New Isoflavanes from Spatholobus suberectus and Their Cytotoxicity against Human Breast Cancer Cell Lines
Abstract
:1. Introduction
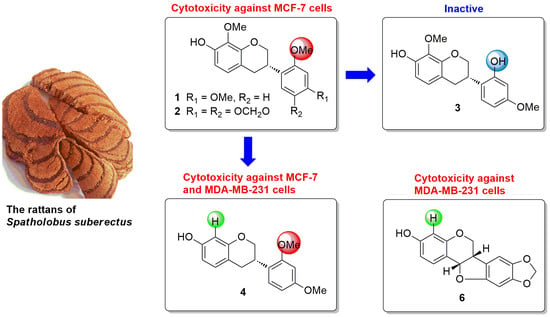
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Cytotoxic Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Z.Y.; Wang, D.M.; Loo, T.Y.; Cheng, Y.; Chen, L.L.; Shen, J.G.; Yang, D.P.; Chow, L.W.C.; Guan, X.Y.; Chen, J.P. Spatholobus suberectus inhibits cancer cell growth by inducing apoptosis and arresting cell cycle at G2/M checkpoint. J. Ethnopharmacol. 2011, 133, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.; Han, S.; Wang, N.; Mo, F.; Loo, T.Y.; Shen, J.; Huang, H.; Chen, J. Bioactivity-guided identification and cell signaling technology to delineate the lactate dehydrogenase A inhibition effects of Spatholobus suberectus on breast cancer. PLoS ONE 2013, 8, e56631. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Q.; Zhang, G.L.; Zhang, Y.; Nan, N.; Sun, X.; Yu, M.W.; Wang, H.; Li, J.P.; Wang, X.M. Spatholobus suberectus column extract inhibits estrogen receptor positive breast cancer via suppressing ER MAPK PI3K/AKT pathway. Evid. Based Complement. Altern. Med. 2016, 2016, 2934340. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cheng, Y.; Chen, J.P.; Wang, D.M. Advances in studies on chemical constituents in Spatholobi Caulis and their pharmacological activities. Chin. Tradit. Herb. Drug 2011, 42, 1229–1234. [Google Scholar]
- Tang, R.N.; Qu, X.B.; Guan, S.H.; Xu, P.P.; Shi, Y.Y.; Guo, D.A. Chemical constituents of Spatholobus suberectus. Chin. J. Nat. Med. 2012, 10, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xuan, L. New phenolic constituents from the stems of Spatholobus suberectus. Helv. Chim. Acta 2006, 89, 1241–1245. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, S.; Li, X.X.; Liu, L.L.; Han, L.F.; Wang, T. Isolation and identification of chemical constituents from Spatholobus suberectus Dunn. J. Shenyang Pharm. Univ. 2014, 31, 174–178. [Google Scholar]
- Cui, Y.J.; Liu, P.; Chen, R.Y. Studies on the active constituents in vine stem of Spatholobus suberectus. China J. Chin. Mater. Med. 2005, 30, 121–123. [Google Scholar]
- Peng, F.; Meng, C.W.; Zhou, Q.M.; Chen, J.P.; Xiong, L. Cytotoxic evaluation against breast cancer cells of isoliquiritigenin analogues from Spatholobus suberectus and their synthetic derivatives. J. Nat. Prod. 2016, 79, 248–251. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, L.; Zhao, W.; Xi-nan, M.; Huang, S.; Yang, J.; Hu, T.; Chen, H. Immunomodulatory and antioxidant effects of total flavonoids of Spatholobus suberectus Dunn on PCV2 infected mice. Sci. Rep. 2017, 7, 8676. [Google Scholar] [CrossRef]
- Li, Y.; Fang, H.; Xu, W. Recent advance in the research of flavonoids as anticancer agents. Mini-Rev. Med. Chem. 2007, 7, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Al-Maharik, N. Isolation of naturally occurring novel isoflavonoids: An update. Nat. Prod. Rep. 2019, 36, 1156–1195. [Google Scholar] [CrossRef] [PubMed]
- Kaennakam, S.; Siripong, P.; Tip-pyang, S. Cytotoxicities of two new isoflavanes from the roots of Dalbergia velutina. J. Nat. Med. 2017, 71, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Parveen, M.; Azaz, S.; Zafar, A.; Ahmad, F.; Silva, M.R.; Silva, P.S.P. Structure elucidation, DNA binding specificity and antiproliferative proficiency of isolated compounds from Garcinia nervosa. J. Photochem. Photobiol. B 2017, 167, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Porter, J.R. An isoflavone from Leiophyllum buxifolium and its antiproliferative effect. J. Nat. Prod. 2015, 78, 1748–1751. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Li, Y.K.; Du, G.; Yang, H.Y.; Gao, X.M.; Hu, Q.F. Isoflavanones from Desmodium oxyphyllum and their cytotoxicity. J. Asian Nat. Prod. Res. 2014, 16, 735–740. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kaneko, Y.; Takashima, Y. Synthetic access to optically active isoflavans by using allylic substitution. Tetrahedron 2010, 66, 197–207. [Google Scholar] [CrossRef]
- Li, Y.; Li, G.; Yu, H.; Jiao, X.; Gao, K. Antifungal activities of isoflavonoids from Uromyces striatus infected Alfalfa. Chem. Biodivers. 2018, 15, e1800407. [Google Scholar] [CrossRef]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Küpeli Akkol, E. A new isoflavane-4-ol derivative from Melilotus officinalis (L.) Pall. Nat. Prod. Res. 2019, 33, 1856–1861. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Campo Fernandez, M.; Cuesta-Rubio, O.; Márquez Hernández, I.; De Simone, F.; Rastrelli, L. Isoflavonoids isolated from Cuban propolis. J. Agric. Food Chem. 2005, 53, 9010–9016. [Google Scholar] [CrossRef] [PubMed]
- Ingham, J.L.; Dewick, P.M. A new isoflavan phytoalexin from leaflets of Lotus hispidus. Phytochemistry 1979, 18, 1711–1714. [Google Scholar] [CrossRef]
- El-Hawiet, A.M.; Toaima, S.M.; Asaad, A.M.; Radwan, M.M.; El-Sebakhy, N.A. Chemical constituents from Astragalus annularis Forssk. and A. trimestris L., Fabaceae. Braz. J. Pharmacogn. 2010, 20, 860–865. [Google Scholar] [CrossRef]
- Rauhamäki, S.; Postila, P.A.; Niinivehmas, S.; Kortet, S.; Schildt, E.; Pasanen, M.; Manivannan, E.; Ahinko, M.; Koskimies, P.; Nyberg, N.; et al. Structure-activity relationship analysis of 3-phenylcoumarin-based monoamine oxidase B inhibitors. Front. Chem. 2018, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Ichinose, K.; Takahashi, C.; Ho, F.C.; Wu, J.B.; Sankawa, U. Chemical studies on Sophora tomentosa: The isolation of a new class of isoflavonoid. Chem. Pharm. Bull. 1990, 38, 2756–2759. [Google Scholar] [CrossRef]
- Fokialakis, N.; Alexi, X.; Aligiannis, N.; Siriani, D.; Meligova, A.K.; Pratsinis, H.; Mitakou, S.; Alexis, M.N. Ester and carbamate ester derivatives of Biochanin A: Synthesis and in vitro evaluation of estrogenic and antiproliferative activities. Bioorg. Med. Chem. 2012, 20, 2962–2970. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Chang, M.; Choi, S.H.; Oh, S.R.; Wu, H.H.; Zhu, Y.; Gao, X.M.; Wang, X.; Zhang, B.; Lim, D.S.; et al. Estrogenic effects of phytoestrogens derived from Flemingia strobilifera in MCF-7 cells and immature rats. Arch. Pharm. Res. 2018, 41, 519–529. [Google Scholar] [CrossRef]
- Liu, F.; Chen, J.F.; Wang, Y.; Guo, L.; Zhou, Q.M.; Peng, C.; Xiong, L. Cytotoxicity of lanostane-type triterpenoids and ergosteroids isolated from Omphalia lapidescens on MDA-MB-231 and HGC-27 cells. Biomed. Pharmacother. 2019, 118, 109273. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| No. | 1 | 2 | 3 | 5 |
|---|---|---|---|---|
| 2a | 4.40 ddd (10.2, 3.6, 1.8) | 4.39 ddd (10.2, 2.4, 1.2) | 4.44 ddd (10.2, 3.6, 1.8) | |
| 2b | 4.03 t (10.2) | 4.02 t (10.2) | 4.07 t (10.2) | |
| 3 | 3.56 m | 3.60 m | 3.51 m | |
| 4a | 2.98 ddd (15.6, 11.4, 0.6) | 2.94 dd (15.6, 10.2) | 3.02 ddd (15.6, 10.8, 1.2) | 7.79 s |
| 4b | 2.86 ddd (15.6, 4.8, 1.2) | 2.88 dd (15.6, 4.8) | 2.90 ddd (15.6, 4.8, 1.2) | |
| 5 | 6.71 d (8.4) | 6.73 d (8.4) | 6.72 brd (8.4) | 7.23 d (8.4) |
| 6 | 6.51 d (8.4) | 6.53 d (8.4) | 6.52 d (8.4) | 7.00 d (8.4) |
| 3′ | 6.49 d (2.4) | 6.58 s | 6.36 d (2.4) | 6.61 s |
| 5′ | 6.46 dd (8.4, 2.4) | 6.48 dd (8.4, 2.4) | ||
| 6′ | 7.02 d (8.4) | 6.66 s | 7.02 d (8.4) | 6.72 s |
| OH-7 | 5.63 s | 5.66 s | 5.63 s | 6.25 s |
| OH-2′ | 4.88 brs | 7.42 s | ||
| OMe-8 | 3.91 s | 3.93 s | 3.91 s | 4.18 s |
| OMe-2′ | 3.82 s | 3.81 s | ||
| OMe-4′ | 3.80 s | 3.77 s | ||
| OCH2O | 5.90 s | 5.97 s |
| No. | 1 | 2 | 3 | 5 |
|---|---|---|---|---|
| 2 | 70.3 | 70.3 | 69.9 | 162.8 |
| 3 | 31.6 | 31.7 | 31.6 | 123.4 |
| 4 | 30.7 | 30.8 | 30.4 | 144.2 |
| 5 | 124.4 | 124.4 | 124.2 | 123.7 |
| 6 | 107.0 | 107.1 | 106.9 | 113.4 |
| 7 | 147.6 | 147.7 | 147.4 | 152.2 |
| 8 | 135.0 | 135.0 | 134.8 | 133.5 |
| 9 | 147.4 | 147.3 | 147.1 | 146.0 |
| 10 | 115.7 | 115.4 | 115.3 | 114.2 |
| 1′ | 121.8 | 121.6 | 119.7 | 115.2 |
| 2′ | 158.4 | 152.5 | 154.2 | 150.7 |
| 3′ | 98.9 | 95.0 | 102.1 | 101.6 |
| 4′ | 159.9 | 146.9 | 159.4 | 149.8 |
| 5′ | 104.3 | 141.4 | 106.0 | 142.6 |
| 6′ | 127.7 | 107.2 | 128.2 | 108.9 |
| OMe-8 | 61.1 | 61.0 | 60.9 | 62.1 |
| OMe-2′ | 55.5 | 56.6 | ||
| OMe-4′ | 55.5 | 55.3 | ||
| OCH2O | 101.3 | 101.8 |
| No. | IC50 (μM) | |
|---|---|---|
| MCF-7 | MDA-MB-231 | |
| 1 | 59.0 ± 8.1 | >100 |
| 2 | 93.6 ± 17.3 | >100 |
| 3 | >100 | >100 |
| 4 | 60.1 ± 7.4 | 34.1 ± 6.3 |
| 5 | >100 | >100 |
| 6 | >100 | 25.1 ± 7.7 |
| 7 | >100 | >100 |
| 8 | >100 | >100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, F.; Zhu, H.; Meng, C.-W.; Ren, Y.-R.; Dai, O.; Xiong, L. New Isoflavanes from Spatholobus suberectus and Their Cytotoxicity against Human Breast Cancer Cell Lines. Molecules 2019, 24, 3218. https://doi.org/10.3390/molecules24183218
Peng F, Zhu H, Meng C-W, Ren Y-R, Dai O, Xiong L. New Isoflavanes from Spatholobus suberectus and Their Cytotoxicity against Human Breast Cancer Cell Lines. Molecules. 2019; 24(18):3218. https://doi.org/10.3390/molecules24183218
Chicago/Turabian StylePeng, Fu, Huan Zhu, Chun-Wang Meng, Yan-Rui Ren, Ou Dai, and Liang Xiong. 2019. "New Isoflavanes from Spatholobus suberectus and Their Cytotoxicity against Human Breast Cancer Cell Lines" Molecules 24, no. 18: 3218. https://doi.org/10.3390/molecules24183218
APA StylePeng, F., Zhu, H., Meng, C.-W., Ren, Y.-R., Dai, O., & Xiong, L. (2019). New Isoflavanes from Spatholobus suberectus and Their Cytotoxicity against Human Breast Cancer Cell Lines. Molecules, 24(18), 3218. https://doi.org/10.3390/molecules24183218