Green Approach Extraction of Perezone from the Roots of Acourtia platyphilla (A. Grey): A Comparison of Four Activating Modes and Supercritical Carbon Dioxide
Abstract
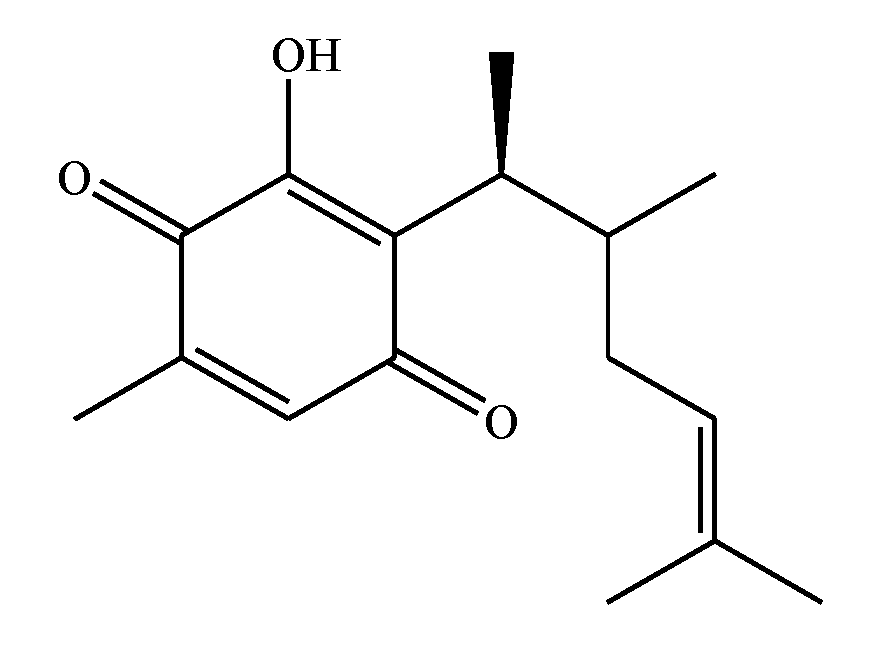
:1. Introduction
2. Results and Discussion
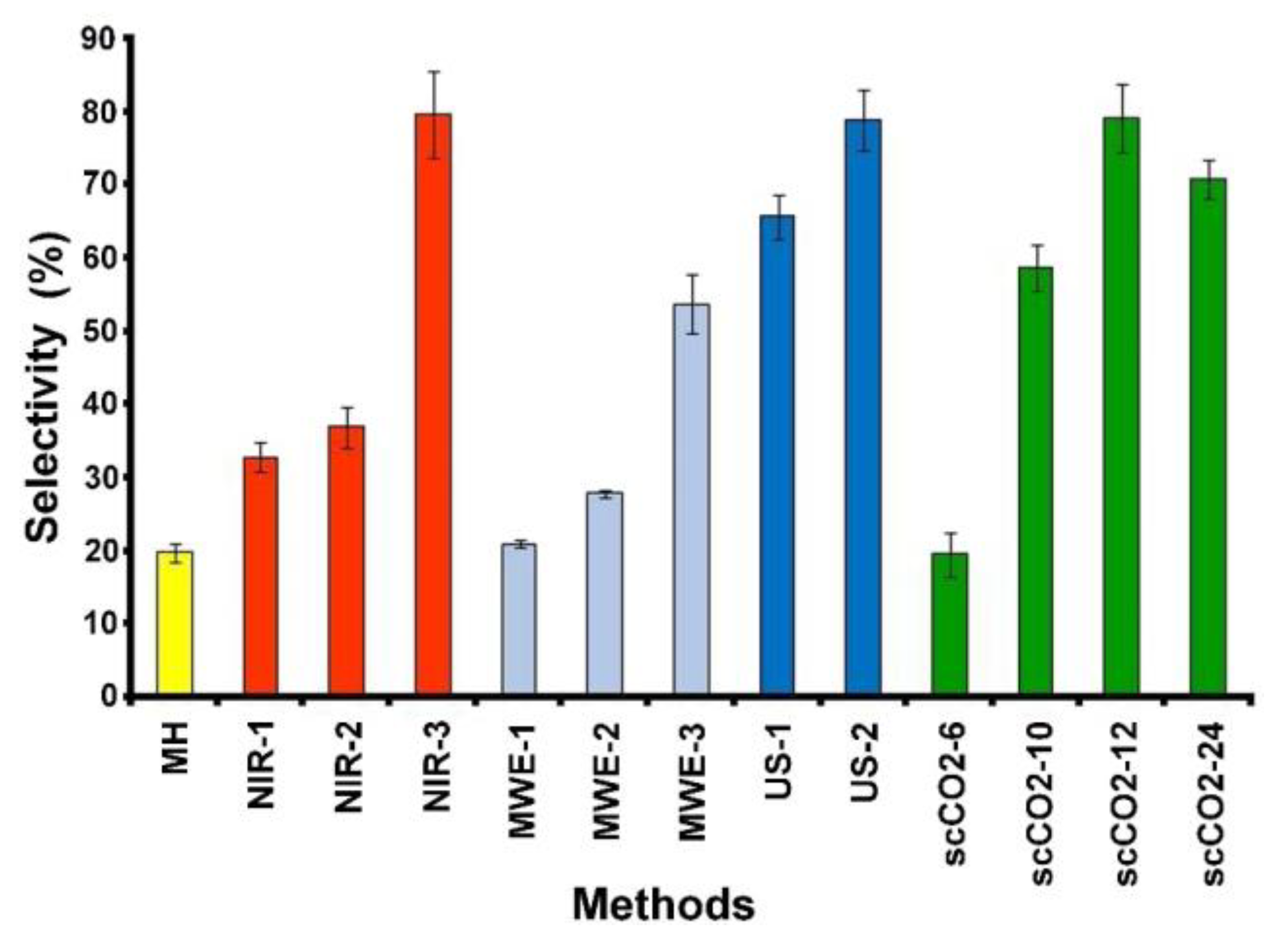
2.1. Conventional Thermal Extraction
2.2. Near-Infrared Promoted Extraction
2.3. Microwave Promoted Extraction
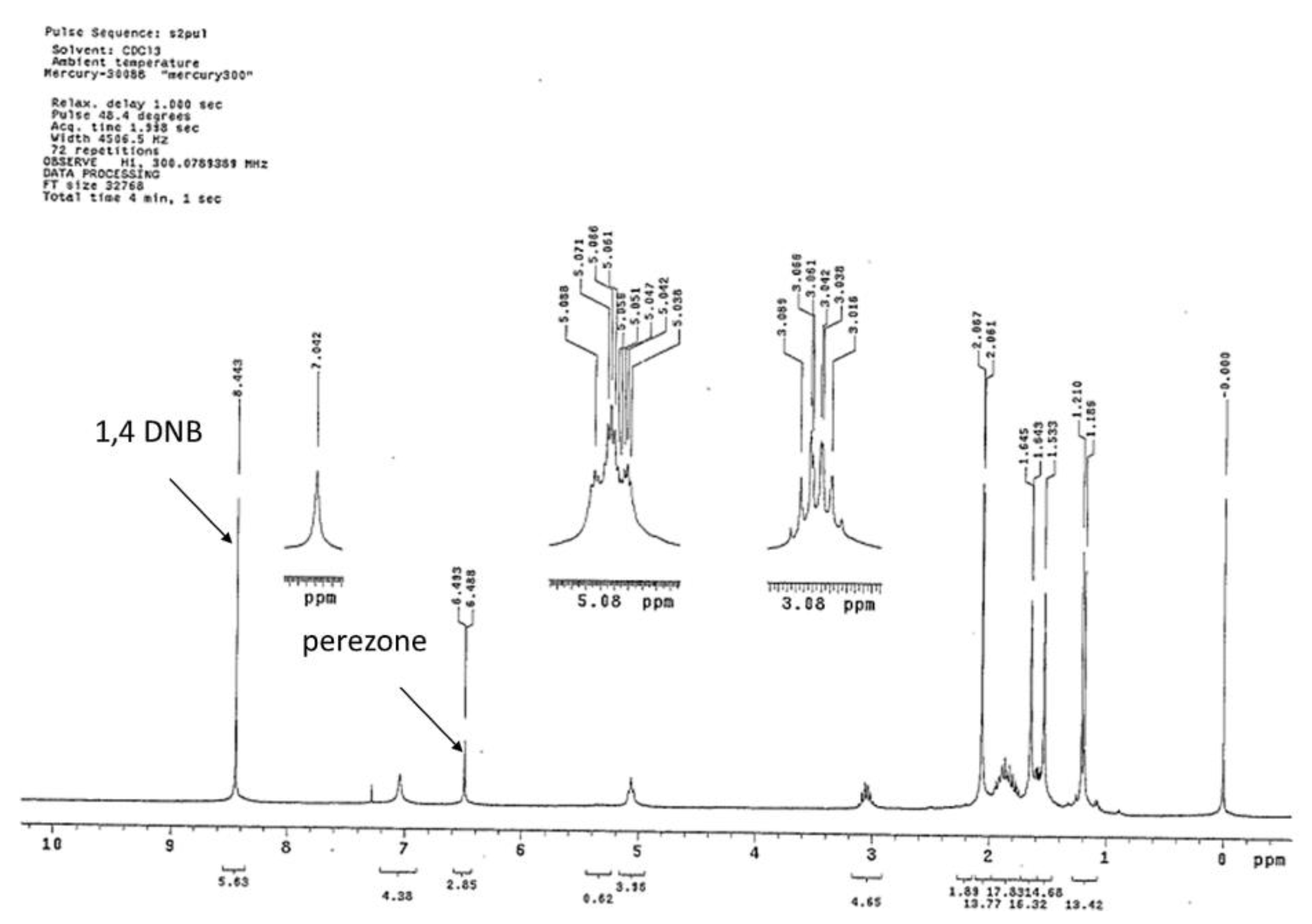
2.4. Ultrasound Promoted Extraction
2.5. Supercritical Dioxide Perezone Extraction
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Typical Extraction Using Thermal Conditions
3.4. Typical Extraction Using Non-Conventional Activating Sources
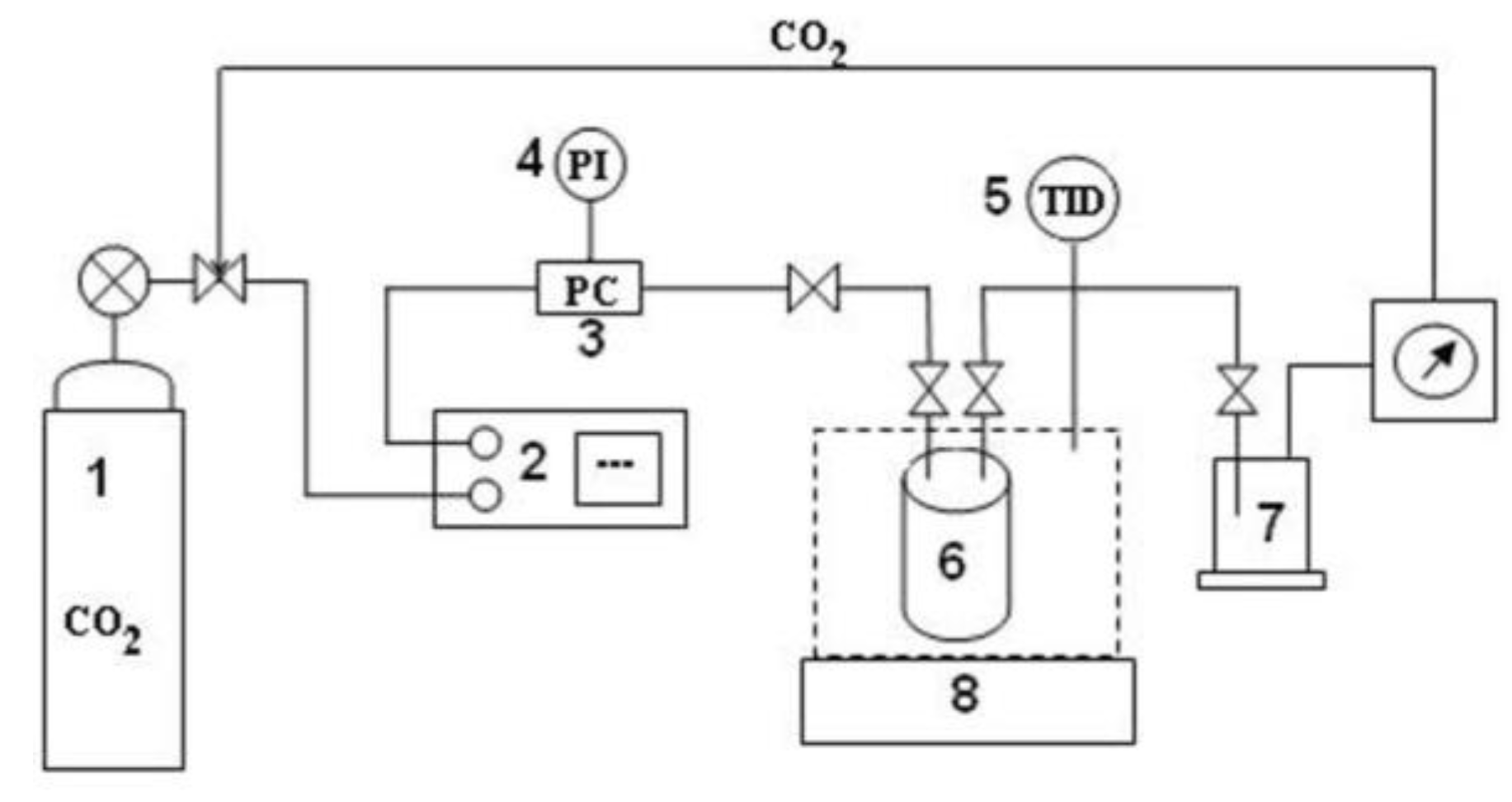
3.5. Perezone Extraction with Supercritical Carbon Dioxide
3.5.1. Perezone Solubility in scCO2
3.5.2. Perezone Extraction with scCO2
3.5.3. Perezone Quantification in the Extract
3.6. Statistical Process Control
3.7. Perezone Spectral Data
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FIR | Far infrared |
| 1H NMR | Proton nuclear magnetic resonance |
| IR | Infrared |
| MH | Mantle heating |
| MIR | Middle infrared |
| MM | Tribochemistry-mechanical milling |
| MW | Microwave |
| MWE | Microwave extraction |
| NIR | Near-infrared |
| scCO2 | Supercritical carbon dioxide |
| scCO2-6 | Supercritical carbon dioxide extraction at 6 h |
| scCO2-10 | Supercritical carbon dioxide extraction at 10 h |
| scCO2-12 | Supercritical carbon dioxide extraction at 12 h |
| scCO2-24 | Supercritical carbon dioxide extraction at 24 h |
| US | Ultrasound |
References
- Moss, G. Nomenclature of Quinones with Isoprenoid Chain-Side. Available online: http://www.chem.qmul.ac.uk/iupac/misc/quinone.html (accessed on 2 November 2015).
- Rio de la Loza, L. Discurso Pronunciado por el Catedrático de Química Médica de la Escuela de Medicina 23 November 1852; Noriega, J., Ed.; Imprenta de Ignacio Escalante: Mexico City, Mexico, 1911. [Google Scholar]
- Sanchez, I.H.; Yanez, R.; Enriquez, R.; Joseph-Nathan, P. Reaction mechanism change in the Lewis acid catalyzed perezone-pipitzol transformation. J. Org. Chem. 1981, 46, 2818–2819. [Google Scholar] [CrossRef]
- Sanchez, I.H.; Basurto, F.; Joseph-Nathan, P. The stereocontrol of the perezone to pipitzol transformation. J. Nat. Prod. Prod. 1984, 47, 382–383. [Google Scholar] [CrossRef]
- Joseph-Nathan, P.; Santillan, R. The Chemistry of Perezone and Its Consequences; Atta-ur-Rahman, Ed.; El Servier: Amsterdam, The Netherlands, 1989; Volume 5, pp. 763–813. [Google Scholar]
- Rodríguez-Hernández, A.; Barrios, H.; Collera, O.; Enríquez, R.G.; Ortiz, B.; Sánchez-obregón, R.; Walls, F.; Yuste, F.; Reynolds, W.F.; Yu, M. Isomerization of perezone into isoperezone and preparation of dihydroisoperezinone. Nat. Prod. Lett. 1994, 4, 133–139. [Google Scholar] [CrossRef]
- Escobedo-González, R.G.; Pérez Martínez, H.; Nicolás-Vázquez, M.I.; Martínez, J.; Gómez, G.; Serrano, J.N.; Carranza Téllez, V.; Vargas-Requena, C.L.; Miranda Ruvalcaba, R. Green production of indolylquinones, derivatives of perezone, and related molecules, promising antineoplastic compounds. J. Chem. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Martínez, J.; Velasco-Bejarano, B.; Delgado-Reyes, F.; Pozas, R.; Torres-Domínguez, H.; Trujillo-Ferrara, J.; Arroyo-Razo, G.; Miranda, R. Eco-contribution to the chemistry of perezone, a comparative study, using different modes of activation and solventless conditions. Nat. Prod. Commun. 2008, 3, 1465–1468. [Google Scholar] [CrossRef]
- Arellano, J.; Vázquez, F.; Villegas, T.; Hernández, G. Establishment of transformed root cultures of Perezia cuernavacana producing the sesquiterpene quinone perezone. Plant Cell Rep. 1996, 15, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Burgueño-Tapia, E.; Joseph-Nathan, P. Detailed studies of perezone rearrangements. Monatshefte Chem. Chem. Mon. 1997, 128, 651–658. [Google Scholar] [CrossRef]
- Burgueño-Tapia, E.; Joseph-Nathan, P. 13C NMR substituent chemical shifts in hydroxy-p-benzoquinones. Magn. Reson. Chem. 2000, 38, 390–393. [Google Scholar] [CrossRef]
- Enríquez, R.; Ortega, J.; Lozoya, X. Active components in Perezia roots. J. Ethnopharmacol. 1980, 2, 389–393. [Google Scholar] [CrossRef]
- Alarcon-Aguilar, F.J.; Roman-Ramos, R.; Jimenez-Estrada, M.; Reyes-Chilpa, R.; Gonzalez-Paredes, B.; Flores-Saenz, J.L. Effects of three Mexican medicinal plants (Asteraceae) on blood glucose levels in healthy mice and rabbits. J. Ethnopharmacol. 1997, 55, 171–177. [Google Scholar] [CrossRef]
- Burgueño-Tapia, E.; Castillo, L.; González-Coloma, A.; Joseph-Nathan, P. Antifeedant and phytotoxic activity of the sesquiterpene p-benzoquinone perezone and some of its derivatives. J. Chem. Ecol. 2008, 34, 766–771. [Google Scholar] [CrossRef]
- De La Peña, A.; Izaguirre, R.; Baños, G.; Viveros, M.; Enriquez, R.G.; Fernandez, J.M. Effect of perezone, aminoperezone and their corresponding isomers isoperezone and isoaminoperezone upon in vitro platelet aggregation. Phytomedicine 2001, 8, 465–468. [Google Scholar]
- Téllez, J.; Carvajal, K.; Cruz, D.; Cárabez, A.; Chávez, E. Effect of perezone on arrhythmias and markers of cell injury during reperfusion in the anesthetized rat. Life Sci. 1999, 65, 1615–1623. [Google Scholar] [CrossRef]
- Concepción Lozada, M.; Soria-Arteche, O.; Teresa Ramírez Apan, M.; Nieto-Camacho, A.; Enríquez, R.G.; Izquierdo, T.; Jiménez-Corona, A. Synthesis, cytotoxic and antioxidant evaluations of amino derivatives from perezone. Bioorg. Med. Chem. 2012, 20, 5077–5084. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-González, R.G.; Bahena, L.; Arias Tellez, J.L.; Hinojosa Torres, J.; Ruvalcaba, R.M.; Aceves-Hernández, J.M. Characterization and comparison of perezone with some analogues. Experimental and theoretical study. J. Mol. Struct. 2015, 1097, 98–105. [Google Scholar] [CrossRef]
- Sánchez-Torres, L.E.; Torres-Martínez, J.A.; Godínez-Victoria, M.; Omar, J.-M.; Velasco-Bejarano, B. Perezone and its isomer isoperezone induce caspase-dependent and caspase-independent cell death. Phytomedicine 2010, 17, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, NY, USA, 1998. [Google Scholar]
- Clark, J.H.; Macquarrie, D.J. Handbook of Green Chemistry and Technology; The Blackwell Science: Hoboken, NJ, USA; John Wiley & Sons: Oxford, NY, USA, 2002. [Google Scholar]
- Victor, D.G. Strategies for cutting carbon. Nature 1998, 395, 837. [Google Scholar] [CrossRef]
- Doble, M.; Rollins, K.; Kumar, A. Green Chemistry and Engineering; Academic Press: London, UK, 2007. [Google Scholar]
- Escobedo, R.; Miranda, R.; Martínez, J. Infrared irradiation: Toward green chemistry, a review. Int. J. Mol. Sci. 2016, 17, 453. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.; Miranda, R. Infrared irradiation, an excellent, alternative green energy source. In Green Chemistry; IntechOpen: London, UK, 2019; ISBN 978-1-78984-124-4. [Google Scholar]
- Koel, M.; Ljovin, S.; Hollis, K.; Rubin, J. Using neoteric solvents in oil shale studies. Pure Appl. Chem. 2001, 73, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Brunner, G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Cruz-Olivares, J.; Ortiz-Estrada, C.H.; Pérez-Alonso, C.; Chaparro-Mercado, M.C.; Barrera-Diíaz, C. Solubility of mesquite gum in supercritical carbon dioxide. J. Chem. Eng. Data 2011, 56, 2449–2452. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S. Measurement and correlation of the solubility of Ligusticum Chuanxiong oil in supercritical CO2. Chin. J. Chem. Eng. 2005, 13, 796–799. [Google Scholar]
- Kerton, F.M.; Marriott, R. Alternative Solvents for Green Chemistry, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2013; ISBN 9781849735957. [Google Scholar]
- McHugh, M.; Krukonis, V. Supercritical Fluid Extraction: Principles and Practice, 2nd ed.; Elsevier: Stoneham, MA, USA, 2013. [Google Scholar]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Koulouri, S.; Malamidou-Xenikaki, E.; Spyroudis, S. Acid-catalyzed addition of indoles to hydroxyquinones. Tetrahedron 2005, 61, 10894–10902. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Swamy, T. Bi(OTf)3-catalyzed conjugate addition of indoles to p-quinones: A facile synthesis of 3-indolyl quinones. Tetrahedron Lett. 2003, 44, 9121–9124. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Liu, L.; Chen, Y.-J.; Wang, D.; Li, C.-J. “On Water”-promoted direct coupling of indoles with 1,4-benzoquinones without catalyst. Eur. J. Org. Chem. 2006, 2006, 869–873. [Google Scholar] [CrossRef]
- Anastas, P.T.; Kirchhoff, M.M. Origins, current status, and future challenges of green chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Gomez Pliego, R.; Ramirez-San Juan, E.; Miranda, R.; Villalobos-Molina, R.; Delgado, F.; Osnaya, R.; Trujillo Ferrara, J. Vasodilator effects of bis-dihydropyridines structurally related to nifedipine. Med.Chem. 2006, 2, 527–534. [Google Scholar] [CrossRef]
- Martínez, J.; Romero-Vega, S.; Abeja-Cruz, R.; Álvarez-Toledano, C.; Miranda, R. Green approach—Multicomponent production of boron—Containing Hantzsch and Biginelli esters. Int. J. Mol. Sci. 2013, 14, 2903–2915. [Google Scholar] [CrossRef] [PubMed]
- Noguez, M.O.; García, A.; Ibarra, C.; Cabrera, A.; Aceves, J.M.; Nicolas, M.I.; Miranda, R. Green synthesis of bis-Biginelli esters, with vasodilatory effects, their mass spectrometric and physical studies. Trends Org. Chem. 2009, 13, 75–82. [Google Scholar]
- Reyes, L.; Corona, S.; Arroyo, G.; Delgado, F.; Miranda, R. Eco-contribution for the Production of N-arylnitrones: Solvent-free and assisted by microwaves. Int. J. Mol. Sci. 2010, 11, 2576–2583. [Google Scholar] [CrossRef]
- Velasco, B.; Trujillo-Ferrara, J.G.; Castillo, L.H.F.; Miranda, R.; Sánchez-Torres, L.E. In vitro apoptotic activity of 2,2-diphenyl-1,3,2-oxazaborolidin-5-ones in L5178Y cells. Life Sci. 2007, 80, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Zarco Juarez, M.; Martínez, J.O.; Noguez Cordova, O.; Nicolás Vazquez, M.I.; Ramírez-Apan, T.; Pérez Flores, J.; Miranda Ruvalcaba, R.; Arroyo Razo, G.A. A green approach to the production of hybrid diindolylmethane-phenylboronic acids via a 3MCR: Promising antineoplasic molecules. J. Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Martínez, J.; Rosas, J.; Pérez, J.; Saavedra, Z.; Carranza, V.; Alonso, P. Green approach to the extraction of major capsaicinoids from habanero pepper using near-infrared, microwave, ultrasound and Soxhlet methods, a comparative study. Nat. Prod. Res. 2019, 33, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Nathan, P.; González, M.P.; Rodríguez, V.M. Terpenoids of Perezia hebeclada. Phytochemistry 1972, 11, 1803–1808. [Google Scholar] [CrossRef]
- Joseph-Nathan, P.; Hernández, J.D.; Román, L.U.; García, G.E.; Mendoza, V. Sesquiterpenes from Perezia carpholepis. Phytochemistry 1982, 21, 669–672. [Google Scholar] [CrossRef]
- Hao, J.; Han, W.; Huang, S.; Xue, B.; Deng, X. Microwave-assisted extraction of artemisinin from Artemisia annua L. Sep. Purif. Technol. 2002, 28, 191–196. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction–an innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Pan, X.; Liu, H.; Jia, G.; Shu, Y.Y. Microwave-assisted extraction of glycyrrhizic acid from licorice root. Biochem. Eng. J. 2000, 5, 173–177. [Google Scholar] [CrossRef]
- Pan, X.; Niu, G.; Liu, H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process. Process Intensif. 2003, 42, 129–133. [Google Scholar] [CrossRef]
- Brittany, L.; Hayes, D. Microwave Synthesis: Chemistry at the Speed of Light; CEM publishing: Matthews, NC, USA, 2002. [Google Scholar]
- Galanakis, C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Kobus, Z. Dry matter extraction from valerian roots (Valeriana officinalis L.) with the help of pulsed acoustic field. Int. Agrophys. 2008, 22, 133–137. [Google Scholar]
- Lagnika, C.; Zhang, M.; Nsor-Atindana, J.; Tounkara, F. Extension of mushroom shelf-life by ultrasound treatment combined with high pressure argon. Int. Agrophys. 2014, 28, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Roselló-Soto, E.; Galanakis, C.M.; Brnčić, M.; Orlien, V.; Trujillo, F.J.; Mawson, R.; Knoerzer, K.; Tiwari, B.K.; Barba, F.J. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci. Technol. 2015, 42, 134–149. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, T.; He, J.; Barba, F.; Cravotto, G.; Koubaa, M. Ultrasound-assisted extraction, centrifugation and ultrafiltration: Multistage process for polyphenol recovery from purple sweet potatoes. Molecules 2016, 21, 1584. [Google Scholar] [CrossRef]
- Gupta, R.B.; Shim, J.-J. Solubility in Supercritical Carbon Dioxide; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9781420005998. [Google Scholar]
- Foster, N.R.; Lucien, F.P.; Mammucari, R. Basic physical properties, phase behaviour and solubility. In Handbook of Green Chemistry; Leitner, W., Jessop, P.G., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Hintermair, U.; Leitner, W.; Jessop, P.; Hintermair, U.; Leitner, W.; Jessop, P. Expanded liquid phases in catalysis: Gas-expanded liquids and liquid-supercritical fluid biphasic systems. In Handbook of Green Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; ISBN 9783527628698. [Google Scholar]
- Li, Z.; Welbeck, E.; Yang, L.; He, C.; Hu, H.; Song, M.; Bi, K.; Wang, Z. A quantitative 1H nuclear magnetic resonance (qHNMR) method for assessing the purity of iridoids and secoiridoids. Fitoterapia 2015, 100, 187–194. [Google Scholar] [CrossRef]
- Li, Z.Y.; Welbeck, E.; Wang, R.F.; Liu, Q.; Yang, Y.B.; Chou, G.X.; Bi, K.S.; Wang, Z.T. A universal quantitative 1H nuclear magnetic resonance (qNMR) method for assessing the purity of Dammarane-type Ginsenosides. Phytochem. Anal. 2015, 26, 8–14. [Google Scholar] [CrossRef]
- Mojsiewicz-Pieńkowska, K.; Jamrógiewicz, Z.; Łukasiak, J. Determination of polydimethylsiloxanes by 1H-NMR in wine and edible oils. Food Addit. Contam. 2003, 20, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Desai, N.H.; Bhatt, A.; Shivramchandra, K.; Kamath, B.V. 1H qNMR approach for estimation of a toxic compound N,N-dimethylamine in metformin hydrochloride: An equivalent efficient method over reversed phase derivatization HPLC method. Iran. J. Anal. Chem. 2015, 2, 112–119. [Google Scholar]
Sample Availability: Samples of perezone are available from the authors. |
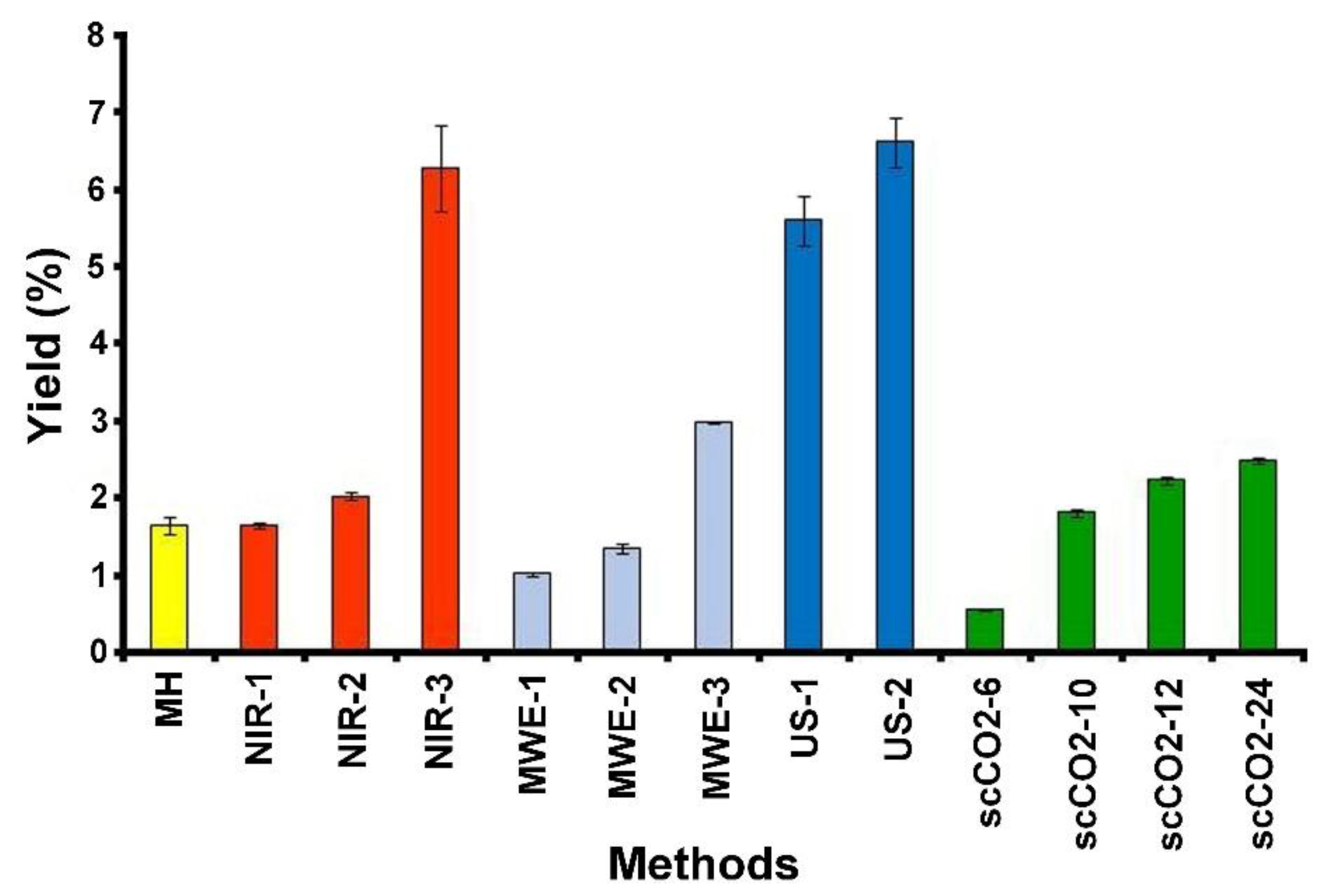


| Methods | Yield (%) | Standard Deviation |
|---|---|---|
| MH 1 | 1.652 | 0.119 |
| NIR-1 2 | 1.656 | 0.027 |
| NIR-2 3 | 2.036 | 0.043 |
| NIR-3 4 | 6.271 | 0.554 |
| MWE-1 5 | 1.027 | 0.026 |
| MWE-2 6 | 1.353 | 0.065 |
| MWE-3 7 | 2.996 | 0.008 |
| US-1 8 | 5.609 | 0.322 |
| US-2 9 | 6.623 | 0.322 |
| scCO2-6 10 | 0.564 | 0.018 |
| scCO2-10 11 | 1.818 | 0.045 |
| scCO2-12 12 | 2.243 | 0.046 |
| scCO2-24 13 | 2.495 | 0.043 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobedo-González, R.; Vázquez Cabañas, A.V.; Martínez González, A.; Mendoza Sánchez, P.; Saavedra-Leos, Z.; Cruz-Olivares, J.; Nava Serrano, J.; Martínez, J.; Miranda Ruvalcaba, R. Green Approach Extraction of Perezone from the Roots of Acourtia platyphilla (A. Grey): A Comparison of Four Activating Modes and Supercritical Carbon Dioxide. Molecules 2019, 24, 3035. https://doi.org/10.3390/molecules24173035
Escobedo-González R, Vázquez Cabañas AV, Martínez González A, Mendoza Sánchez P, Saavedra-Leos Z, Cruz-Olivares J, Nava Serrano J, Martínez J, Miranda Ruvalcaba R. Green Approach Extraction of Perezone from the Roots of Acourtia platyphilla (A. Grey): A Comparison of Four Activating Modes and Supercritical Carbon Dioxide. Molecules. 2019; 24(17):3035. https://doi.org/10.3390/molecules24173035
Chicago/Turabian StyleEscobedo-González, René, Andrea Vázquez Vázquez Cabañas, Armando Martínez González, Pablo Mendoza Sánchez, Zenaida Saavedra-Leos, Julián Cruz-Olivares, Juan Nava Serrano, Joel Martínez, and René Miranda Ruvalcaba. 2019. "Green Approach Extraction of Perezone from the Roots of Acourtia platyphilla (A. Grey): A Comparison of Four Activating Modes and Supercritical Carbon Dioxide" Molecules 24, no. 17: 3035. https://doi.org/10.3390/molecules24173035







