 |
| Parameters | CL in PEG 300 | CL-SFNP |
|---|---|---|
| C0 (µg mL−1) | 0.25 | 1.09 |
| AUC0–inf (µg h mL−1) | 0.18 ± 0.31 | 0.47 ± 0.20 |
| CL (mL h−1) | 1.78 ± 0.94 | 0.71 ± 0.33 |
| MRT (h) | 0.26 ± 0.44 | 0.51 ± 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onyeabor, F.; Paik, A.; Kovvasu, S.; Ding, B.; Lin, J.; Wahid, M.A.; Prabhu, S.; Betageri, G.; Wang, J. Optimization of Preparation and Preclinical Pharmacokinetics of Celastrol-Encapsulated Silk Fibroin Nanoparticles in the Rat. Molecules 2019, 24, 3271. https://doi.org/10.3390/molecules24183271
Onyeabor F, Paik A, Kovvasu S, Ding B, Lin J, Wahid MA, Prabhu S, Betageri G, Wang J. Optimization of Preparation and Preclinical Pharmacokinetics of Celastrol-Encapsulated Silk Fibroin Nanoparticles in the Rat. Molecules. 2019; 24(18):3271. https://doi.org/10.3390/molecules24183271
Chicago/Turabian StyleOnyeabor, Felicia, Amy Paik, Surya Kovvasu, Baoyue Ding, Jelissa Lin, Md Arif Wahid, Sunil Prabhu, Guru Betageri, and Jeffrey Wang. 2019. "Optimization of Preparation and Preclinical Pharmacokinetics of Celastrol-Encapsulated Silk Fibroin Nanoparticles in the Rat" Molecules 24, no. 18: 3271. https://doi.org/10.3390/molecules24183271
APA StyleOnyeabor, F., Paik, A., Kovvasu, S., Ding, B., Lin, J., Wahid, M. A., Prabhu, S., Betageri, G., & Wang, J. (2019). Optimization of Preparation and Preclinical Pharmacokinetics of Celastrol-Encapsulated Silk Fibroin Nanoparticles in the Rat. Molecules, 24(18), 3271. https://doi.org/10.3390/molecules24183271