Effect of Different Post-Harvest Processing Methods on the Chemical Constituents of Notopterygium franchetii by an UHPLC-QTOF-MS-MS Metabolomics Approach
Abstract
:1. Introduction
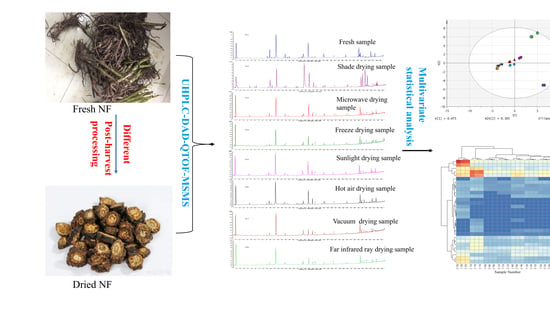
2. Results and Discussion
2.1. Method Validation and Optimization
2.1.1. Sample Preparation Optimization
2.1.2. Chromatography and MS Parameter Optimization
2.2. Methodological Validation of UHPLC
2.3. Quantification of Six Analytes in Fresh NF and NF Dried by Different Methods
2.4. Identification of Chemical Constituents of N. Franchetii
2.5. Chemical Transformation of NF during Drying
2.6. Multivariate Analyses
3. Materials and Methods
3.1. Materials and Reagents
3.2. Sample Preparation
3.2.1. Preparation of Standard Solutions
3.2.2. Sample Processing
3.2.3. Sample Preparation
3.3. UHPLC-DAD-QTOF-MS/MS Analysis
3.4. UHPLC Method Validation
3.5. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, S.; Shirakawa, A.; Shi, Y.; Yu, X.; Tamura, T.; Shibahara, N.; Yoshimatsu, K.; Komatsu, K. Impact of different post-harvest processing methods on the chemical compositions of peony root. J. Nat. Med. 2018, 72, 757–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busse, J.S.; Wiberley-Bradford, A.E.; Bethke, P.C. Transient heat stress during tuber development alters post-harvest carbohydrate composition and decreases processing quality of chipping potatoes. J. Sci. Food Agric. 2019, 99, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Dai, Y.; Cheng, H.Y.; Lam, W.; Cheng, Y.C.; Li, K.; Peng, W.H.; Pao, L.H.; Hsieh, M.T.; Qin, X.M.; et al. Improving the Concentrations of the Active Components in the Herbal Tea Ingredient, Uraria crinita: The Effect of Post-harvest Oven-drying Processing. Sci. Rep. 2017, 7, 38763. [Google Scholar] [CrossRef] [PubMed]
- Azietaku, J.T.; Ma, H.; Yu, X.A.; Li, J.; Oppong, M.B.; Cao, J.; An, M.; Chang, Y.X. A review of the ethnopharmacology, phytochemistry and pharmacology of Notopterygium incisum. J. Ethnopharmacol. 2017, 202, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Q.; Dong, J.; Wang, H.; Hashi, Y.; Chen, S.Z. Comparison of two species of Notopterygium by high-performance liquid chromatography-photodiode array detection-electrospray ionization tandem mass spectrometry. Eur. J. Mass Spectrom (Chichester) 2012, 18, 59–69. [Google Scholar] [CrossRef]
- Xu, K.; Jiang, S.; Sun, H.; Zhou, Y.; Xu, X.; Peng, S.; Ding, L. New alkaloids from the seeds of Notopterygium incisum. Nat. Prod. Res. 2012, 26, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Xiong, J.; Zhao, Y.; Cao, L.; Wu, S.B.; Xia, G.; Hu, J.F. Glycosides from the methanol extract of Notopterygium incisum. Planta Med. 2011, 77, 1939–1943. [Google Scholar] [CrossRef]
- Wu, X.W.; Wei, W.; Yang, X.W.; Zhang, Y.B.; Xu, W.; Yang, Y.F.; Zhong, G.Y.; Liu, H.N.; Yang, S.L. Anti-Inflammatory Phenolic Acid Esters from the Roots and Rhizomes of Notopterygium incisium and Their Permeability in the Human Caco-2 Monolayer Cell Model. Molecules 2017, 22, 935. [Google Scholar] [CrossRef]
- Bi, J.P.; Li, P.; Xu, X.X.; Wang, T.; Li, F. Anti-rheumatoid arthritic effect of volatile components in Notopterygium incisum in rats via anti-inflammatory and anti-angiogenic activities. Chin. J. Nat. Med. 2018, 16, 926–935. [Google Scholar] [CrossRef]
- Tang, S.Y.; Cheah, I.K.; Wang, H.; Halliwell, B. Notopterygium forbesii Boiss extract and its active constituent phenethyl ferulate attenuate pro-inflammatory responses to lipopolysaccharide in RAW 264.7 macrophages. A “protective” role for oxidative stress? Chem. Res. Toxicol. 2009, 22, 1473–1482. [Google Scholar] [CrossRef]
- Tang S., Y.; Wang, H.; Zhang, W.; Halliwell, B. Notopterygium forbesii Boiss extract and its active constituents increase reactive species and heme oxygenase-1 in human fetal hepatocytes: mechanisms of action. Chem. Res. Toxicol. 2008, 21, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, S.; Xu, K.; Sun, H.; Zhou, Y.; Xu, X.; Yi, J.; Gu, Y.; Ding, L.S. Quantitative analysis of chemical constituents in different commercial parts of Notopterygium incisum by HPLC-DAD-MS. J. Ethnopharmacol. 2009, 126, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.S.; Wang, Q.; Leung, K.S.; Qin, Y.; Zhao, Z.; Jiang, Z.H. Quality assessment of Rhizoma et Radix Notopterygii by HPTLC and HPLC fingerprinting and HPLC quantitative analysis. J. Pharm. Biomed. Anal. 2007, 44, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Jiang, S.Y.; Guan, X.L.; Zhou, Y.; Huang, S.X. Quantitative Determination of the Chemical Profile of the Plant Material “Qiang-huo” by LC-ESI-MS-MS. Chromatographia. 2006, 64, 405–410. [Google Scholar] [CrossRef]
- Li, Y.; Hong, Y.; Han, Y.; Wang, Y.; Xia, L. Chemical characterization and antioxidant activities comparison in fresh, dried, stir-frying and carbonized ginger. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1011, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Krüger, S.; Mirgos, M.; Morlock, G.E. Effect-directed analysis of fresh and dried elderberry (Sambucus nigra L.) via hyphenated planar chromatography. J. Chromatogr. A 2015, 1426, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Kaškonienė, V.; Stankevičius, M.; Drevinskas, T.; Akuneca, I.; Kaškonas, P.; Bimbiraitė-Survilienė, K.; Maruška, A.; Ragažinskienė, O.; Kornyšova, O.; Briedis, V.; et al. Evaluation of phytochemical composition of fresh and dried raw material of introduced Chamerion angustifolium L. using chromatographic, spectrophotometric and chemometric techniques. Phytochemistry 2015, 115, 184–193. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, D.; Dong, Y.; Ju, H.; Wu, C.; Lin, S. Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried Tricholoma matsutake Singer by HS-GC-IMS and HS-SPME-GC-MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1099, 46–55. [Google Scholar] [CrossRef]
- Yuan, M.; Yan, Z.G.; Liu, Y.; Chen, D.Q.; Yang, Z.J.; He, L.L.; Zhang, Z.F. Chemical profiles, antioxidant activity and acute toxicity of raw and sulfur-fumigated Smilacis Glabrae Rhizoma. J. Ethnopharmacol. 2019, 234, 76–84. [Google Scholar] [CrossRef]
- Li, X.B.; Wang, W.; Zhou, G.J.; Li, Y.; Xie, X.M.; Zhou, T.S. Production of salvianolic acid B in roots of Salvia miltiorrhiza (Danshen) during the post-harvest drying process. Molecules 2012, 17, 2388–2407. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L. Comparison of two species of Notopterygium by GC-MS and HPLC. Molecules 2015, 20, 5062–5073. [Google Scholar] [CrossRef] [PubMed]
- Teye Azietaku, J.; Yu, X.A.; Li, J.; Hao, J.; Cao, J.; An, M.; Tan, Z.; Chang, Y.X. Simultaneous Determination of Bergapten, Imperatorin, Notopterol, and Isoimperatorin in Rat Plasma by High Performance Liquid Chromatography with Fluorescence Detection and Its Application to Pharmacokinetic and Excretion Study after Oral Administration of Notopterygium incisum Extract. Int. J. Anal. Chem. 2016, 2016, 9507246. [Google Scholar] [PubMed]
- Xu, K.; Jiang, S.; Zhou, Y.; Zhang, Y.; Xia, B.; Xu, X.; Zhou, Y.; Li, Y.; Wang, M.; Ding, L. Discrimination of the seeds of Notopterygium incisum and Notopterygium franchetii by validated HPLC-DAD-ESI-MS method and principal component analysis. J. Pharm. Biomed. Anal. 2011, 56, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Dionísio, A.P.; Abreu, F.; Brito, E.S.; Wurlitzer, N.J.; Silva, L.; Ribeiro, P.; Rodrigues, S.; Taniguchi, C.; Pontes, D.F. Evaluation of nutritional and chemical composition of yacon syrup using 1H-NMR and UPLC-ESI-Q-TOF-MSE. Food Chem. 2018, 245, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Q.; Zheng, X.X.; Chen, X.; Yang, D.Z.; Du, Q. Quantitative and qualitative analysis of common peaks in chemical fingerprint of Yuanhu Zhitong tablet by HPLC-DAD-MS/MS. J. Pharm. Anal. 2014, 4, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cai, H.; Cao, G.; Duan, Y.; Pei, K.; Tu, S.; Zhou, J.; Xie, L.; Sun, D.; Zhao, J.; et al. Profiling and analysis of multiple constituents in Baizhu Shaoyao San before and after processing by stir-frying using UHPLC/Q-TOF-MS/MS coupled with multivariate statistical analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1083, 110–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, S.; Xu, K.; Shi, H.; Zhou, Y.; Deng, W.; Ding, L.; Peng, S. Chemical constituents contained in seeds of Notopterygium franchetii. Zhongguo Zhong Yao Za Zhi. 2012, 37, 941–945. [Google Scholar]
- Wang, L.; Sang, M.; Liu, E.; Banahene, P.O.; Zhang, Y.; Wang, T.; Han, L.; Gao, X. Rapid profiling and pharmacokinetic studies of major compounds in crude extract from Polygonum multiflorum by UHPLC-Q-TOF-MS and UPLC-MS/MS. J. Pharm. Biomed. Anal. 2017, 140, 45–61. [Google Scholar] [CrossRef]
- De la Luz Cádiz-Gurrea, M.; Fernández-Arroyo, S.; Joven, J.; Segura-Carretero, A. Comprehensive characterization by UHPLC-ESI-Q-TOF-MS from an Eryngium bourgatii extract and their antioxidant and anti-inflammatory activities. Food Res. Int. 2013, 50, 197–204. [Google Scholar] [CrossRef]
- Wu, X.W.; Zhang, Y.B.; Zhang, L.; Yang, X.W. Simultaneous quantification of 33 active components in Notopterygii Rhizoma et Radix using ultra high performance liquid chromatography with tandem mass spectrometry. J. Chromatogr. B 2018, 1092, 244–251. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.W. Studies on chemical constituents in roots and rhizomes of Notopterygium incisum. Zhongguo Zhong Yao Za Zhi. 2008, 33, 2918–2921. [Google Scholar] [PubMed]
- Ismail, B.B.; Pu, Y.; Guo, M.; Ma, X.; Liu, D. LC-MS/QTOF identification of phytochemicals and the effects of solvents on phenolic constituents and antioxidant activity of baobab (Adansonia digitata) fruit pulp. Food Chem. 2019, 277, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Kaur, N. Metabolic fingerprinting of different populations of Phyllanthus niruri L. from Punjab using electrospray ionization mass spectrometry (ESI–MS). Med. Chem. Res. 2016, 25, 2798–2821. [Google Scholar] [CrossRef]
- Zhao, P.; Duan, L.; Guo, L.; Dou, L.L.; Dong, X.; Zhou, P.; Liu, E.H. Chemical and biological comparison of the fruit extracts of Citrus wilsonii Tanaka and Citrus medica L. Food Chem. 2015, 173, 54–60. [Google Scholar] [CrossRef]
- Han, J.; Ye, M.; Yang, M.; Wang, B.R.; Guo, D.A. Analysis of multiple constituents in a Chinese herbal preparation Shuang-Huang-Lian oral liquid by HPLC-DAD-ESI-MSn. J. Pharm. Biomed. Anal. 2007, 140, 45–61. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, X.; Dong, F.; Watanabe, N.; Yang, Z. Increasing postharvest high-temperatures lead to increased volatile phenylpropanoids/benzenoids accumulation in cut rose (Rosa hybrida) flowers. Postharvest Biol. Tec. 2019, 148, 68–75. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (1–30) are not available from the authors. |






| Analyte | Y = ax + b | r | Range (μg/mL) | LOD (ng) | LOQ (ng) | Precision (n = 6) RSD | Stability (n = 6) RSD | Repeatability (n = 6) RSD | Recovery (n = 6) RSD | |
|---|---|---|---|---|---|---|---|---|---|---|
| a | b | |||||||||
| nodakenin | 2.95 × 106 | −1127 | 0.9996 | 1.18–472 | 26 | 78 | 1.09% | 1.46% | 1.23% | 2.56% |
| psoralen | 2.43 × 107 | 821 | 0.9999 | 0.44–176 | 15 | 44 | 1.11% | 2.25% | 1.79% | 1.75% |
| bergapten | 5.48 × 107 | 873 | 0.9999 | 0.35–140 | 10 | 28 | 1.12% | 0.95% | 1.51% | 0.74% |
| notopterol | 2.97 × 107 | 240 | 0.9999 | 0.58–234 | 19 | 58 | 0.57% | 2.41% | 1.03% | 1.43% |
| imperatorin | 2.88 × 107 | 725 | 0.9999 | 0.30–120 | 16 | 48 | 0.74% | 2.87% | 2.03% | 2.06% |
| isoimperatorin | 1.42 × 107 | 6764 | 0.9999 | 0.20–80 | 9 | 26 | 0.42% | 0.95% | 1.42% | 0.99% |
| No. | Drying Method | Nodakenin | Psoralen | Bergapten | Notopterol | Imperatorin | Isoimperatorin | Summary | Reduction Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| S1 | Fresh sample | 24.31 | 0.17 | 0.30 | 0.13 | 0.57 | 9.79 | 35.27 | - |
| S2 | Shade drying | 14.84 | 0.08 | 0.31 | 0.06 | 5.17 | 3.06 | 23.52 | 33.31 |
| S3 | Microwave drying | 7.18 | 0.04 | 0.40 | 0.07 | 0.21 | 5.00 | 12.90 | 63.44 |
| S4 | Freeze drying | 10.4 | 0.05 | 0.22 | 0.07 | 0.28 | 4.92 | 15.94 | 54.80 |
| S5 | Sunlight drying | 10.94 | 0.07 | 0.39 | 0.08 | 0.39 | 5.55 | 17.42 | 50.61 |
| S6 | Hot air drying | 14.22 | 0.08 | 0.21 | 0.11 | 0.53 | 8.99 | 24.14 | 31.55 |
| S7 | Vacuum drying | 8.75 | 0.05 | 0.16 | 0.06 | 0.19 | 5.08 | 14.29 | 59.47 |
| S8 | Far infrared ray drying | 10.12 | 0.03 | 0.10 | 0.06 | 0.22 | 4.27 | 14.80 | 58.04 |
| Peak No. | Retention (min) | Molecular Formula | Quasi-molecular[M-H] = [M + Cl/COOH]−(error, ppm) | Quasi-Molecular[M + H/Na]+(error, ppm) | MS/MS Fragments Ions | Identification |
|---|---|---|---|---|---|---|
| 1 | 4.495 | C16H18O9 | 353.0878(0.02) | 191.0466,173.0452,135.0427 | Chlorogenic acid [24] | |
| 2 | 5.754 | C29H50O | 415.3959(−5.92) | 399.1085,255.0318,211.9974 | β-Sitosterol [25] | |
| 3 | 6.11 | C28H32O15 | 609.1819(−0.77) | 463.1250,301.0724,203.0353 | Diosmin [25] | |
| 4 | 9.17 | C9H6O3 | 161.0244(−0.08) | 133.0297,119.0194,106.2345 | Umbelliferone [26] | |
| 5 | 9.779 | C20H24O9 | 409.1482(2.71) | 246.9364,228.8693,174.5889 | Isomer of nodakenin [27] | |
| 6 | 10.313 | C10H10O4 | 193.0506(1.20) | 178.0160,134.0372,105.0353 | Ferulic acid [28] | |
| 7 | 10.941 | C13H10O5 | 245.0479(−9.6) | 227.0690,211.0387,159.0454 | Isopimpinellin [29] | |
| 8 | 11.463 | C20H24O9 | 409.1482(2.71) | 246.9364,228.8695,174.5887 | Nodakenin [27] | |
| 9 | 13.416 | C21H32O2 | 317.2474(0.34) | 183.0106,119.0331,102.0110 | Pregnenolone [25] | |
| 10 | 13.847 | C11H6O3 | 187.0402(−6.57) | 143.0504,131.0500,115.0546 | Psoralen [25] | |
| 11 | 14.505 | C20H24O10 | 447.1233(6.41) | 241.0875,179.0853,127.0393 | Decuroside V [30] | |
| 12 | 14.978 | C25H24O12 | 517.1314(5.13) | 355.1988,200.7070,156.4883 | 3,5-Dicaffeoylquinic acid [31] | |
| 13 | 16.581 | C21H24O11 | 453.1364(6.04) | 322.2452,283.1529,208.8737 | (−)-Catechin-7-O-glucoside [32] | |
| 14 | 16.894 | C16H16O4 | 271.0970(−0.43) | 225.2215,137.0230,106.0417 | p-Hydroxyphenethyl anisate [25] | |
| 15 | 17.442 | C26H26O12 | 529.1315(6.9) | 363.1776,247.1300,159.0230 | 1-Caffeoyl-5-feruloylquinic acid [33] | |
| 16 | 18.314 | C16H16O6 | 305.0991(9.39) | 202.7124,174.58312,158.5002 | Oxypeucedanin hydrate [34] | |
| 17 | 18.761 | C11H6O4 | 203.0354(−7.46) | 159.0448,147.0450,131.0502 | Bergaptol [14] | |
| 18 | 19.943 | C30H32O12 | 585.1998(−5.38) | 405.1338,247.0979,177.0560 | 6′-O-trans-feruloyl nodakenin [27] | |
| 19 | 20.933 | C16H14O4 | 271.0959(2.16) | 201.0558,173.0608,145.0658 | Isomer of imperatorin [35] | |
| 20 | 21.614 | C12H8O4 | 261.0424(−7.43) | 201.7049,176.6158,145.4164 | Bergapten [35] | |
| 21 | 23.623 | C16H12O6 | 299.0566(−1.63) | 284.0314,256.0362,183.0440 | Diosmetin [14] | |
| 22 | 24.016 | C21H22O4 | 337.1440(1.58) | 201.0172,173.0205,109.0287 | Anhydronotoptol [7] | |
| 23 | 26.571 | C21H22O5 | 355.1566(−7.32) | 172.5619,216.7848,272.8885 | Notopterol [35] | |
| 24 | 29.892 | C16H14O5 | 287.0936(−7.66) | 203.0355,175.0402,147.0450 | Oxypeucedanin [7] | |
| 25 | 33.391 | C18H18O5 | 349.0858(−2.79) | 149.0610,134.0364,117.0335 | p-Hydroxyphenethyl ferulate [31] | |
| 26 | 33.725 | C18H18O4 | 297.1137(−1.57) | 183.0132,160.8449,136.9432 | Phenethyl ferulate [35] | |
| 27 | 34.376 | C20H26O4 | 329.1756(0.71) | 314.1504,177.0181,133.0289 | Bornyl ferulate [35] | |
| 28 | 34.948 | C16H14O4 | 271.0959(2.16) | 201.0557,173.0609,145.0658 | Imperatorin [35] | |
| 29 | 36.501 | C16H14O4 | 271.0959(2.16) | 201.0559,173.0608,145.0658 | Isoimperatorin [35] | |
| 30 | 37.301 | C19H22O3 | 299.1628(4.58) | 175.0400,145.0295,119.0498 | 7-Geranyloxycoumarin [35] |
| Peak No. | Retention (min) | Fold Change | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fresh Sample | Shade Drying | Microwave Drying | Freeze Drying | Sun Drying | Hot Air Drying | Vacuum Drying | Far infrared Ray Drying | ||
| 1 | 4.495 | 1.00 | 3.47 | 3.31 | 0.29 | 1.85 | 1.76 | 0.89 | 0.00 |
| 2 | 5.754 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 3 | 6.110 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 4 | 9.170 | 1.00 | 0.88 | 0.42 | 0.28 | 0.52 | 0.84 | 0.30 | 0.39 |
| 5 | 9.779 | 1.00 | 0.76 | 0.06 | 0.16 | 0.13 | 0.20 | 0.08 | 0.07 |
| 6 | 10.313 | 1.00 | 1.13 | 0.98 | 0.41 | 0.87 | 0.95 | 0.00 | 0.00 |
| 7 | 10.941 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 8 | 11.463 | 1.00 | 0.61 | 0.30 | 0.43 | 0.45 | 0.58 | 0.36 | 0.42 |
| 9 | 13.416 | 1.00 | 1.90 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | 13.847 | 1.00 | 0.47 | 0.24 | 0.29 | 0.41 | 0.47 | 0.29 | 0.18 |
| 11 | 14.505 | 1.00 | 0.00 | 0.38 | 0.00 | 0.77 | 0.81 | 0.00 | 0.00 |
| 12 | 14.978 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 13 | 16.581 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 14 | 16.894 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 1.09 | 0.00 | 0.00 |
| 15 | 17.442 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.88 | 0.00 | 0.00 |
| 16 | 18.314 | 1.00 | 0.00 | 0.90 | 0.62 | 0.00 | 0.58 | 0.00 | 0.39 |
| 17 | 18.761 | 1.00 | 6.17 | 0.27 | 0.29 | 0.41 | 0.39 | 0.33 | 0.32 |
| 18 | 19.943 | 1.00 | 0.73 | 0.19 | 0.33 | 0.33 | 0.48 | 0.08 | 0.12 |
| 19 | 20.933 | 1.00 | 9.38 | 8.62 | 1.19 | 4.59 | 6.94 | 1.61 | 1.07 |
| 20 | 21.614 | 1.00 | 1.03 | 1.33 | 0.73 | 1.30 | 0.67 | 0.53 | 0.33 |
| 21 | 23.623 | 0.00 | 3.66 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 22 | 24.016 | 1.00 | 0.31 | 0.34 | 0.12 | 0.19 | 0.21 | 0.13 | 0.09 |
| 23 | 26.571 | 1.00 | 0.46 | 0.54 | 0.54 | 0.62 | 0.77 | 0.46 | 0.46 |
| 24 | 29.892 | 1.00 | 0.48 | 0.34 | 0.30 | 0.29 | 0.38 | 0.26 | 0.27 |
| 25 | 33.391 | 1.00 | 0.77 | 0.26 | 0.36 | 0.34 | 0.47 | 0.29 | 0.30 |
| 26 | 33.725 | 1.00 | 2.92 | 0.25 | 0.21 | 0.19 | 1.34 | 0.20 | 0.85 |
| 27 | 34.376 | 1.00 | 25.62 | 0.64 | 0.93 | 1.20 | 2.41 | 0.89 | 0.83 |
| 28 | 34.948 | 1.00 | 8.72 | 0.36 | 0.50 | 0.69 | 0.92 | 0.34 | 0.38 |
| 29 | 36.501 | 1.00 | 0.38 | 0.51 | 0.50 | 0.57 | 0.82 | 0.52 | 0.44 |
| 30 | 37.301 | 1.00 | 1.04 | 0.93 | 0.58 | 0.61 | 0.72 | 0.23 | 0.34 |
| No. | Drying Methods | Drying Parameters | Duration | Moisture (%) |
|---|---|---|---|---|
| Temp. or Power | ||||
| S2 | Shade drying | 22–25 °C | 10 d | 9.35 |
| S3 | Microwave drying | 2450 MHz | 30 min | 8.75 |
| S4 | Freeze drying | −35 °C | 8 h | 9.28 |
| S5 | Sunlight drying | 22–30 °C | 4 d | 10.25 |
| S6 | Hot air drying | 50 °C | 2 d | 10.35 |
| S7 | Vacuum drying | 50 °C | 4 h | 7.82 |
| S8 | Far infrared ray drying | 80–85 °C, 1125 W | 3h | 8.54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Wu, Y.; Li, Y.; Huang, Y.; Liu, Y.; Luo, P.; Zhang, Z. Effect of Different Post-Harvest Processing Methods on the Chemical Constituents of Notopterygium franchetii by an UHPLC-QTOF-MS-MS Metabolomics Approach. Molecules 2019, 24, 3188. https://doi.org/10.3390/molecules24173188
Su X, Wu Y, Li Y, Huang Y, Liu Y, Luo P, Zhang Z. Effect of Different Post-Harvest Processing Methods on the Chemical Constituents of Notopterygium franchetii by an UHPLC-QTOF-MS-MS Metabolomics Approach. Molecules. 2019; 24(17):3188. https://doi.org/10.3390/molecules24173188
Chicago/Turabian StyleSu, Xueyan, Youjiao Wu, Ying Li, Yanfei Huang, Yuan Liu, Pei Luo, and Zhifeng Zhang. 2019. "Effect of Different Post-Harvest Processing Methods on the Chemical Constituents of Notopterygium franchetii by an UHPLC-QTOF-MS-MS Metabolomics Approach" Molecules 24, no. 17: 3188. https://doi.org/10.3390/molecules24173188