Development of 11-DGA-3-O-Gal-Modified Cantharidin Liposomes for Treatment of Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Structure Confirmation of 11-DGA-3-O-Gal
2.2. Characterization of Liposomes
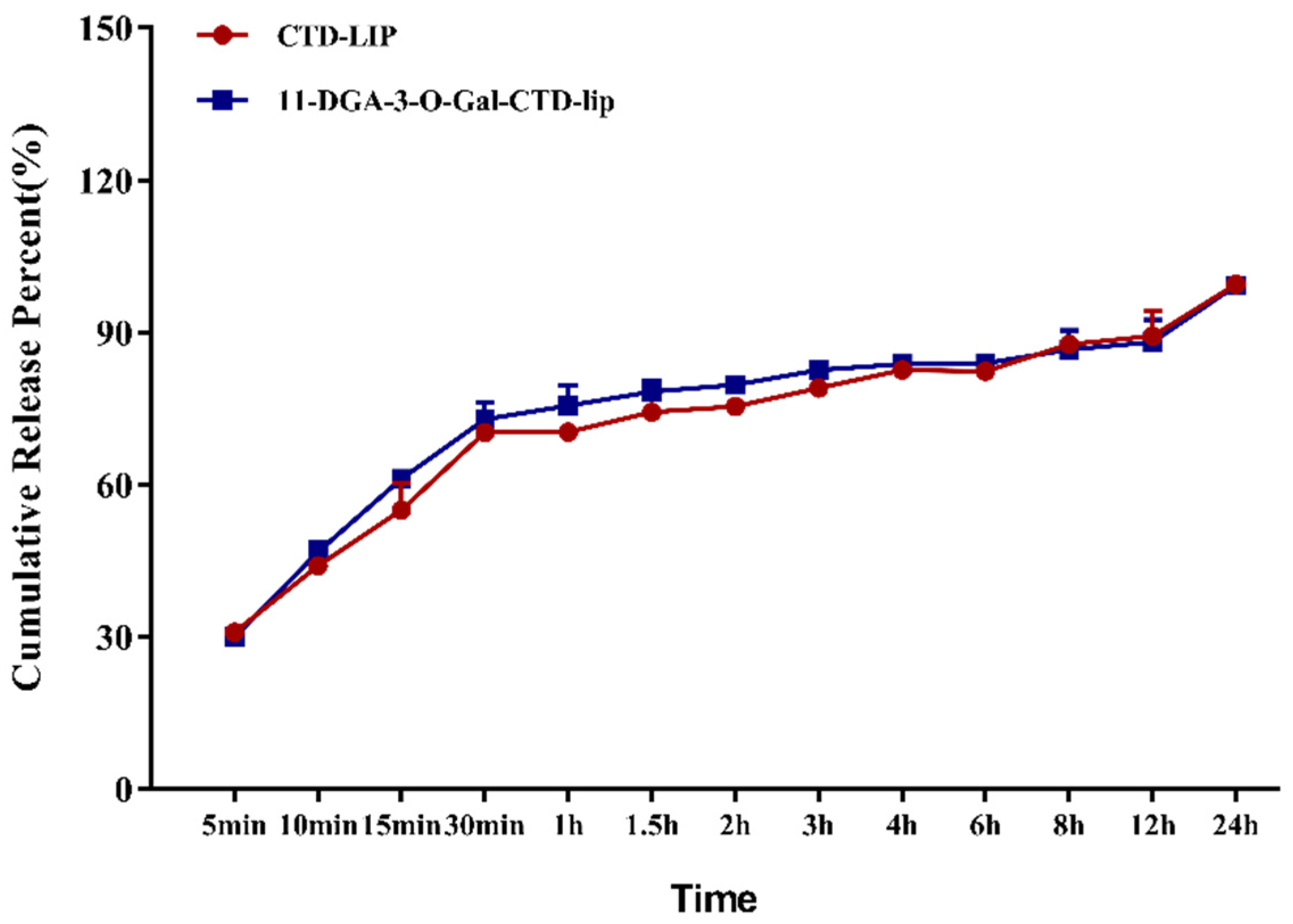
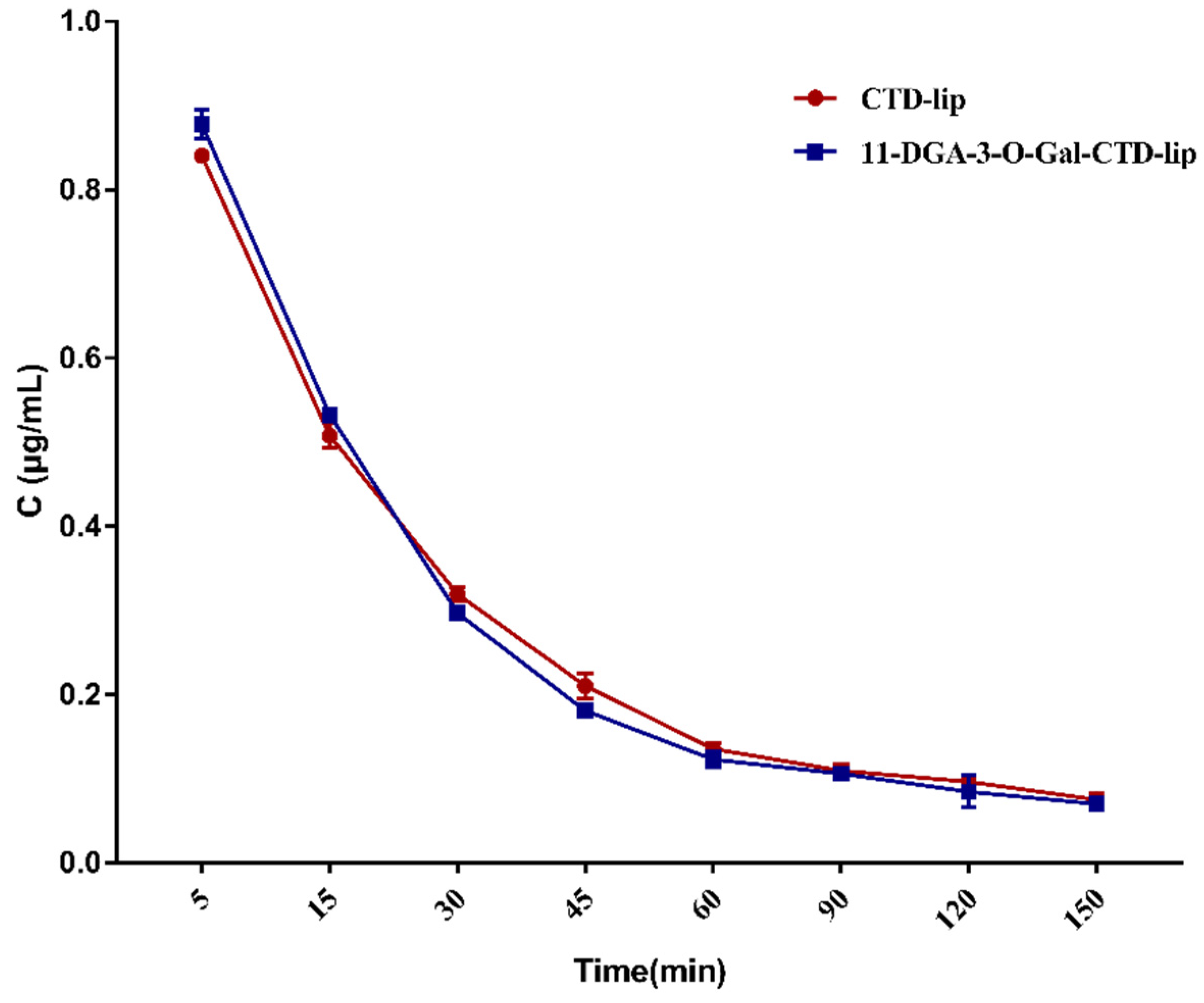
2.3. In Vitro Release of Cantharidin (CTD)
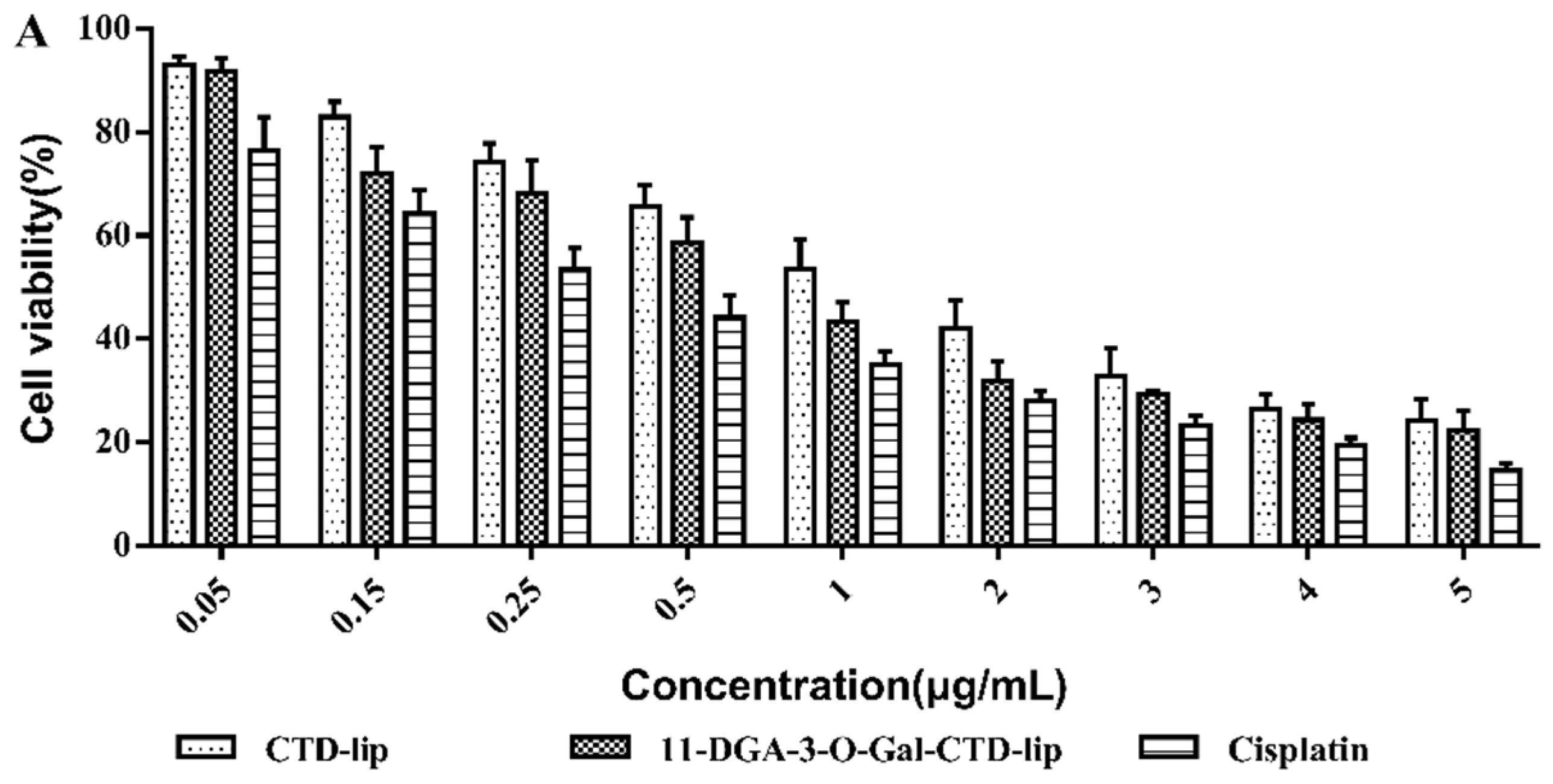
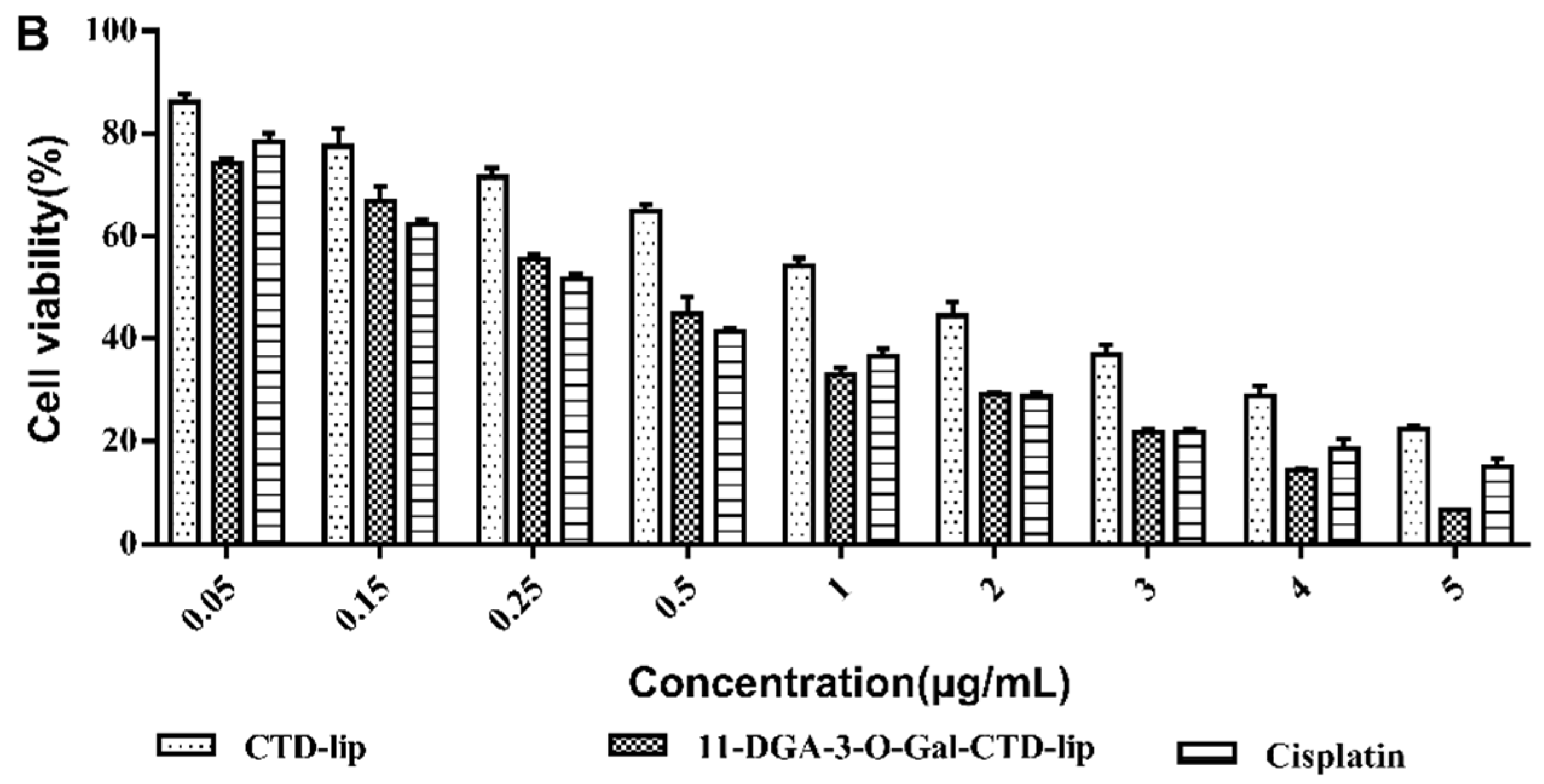
2.4. In Vitro Cytotoxicity Assay
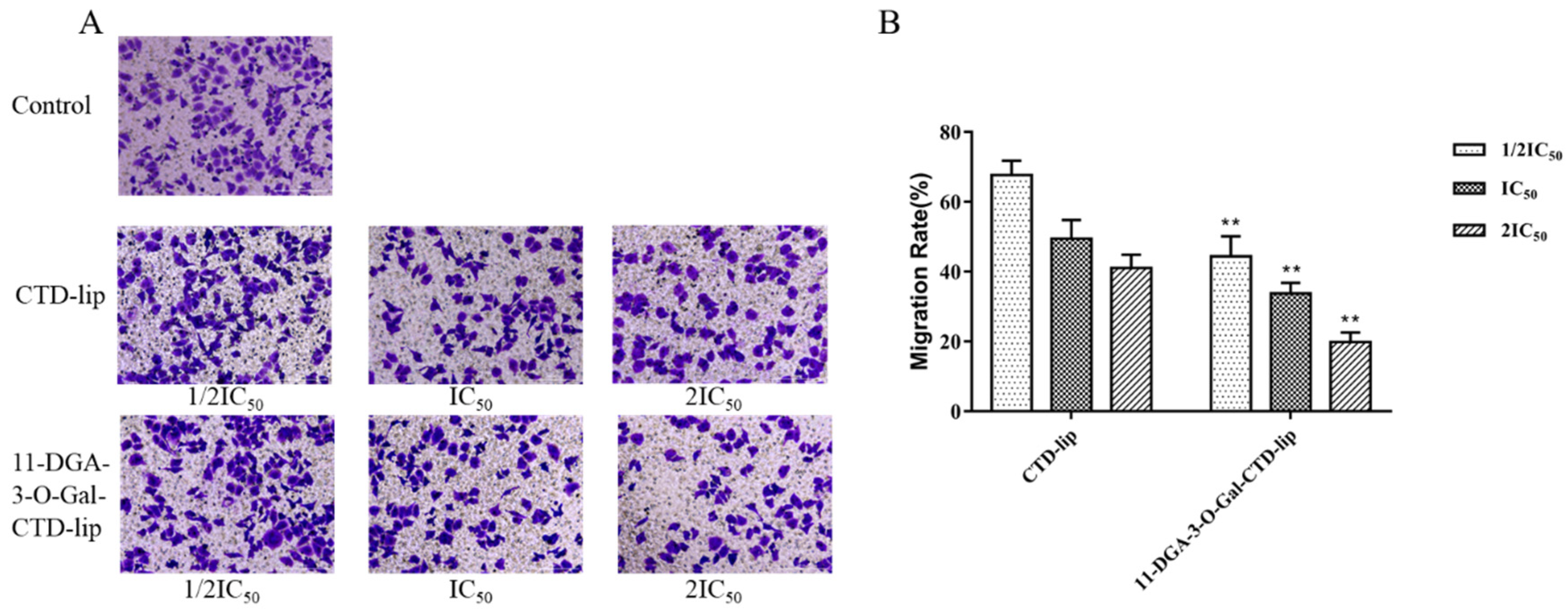
2.5. Cell Migration Assays
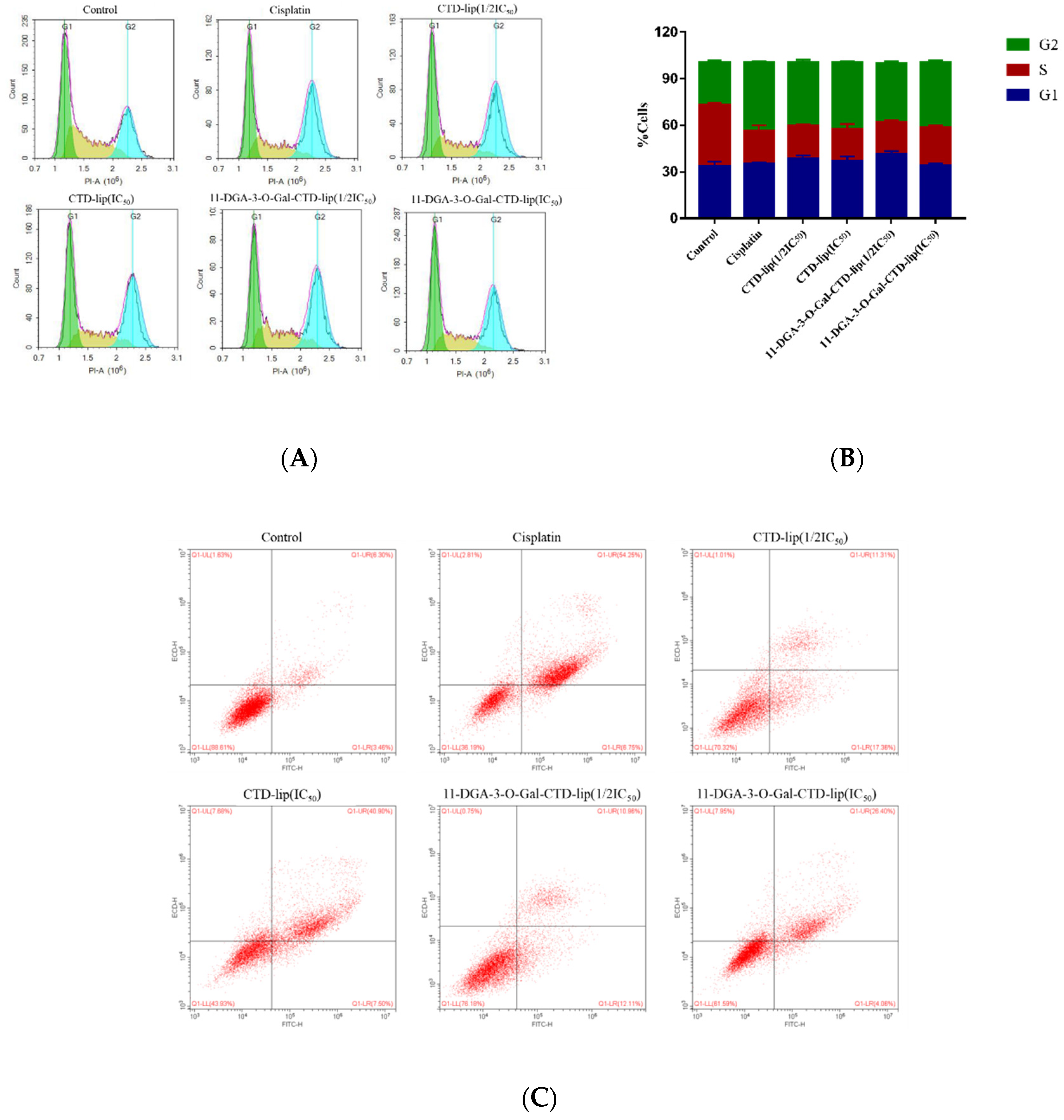
2.6. Inhibition Mechanism of 11-DGA-3-O-Gal-CTD-Lip in HepG2 Cells
2.7. In Vivo Pharmacokinetics Study
2.8. Tissue Distribution Study
3. Materials
4. Methods
4.1. Synthesis of 11-DGA-3-O-Gal
4.2. Preparation of Liposomes
4.3. Physicochemical Characterization of Liposomes
4.4. In Vitro Release of CTD
4.5. In Vitro Cytotoxicity Assay
4.6. Cell Migration Assays
4.7. Cell-Cycle and Cell Apoptosis Detection
4.8. Pharmacokinetic Studies
4.9. Tissue Distributions
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends an Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, A.B.; Eheman, C.R.; Altekruse, S.F.; Ward, J.W.; Jemal, A.; Sherman, R.L.; Henley, S.J.; Holtzman, D.; Lake, A.; Noone, A.M.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016, 122, 1312–1337. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; You, F.Y.; Peng, Q.; Gao, Y. Current Situation and Prospect of Human Primary Hepatocellular Carcinoma Cell Culture. J. Pract. Med. 2018, 34, 169–175. [Google Scholar]
- Siafaka, P.I.; Karavas, E.; Bikiaris, D.N. Surface Modified Multifunctional and Stimuli Responsive Nanoparticles for Drug Targeting: Current Status and Uses. Int. J. Mol. Sci. 2016, 17, 1440. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Lenhard, R.K. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.Y.; Ren, Z.G.; Zhou, J.; Fan, J.; Gao, Q. Critical appraisal of Chinese 2017 guideline on the management of hepatocellular carcinoma. Hepatobiliary Surg. Nutr. 2017, 6, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.L.; Norhaizan, M.E. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef]
- Takara, K.; Sakaeda, T.; Okumura, K. An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Curr. Pharm. Des. 2006, 12, 273–286. [Google Scholar] [CrossRef]
- Zheng, L.H.; Bao, Y.L.; Wu, Y.; Yu, C.L.; Meng, X.; Li, Y.X. Cantharidin reverses multidrug resistance of human hepatoma HepG2/ADM cells via down-regulation of P-glycoprotein expression. Cancer Lett. 2008, 272, 102–109. [Google Scholar] [CrossRef]
- Su, C.C.; Lee, K.I.; Chen, M.K.; Kuo, C.Y.; Tang, C.H.; Liu, S.H. Cantharidin Induced Oral Squamous Cell Carcinoma Cell Apoptosis via the JNK-Regulated Mitochondria and Endoplasmic Reticulum Stress-Related Signaling Pathways. PLoS ONE 2016, 11, e0168095. [Google Scholar] [CrossRef]
- Xu, M.D.; Liu, L.; Wu, M.Y.; Jiang, M.; Shou, L.M.; Wang, W.J.; Wu, J.; Zhang, Y.; Gong, F.R.; Chen, K.; et al. The combination of cantharidin and antiangiogenic therapeutics presents additive antitumor effects against pancreatic cancer. Oncogenesis 2018, 7, 94–103. [Google Scholar] [CrossRef]
- McCluskey, A.; Bowyer, M.C.; Collins, E.; Sim, A.T.; Sakoff, J.A.; Baldwin, M.L. Anhydride modified cantharidin analogues: Synthesis, inhibition of protein phosphatases 1 and 2A and anticancer activity. Bioorganic Med. Chem. Lett. 2000, 10, 1687–1690. [Google Scholar] [CrossRef]
- Li, H.C.; Xia, Z.H.; Chen, Y.F.; Yang, F.; Feng, W.; Cai, H.; Mei, Y.; Jiang, Y.M.; Xu, K.; Feng, D.X. Cantharidin Inhibits the Growth of Triple-Negative Breast Cancer Cells by Suppressing Autophagy and Inducing Apoptosis in Vitro and in Vivo. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 43, 1829–1843. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, Q.; Ni, W.; Wei, Z.; Yu, S.; Jia, Q.; Wang, M.; Wang, A.; Chen, W.; Lu, Y. Breaking Glucose Transporter 1/Pyruvate Kinase M2 Glycolytic Loop Is Required for Cantharidin Inhibition of Metastasis in Highly Metastatic Breast Cancer. Front Pharmacol. 2019, 10, 590–601. [Google Scholar] [CrossRef]
- Chen, C.C.; Chueh, F.S.; Peng, S.F.; Huang, W.W.; Tsai, C.H.; Tsai, F.J.; Huang, C.Y.; Tang, C.H.; Yang, J.S.; Hsu, Y.M.; et al. Cantharidin decreased viable cell number in human osteosarcoma U-2 OS cells through G2/M phase arrest and induction of cell apoptosis. Biosci. Biotechnol. Biochem. 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.P.; Li, L.; Xu, L.; Dai, E.; Chen, W.D. Cantharidin suppresses cell growth and migration, and activates autophagy in human non-small cell lung cancer cells. Oncol. Lett. 2018, 15, 6527–6532. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Z. The Effects of Cantharidin and Cantharidin Derivates on Tumour Cells. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2009, 9, 392–396. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, C.C.; Chan, W.K.N.; Liu, K.L.; Yang, Z.J.; Zhang, H.Q. Augmented Anticancer Effects of Cantharidin with Liposomal Encapsulation: In Vitro and in Vivo Evaluation. Molecules 2017, 22, 1052. [Google Scholar] [CrossRef]
- Zou, J.J.; Zhang, S.Q.; Feng, R.X. The Toxicity and Pharmacokinetics of Cantharidin. J. China Pharm. Univ. 2002, 5, 40–43. [Google Scholar]
- Zhang, Y.; Zhou, X.; Zhang, J.; Guan, C.; Liu, L. Cantharides poisoning: A retrospective analysis from 1996 to 2016 in China. Regul. Toxicol. Pharmacol. 2018, 96, 142–145. [Google Scholar] [CrossRef]
- Zahednezhad, F.; Saadat, M.; Valizadeh, H.; Zakeri-Milani, P.; Baradaran, B. Liposome and immune system interplay: Challenges and potentials. J. Control. Release 2019, 305, 194–209. [Google Scholar] [CrossRef]
- Sriraman, S.K.; Torchilin, V.P. Recent Advances with Liposomes as Drug Carriers. Adv. Biomater. Biodevices 2014, 2, 79–119. [Google Scholar]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Huang, L.; Gauthier, M.; Yang, G.; Wang, Q. Recent advances in liposome surface modification for oral drug delivery. Nanomedicine 2016, 11, 1169–1185. [Google Scholar] [CrossRef]
- Li, C.; Lai, C.; Qiu, Q.; Luo, X.; Hu, L.; Zheng, H.; Lu, Y.; Liu, M.; Zhang, H.; Liu, X.; et al. Dual-Ligand Modification of PEGylated Liposomes Used for Targeted Doxorubicin Delivery to Enhance Anticancer Efficacy. AAPS PharmSciTech 2019, 20, 188–196. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, L.; Cheng, L.; Huang, F.; Hu, Q.; Li, L.; Tian, C.; Wei, L.; Chen, D. Folate and TAT peptide co-modified liposomes exhibit receptor-dependenthighly efficient intracellular transport of payload in vitro and in vivo. Pharm. Res. 2014, 31, 3289–3303. [Google Scholar] [CrossRef]
- Gao, W.; Meng, T.; Shi, N.; Zhuang, H.; Yang, Z.; Qi, X. Targeting and Microenvironment-Responsive Lipid Nanocarrier for the Enhancement of Tumor Cell Recognition and Therapeutic Efficiency. Adv. Healthc. Mater. 2015, 4, 748–759. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, Y.; Zhuang, Q.; Cui, L.; Xu, X.; Xu, R.; He, X. Anti-tumor effect of RGD modified PTX loaded liposome on prostatic cancer. Int. J. Clin. Exp. Med. 2015, 8, 12182–12191. [Google Scholar]
- Gao, Y.; Lu, L.; Xu, L.; Qi, T.; Jin, L.; Xu, L.; Xiao, M. Advances in asialoglycoprotein receptor-mediated liver cancer targeted drug delivery system. J. China Pharm. Univ. 2016, 47, 537–542. [Google Scholar]
- Zhao, R.; Li, T.; Zheng, G.; Jiang, K.; Fan, L.; Shao, J. Simultaneous inhibition of growth and metastasis of hepatocellular carcinoma by co-delivery of ursolic acid and sorafenib using lactobionic acid modified and pH-sensitive chitosan-conjugated mesoporous silica nanocomplex. Biomaterials 2017, 143, 1–16. [Google Scholar] [CrossRef]
- D’Souza, A.A.; Devarajan, P.V. Asialoglycoprotein receptor mediated hepatocyte targeting strategi-es and applications. J. Control. Release 2015, 203, 126–139. [Google Scholar] [CrossRef]
- Turato, C.; Balasso, A.; Carloni, V.; Tiribelli, C.; Mastrotto, F.; Mazzocca, A.; Pontisso, P. New molecular targets for functionalized nanosized drug delivery systems in personalized therapy for hepatocellular carcinoma. J. Control. Release 2017, 268, 184–197. [Google Scholar] [CrossRef]
- Fu, L.; Sun, C.; Yan, L. Galactose Targeted pH-Responsive Copolymer Conjugated with Near Infrared Fluorescence Probe for Imaging of Intelligent Drug Delivery. Acs Appl. Mater. Interfaces 2015, 7, 2104–2115. [Google Scholar] [CrossRef]
- Yeeprae, W.; Kawakami, S.; Higuchi, Y.; Yamashita, F.; Hashida, M. Biodistribution characteristics of mannosylated and fucosylated O/W emulsions in mice. J. Drug Target. 2005, 13, 479–487. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, H.; Wu, Y.; Li, Y.; Gao, Y. A novel glycyrrhetinic acid-modified oxaliplatin liposome for liver targeting and in vitro/vivo evaluation. Drug Des. Dev. Ther. 2015, 9, 2265–2275. [Google Scholar]
- Chen, Q.; Ding, H.; Zhou, J.; Zhao, X.; Zhang, J.; Yang, C.; Li, K.; Qiao, M.; Hu, H.; Ding, P.; et al. Novel glycyrrhetinic acid conjugated pH-sensitive liposomes for the delivery of doxorubicin and its antitumor activities. RSC Adv. 2016, 6, 17782–17791. [Google Scholar] [CrossRef]
- Ying, T.H.; Tsai, J.H.; Wu, T.T.; Tsai, M.T.; Su, W.W.; Hsieh, Y.S.; Liu, J.Y. Immunochemical localization of protein kinase Calpha in the biopsies of human hepatocellular carcinoma. Chin. J. Physiol. 2008, 51, 269–274. [Google Scholar]
- He, Z.Y.; Zheng, X.; Wu, X.H.; Song, X.R.; He, G.; Wu, W.F.; Yu, S.; Mao, S.J.; Wei, Y.Q. Development of glycyrrhetinic acid-modified stealth cationic liposomes for gene delivery. Int. J. Pharm. 2010, 397, 147–154. [Google Scholar] [CrossRef]
- Farina, C.; Pinza, M.; Pifferi, G. Synthesis and anti-ulcer activity of new derivatives of glycyrrhetic, oleanolic and ursolic acids. Farmaco 1998, 53, 22–32. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; The Medicine Science and Technology Press of China: Beijing, China, 2015; Volume 4, p. 371. [Google Scholar]
Sample Availability: Samples of the compounds 3-galactosidase-30-stearyl deoxyglycyrrhetinic acid and 11-Deoxyglycyrrhetinic acid are available from the authors. |


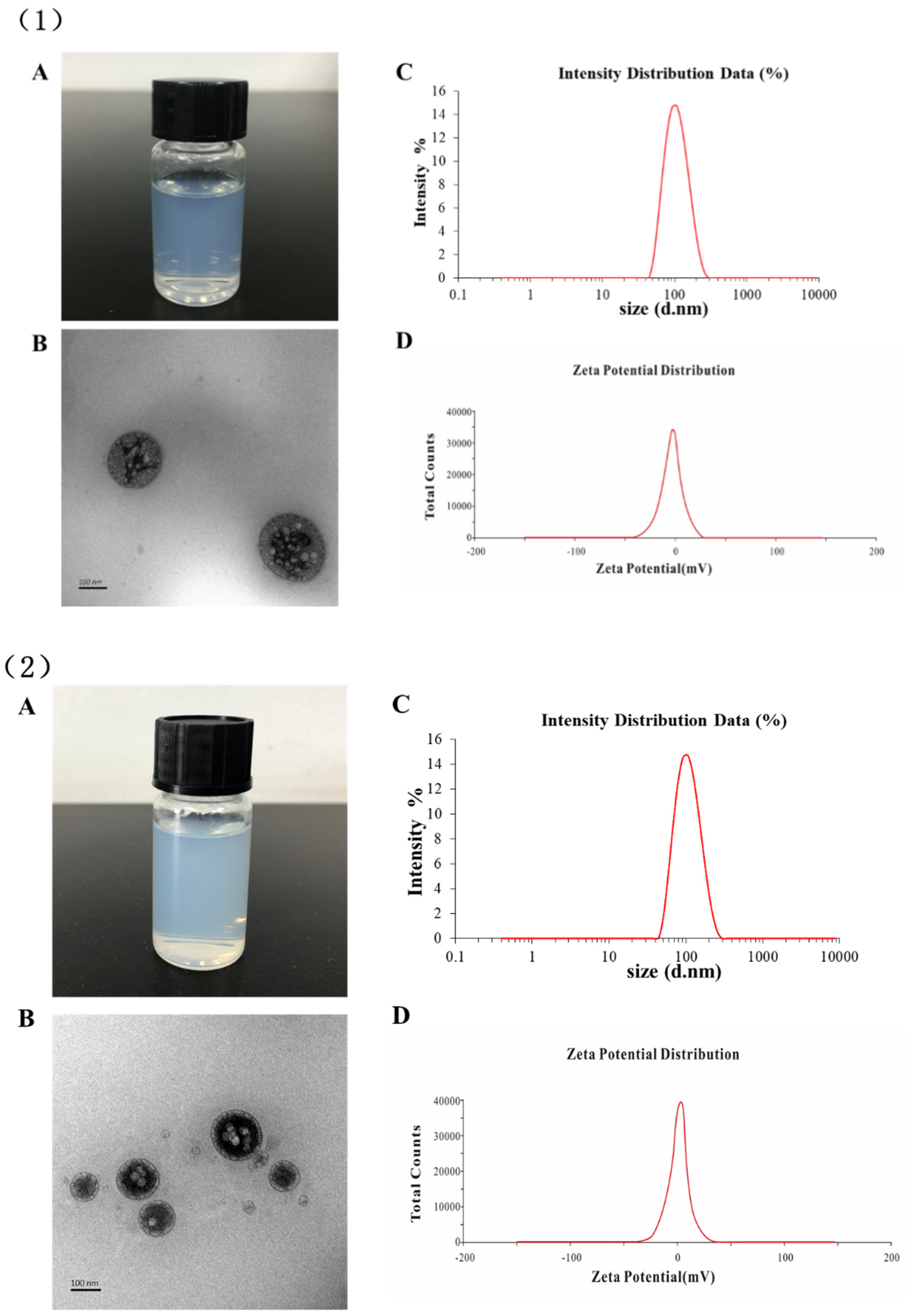



| Type of Liposomes | Particle Size (nm) | PDI | Zeta Potential (mV) | EE % |
|---|---|---|---|---|
| Cantharidin (CTD)-lip | 102.54 ± 3.01 | 0.30 ± 0.02 | −3.07 ± 0.12 | 91.52 ± 1.61 |
| 11-DGA-3-O-Gal-CTD-lip | 107.17 ± 6.12 | 0.26 ± 0.04 | −3.59 ± 0.14 | 90.63 ± 1.66 |
| Type of Liposomes | Weibull Equation | R2 |
|---|---|---|
| CTD-lip | y = 111.0038 × (1 − ) | 0.9903 |
| 11-DGA-3-O-Gal-CTD-lip | y = 97.51614 × (1 − ) | 0.9964 |
| Parameters | CTD-Lip | 11-DGA-3-O-Gal-CTD-Lip |
|---|---|---|
| AUC0-t (μg/L·h) | 595.53 ± 17.91 | 509.34 ± 15.94 |
| AUC0-∞ (μg/L·h) | 756.38 ± 15.12 | 665.11 ± 19.65 |
| T1/2α (h) | 0.25 ± 0.08 | 0.18 ± 0.09 |
| T1/2β (h) | 4.86 ± 2.01 | 2.42 ± 1.03 |
| K12 (L/h) | 1.56 ± 0.87 | 1.90 ± 0.76 |
| K21 (L/h) | 0.45 ± 0.04 | 0.77 ± 0.23 |
| Cl (L/h/kg) | 0.57 ± 0.10 | 0.74 ± 0.13 |
| Vc (L/kg) | 0.46 ± 0.19 | 0.52 ± 0.15 |
| MRT (h) | 5.14 ± 1.16 | 2.47 ± 1.21 |
| Parameters | Tissue | CTD-Lip | 11-DGA-3-O-Gal-CTD-Lip |
|---|---|---|---|
| AUC0-t (μg/L·h) | Heart | 41.38 ± 4.27 | 27.18 ± 3.26 |
| Liver | 61.88 ± 5.94 | 104.63 ± 3.96 | |
| Spleen | 26.93 ± 3.56 | 18.25 ± 5.03 | |
| Lung | 30.70 ± 4.65 | 26.50 ± 5.37 | |
| Kidney | 68.90 ± 3.90 | 77.68 ± 3.74 | |
| Cmax (μg/g) | Heart | 0.35 ± 0.04 | 0.11 ± 0.23 |
| Liver | 0.75 ± 0.08 | 2.01 ± 0.18 | |
| Spleen | 0.19 ± 0.04 | 0.08 ± 0.04 | |
| Lung | 0.15 ± 0.03 | 0.19 ± 0.03 | |
| Kidney | 0.65 ± 0.13 | 1.12 ± 0.17 |
| Parameters | Formulation | Heart | Liver | Spleen | Lung | Kidney |
|---|---|---|---|---|---|---|
| Te% | CTD-lip | 18.01 ± 3.29 | 26.93 ± 2.65 | 11.72 ± 1.77 | 13.36 ± 2.87 | 29.98 ± 2.43 |
| 11-DGA-3-O-Gal-CTD-lip | 10.69 ± 2.04 | 41.15 ± 3.28 | 7.18 ± 2.16 | 10.42 ± 1.73 | 30.55 ± 2.03 | |
| RTe | 11-DGA-3-O-Gal-CTD-lip | 0.59 ± 0.17 | 1.53 ± 0.31 | 0.61 ± 0.12 | 0.78 ± 0.25 | 1.02 ± 0.35 |
| Re | 11-DGA-3-O-Gal-CTD-lip | 0.66 ± 0.26 | 1.69 ± 0.37 | 0.68 ± 0.23 | 0.86 ± 0.25 | 1.13 ± 0.36 |
| Ce | 11-DGA-3-O-Gal-CTD-lip | 0.31 ± 0.08 | 2.68 ± 0.12 | 0.42 ± 0.10 | 1.27 ± 0.32 | 1.72 ± 0.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Zou, M.; Zhu, K.; Ning, S.; Xia, X. Development of 11-DGA-3-O-Gal-Modified Cantharidin Liposomes for Treatment of Hepatocellular Carcinoma. Molecules 2019, 24, 3080. https://doi.org/10.3390/molecules24173080
Zhou L, Zou M, Zhu K, Ning S, Xia X. Development of 11-DGA-3-O-Gal-Modified Cantharidin Liposomes for Treatment of Hepatocellular Carcinoma. Molecules. 2019; 24(17):3080. https://doi.org/10.3390/molecules24173080
Chicago/Turabian StyleZhou, Lili, Manshu Zou, Kun Zhu, Shuangcheng Ning, and Xinhua Xia. 2019. "Development of 11-DGA-3-O-Gal-Modified Cantharidin Liposomes for Treatment of Hepatocellular Carcinoma" Molecules 24, no. 17: 3080. https://doi.org/10.3390/molecules24173080




