VOC Profiles of Saliva in Assessment of Halitosis and Submandibular Abscesses Using HS-SPME-GC/MS Technique
Abstract
:1. Introduction
2. Results and Discussion
2.1. Possible Cause and Origin of Detected Volatiles
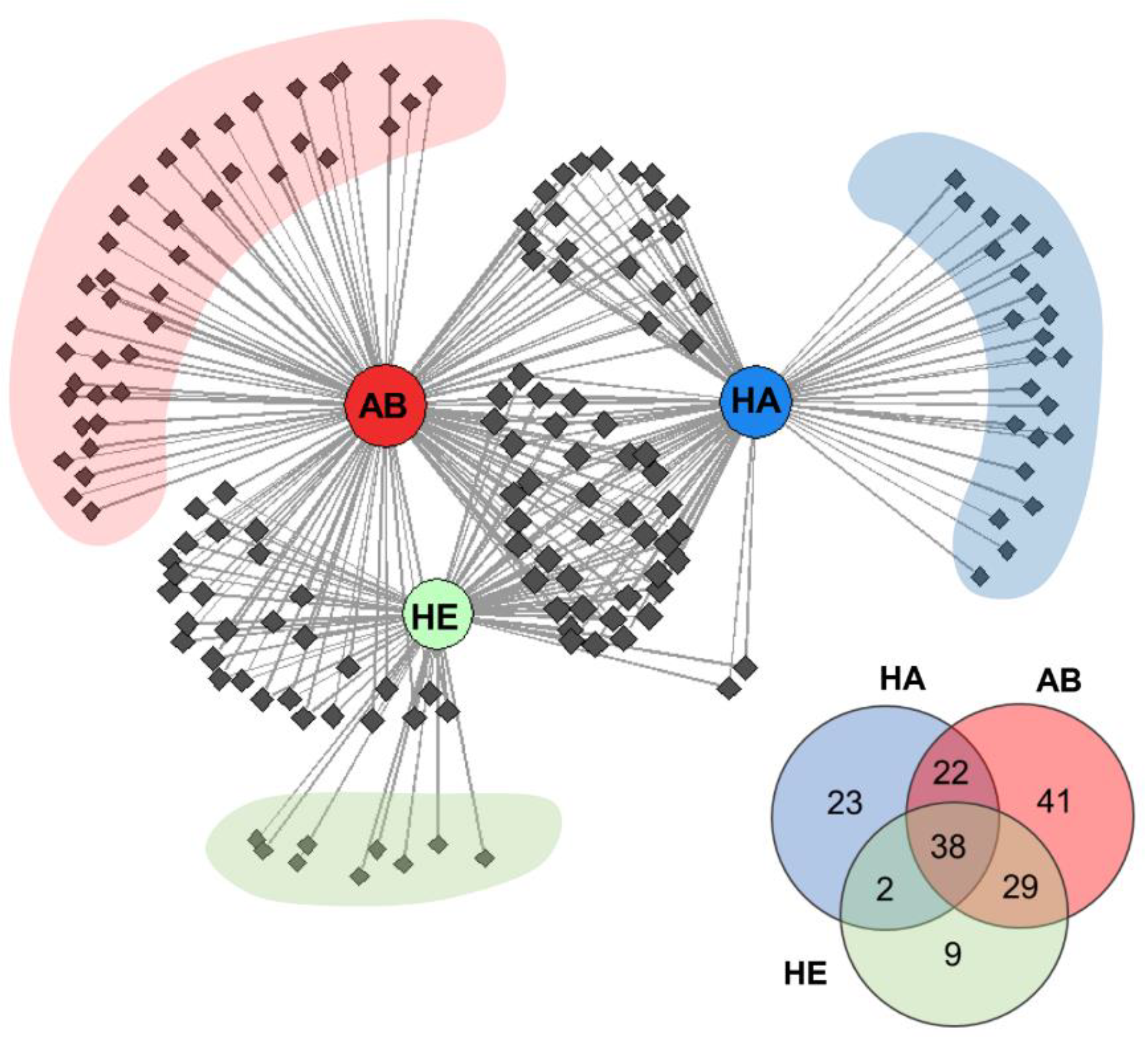
2.2. Distribution of Detected VOCs among the Investigated Groups
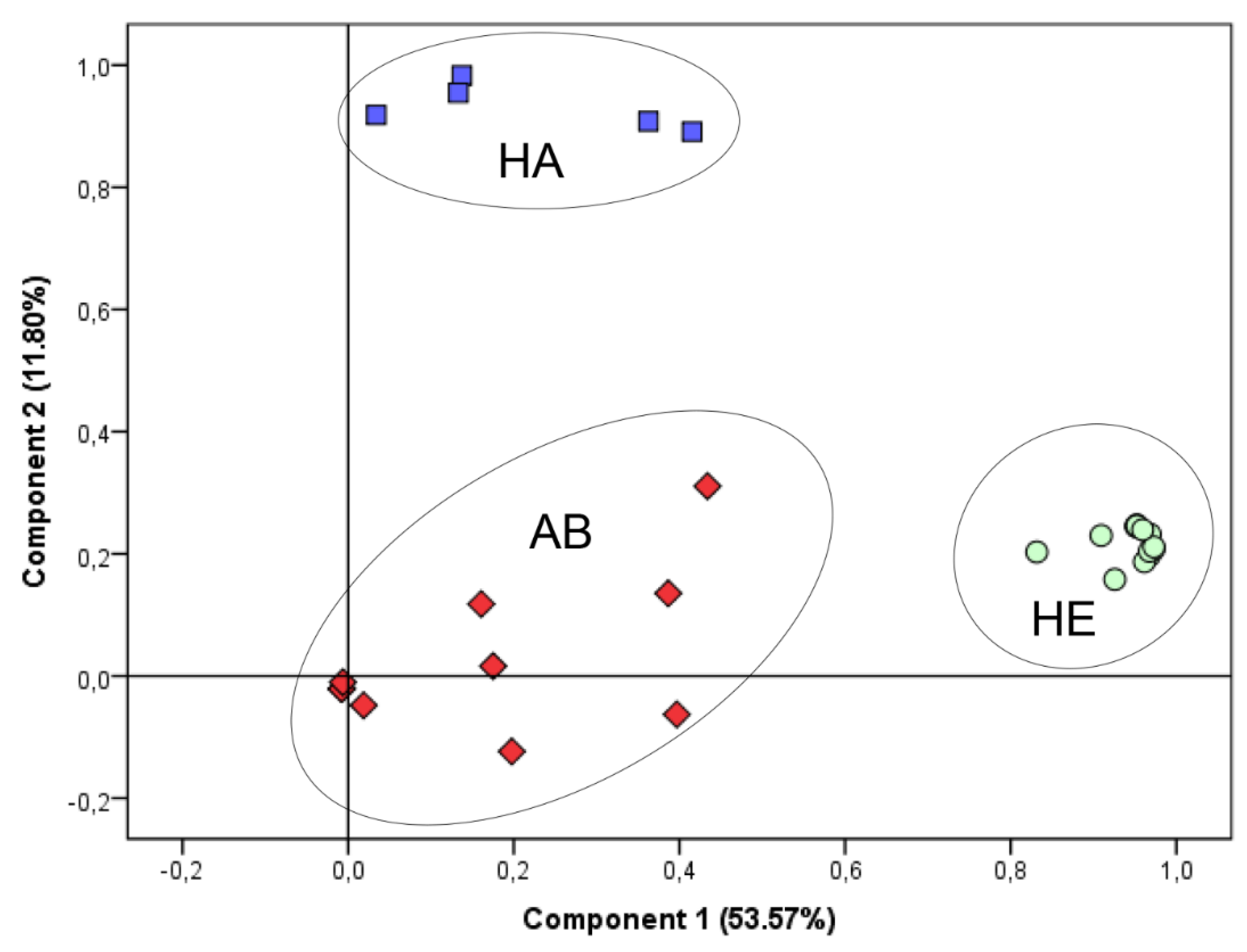
2.3. Statistical Evaluation and Discrimination of VOC Profiles
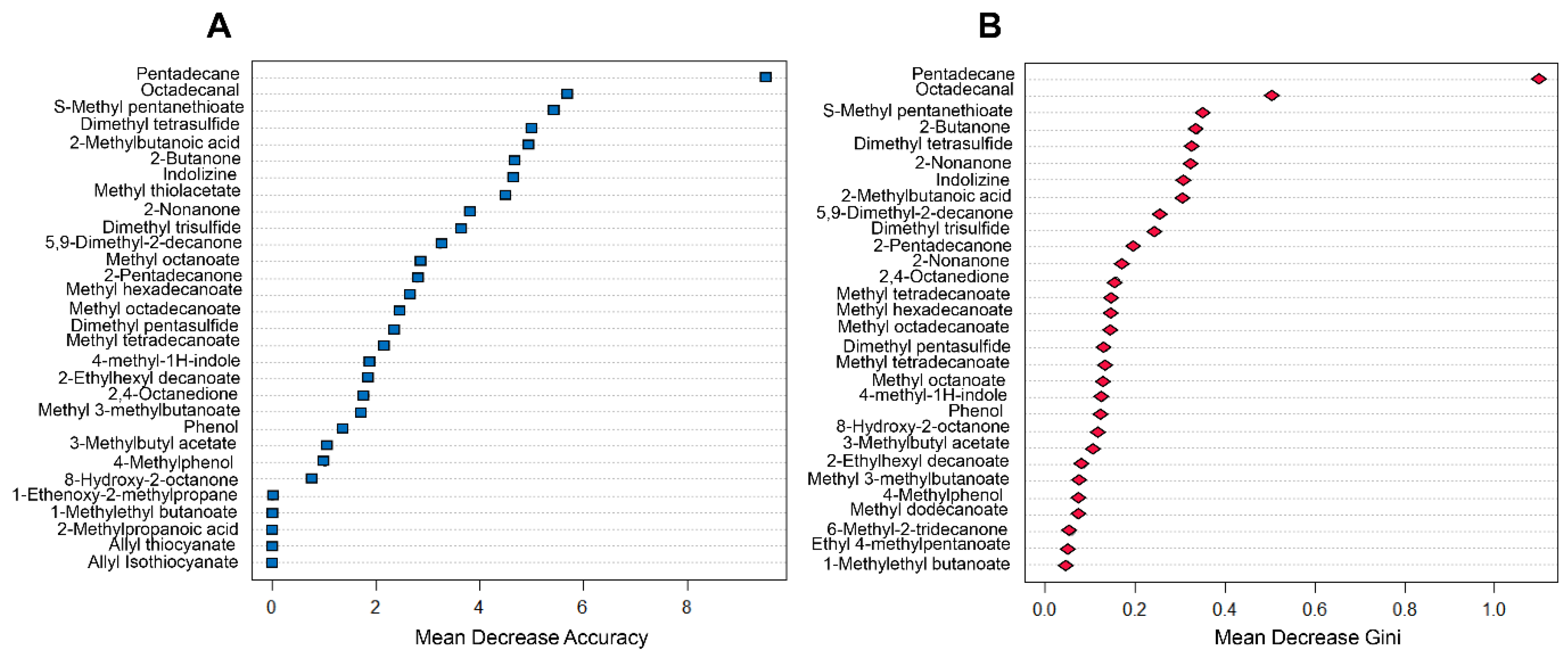
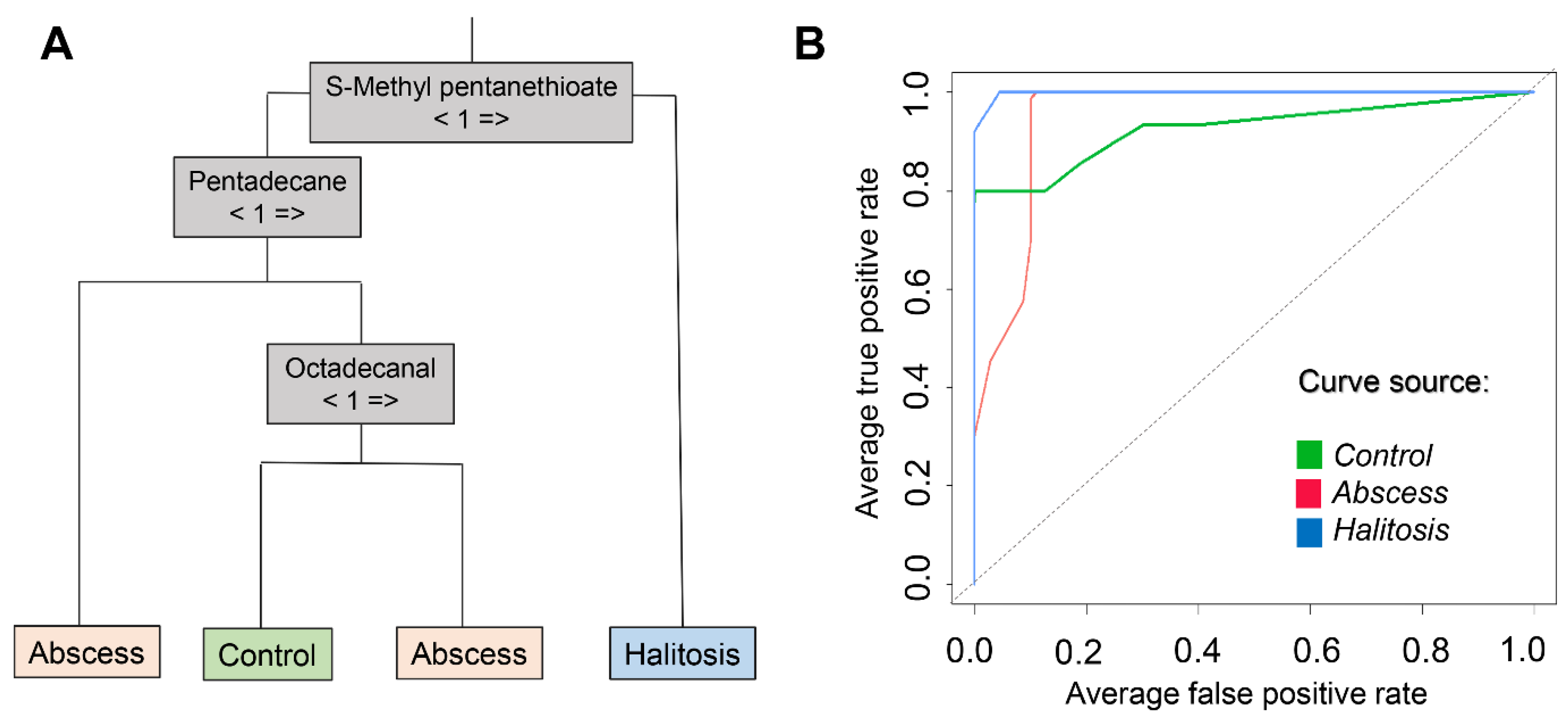
2.4. Diagnosis Prediction Based on Random Forest Model
3. Materials and Methods
3.1. Instruments
3.2. Materials
3.3. Collections of Saliva Samples
3.4. HS-SPME-GC/MS Method
3.5. Statistical Approaches
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cortelli, J.R.; Barbosa, M.D.S.; Westphal, M.A. Halitosis: A review of associated factors and therapeutic approach. Braz. Oral Res. 2008, 22, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.L.; Sensat, M.L.; Stoltenberg, J.L. Halitosis: A review of current literature. J. Dent. Hyg. 2010, 84, 65–74. [Google Scholar] [PubMed]
- Akaji, E.A.; Folaranmi, N.; Ashiwaju, O. Halitosis: A review of the literature on its prevalence, impact and control. Oral Health Prev. Dent. 2014, 12, 297–304. [Google Scholar] [PubMed]
- Aylikci, B.; Çolak, H. Halitosis: From diagnosis to management. J. Nat. Sci. Biol. Med. 2013, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yoneda, M.; Naito, T.; Iwamoto, T.; Hirofuji, T. Relationship between halitosis and psychologic status. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Zhao, H.; Avontroodt, P.; Soers, C.; Pauwels, M.; Coucke, W.; van Steenberghe, D. A salivary incubation test for evaluation of oral malodor: A pilot study. J. Periodontol. 2003, 74, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.R.; Scully, C. Oral malodour (halitosis). BMJ 2006, 333, 632–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, D.; Smith, A.J. The microbiology of the acute dental abscess. J. Med. Microbiol. 2009, 58, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Shweta, S.; Prakash, S.K. Dental abscess: A microbiological review. Dent. Res. J. 2013, 10, 585–591. [Google Scholar]
- Sharma, J.; Patnaik, A.; Banerjee, N.; Sood, R. Management of submandibular abscess with limited resources. Karnataka Anaesth J. 2018, 3, 37–39. [Google Scholar]
- Boscolo-Rizzo, P.; Da Mosto, M.C. Submandibular space infection: A potentially lethal infection. Int. J. Infect. Dis. 2009, 13, 327–333. [Google Scholar] [CrossRef]
- Mohamadi Ardehali, M.; Jafari, M.; Bagheri Hagh, A. Submandibular space abscess: A clinical trial for testing a new technique. Otolaryngol. Head Neck Surg. (USA) 2012, 146, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Raftopulos, M.; Jefferson, N.; Mackay, S.; Ashford, B. A rare case of submandibular abscess complicated by stroke. JRSM Short Rep. 2013, 4, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Milanowski, M.; Pomastowski, P.; Ligor, T.; Buszewski, B. Saliva–Volatile biomarkers and profiles. Crit. Rev. Anal. Chem. 2017, 47, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Milanowski, M.; Ligor, T.; Pomastowski, P. Investigation of bacterial viability from incubated saliva by application of flow cytometry and hyphenated separation techniques. Electrophoresis 2017, 38, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, X. Saliva metabolomics opens door to biomarker discovery, disease diagnosis, and treatment. Appl. Biochem. Biotechnol. 2012, 168, 1718–1727. [Google Scholar] [CrossRef]
- Malathi, N.; Mythili, S.; Vasanthi, H.R. Salivary diagnostics: A brief review. ISRN Dent. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T.W. Salivary biomarkers: Toward future clinical and diagnostic utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef]
- Bean, H.D.; Dimandja, J.-M.D.; Hill, J.E. Bacterial volatile discovery using solid phase microextraction and comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry. J. Chromatogr. B 2012, 901, 41–46. [Google Scholar] [CrossRef]
- Tait, E.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Identification of volatile organic compounds produced by bacteria using HS-SPME-GC-MS. J. Chromatogr. Sci. 2014, 52, 363–373. [Google Scholar] [CrossRef]
- Buszewski, B.; Ratiu, I.A.; Milanowski, M.; Pomastowski, P.; Ligor, T. The effect of biosilver nanoparticles on different bacterial strains’ metabolism reflected in their VOCs profiles. J. Breath Res. 2018, 12, 027105. [Google Scholar] [CrossRef] [PubMed]
- Al-Kateb, H.; De Lacy Costello, B.; Ratcliffe, N. An investigation of volatile organic compounds from the saliva of healthy individuals using headspace-trap/GC-MS. J. Breath Res. 2013, 7, 036004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostelc, J.G.; Preti, G.; Zelson, P.R.; Tonzetich, J.; Huggins, G.R. Volatiles of exogenous origin from the human oral cavity. J. Chromatogr. 1981, 226, 315–323. [Google Scholar] [CrossRef]
- De Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Costello, B.D.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef]
- Hyvärinen, M.J.; Rothberg, M.; Kähkönen, E.; Mielo, T.; Reijula, K. Nicotine and 3-ethenylpyridine concentrations as markers for environmental tobacco smoke in restaurants. Indoor Air 2000, 10, 121–125. [Google Scholar] [CrossRef]
- Vainiotalo, S.; Vaaranrinta, R.; Tornaeus, J.; Aremo, N.; Hase, T.; Peltonen, K. Passive monitoring method for 3-Ethenylpyridine: A marker for environmental tobacco smoke. Environ. Sci. Technol. 2001, 35, 1818–1822. [Google Scholar] [CrossRef]
- Nikolov, P.Y.; Yaylayan, V.A. Role of the ribose-specific marker furfuryl-amine in the formation of aroma active 1-(furan-2-ylmethyl)-1H-pyrrole (or furfuryl-pyrrole) derivatives. J. Agric. Food Chem. 2012, 60, 10155–10161. [Google Scholar] [CrossRef]
- Miki, Y.; Asano, Y. Biosynthetic pathway for the cyanide-free production of phenylacetonitrile in Escherichia coli by utilizing plant cytochrome P450 79A2 and bacterial aldoxime dehydratase. Appl. Environ. Microbiol. 2014, 80, 6828–6836. [Google Scholar] [CrossRef]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Chanotiya, C.S. Chemical composition of leaf and root essential oils of Boenninghausenia albiflora Reichb. from northern India. Nat. Prod. Res. 2012, 26, 2040–2044. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.; Johnen, L. Myrcene as a natural base chemical in sustainable chemistry: A critical review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, N.K.; Jindal, S.K.; Barnes, P.J. Studies on Respiratory Disorders; Humana Press: Totowa, NJ, USA, 2014; ISBN 978-1-4939-0496-9. [Google Scholar]
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Miekisch, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Calenic, B.; Miricescu, D.; Greabu, M.; Kuznetsov, A.V.; Troppmair, J.; Ruzsanyi, V.; Amann, A. Oxidative stress and volatile organic compounds: Interplay in pulmonary, cardio-vascular, digestive tract systems and cancer. Open Chem. 2015, 13, 1020–1030. [Google Scholar] [CrossRef]
- Schnabel, R.; Fijten, R.; Smolinska, A.; Dallinga, J.; Boumans, M.-L.; Stobberingh, E.; Boots, A.; Roekaerts, P.; Bergmans, D.; van Schooten, F.J. Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Sci. Rep. 2015, 5, 17179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.S.; Prada, P.A.; Curran, A.M.; Furton, K.G. Applicability of emanating volatile organic compounds from various forensic specimens for individual differentiation. Forensic Sci. Int. 2013, 226, 173–182. [Google Scholar] [CrossRef]
- Sheng, G.J.; Goldberg, J.M.; Harigaya, R.; Ty, J.C. Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Dartmouth Undergrad. J. Sci. 2006, 8, 47–53. [Google Scholar]
- Nozoe, T.; Goda, S.; Selyanchyn, R.; Wang, T.; Nakazawa, K.; Hirano, T.; Matsui, H.; Lee, S.W. In vitro detection of small molecule metabolites excreted from cancer cells using a Tenax TA thin-film microextraction device. J. Chromatogr. B 2015, 991, 99–107. [Google Scholar] [CrossRef]
- Wood, W.F.; Brandes, M.L.; Watson, R.L.; Jones, R.L.; Largent, D.L. Trans-2-nonenal, the cucumber odor of mushrooms. Mycologia 1994, 86, 561–563. [Google Scholar] [CrossRef]
- Tangerman, A.; Winkel, E.G. Volatile sulfur compounds: The cause of bad breath. Phosphorus. Sulfur. Silicon Relat. Elem. 2013, 188, 396–402. [Google Scholar] [CrossRef]
- Schemel-Suárez, M.; Chimenos-Küstner, E.; Estrugo-Devesa, A.; Jané-Salas, E.; López-López, J. Halitosis assessment and changes in volatile sulfur compounds after chewing gum: A study performed on dentistry students. J. Evid. Based Dent. Pract. 2017, 17, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Haraszthy, V.I.; Zambon, J.J.; Sreenivasan, P.K.; Zambon, M.M.; Gerber, D.; Rego, R.; Parker, C. Identification of oral bacterial species associated with halitosis. J. Am. Dent. Assoc. 2007, 138, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, T.; Ghimenti, S.; Biagini, D.; Bramanti, E.; Onor, M.; Bellagambi, F.G.; Fuoco, R.; Di Francesco, F. The effect of sampling procedures on the urate and lactate concentration in oral fluid. Microchem. J. 2018, 136, 255–262. [Google Scholar] [CrossRef]
- Lomonaco, T.; Ghimenti, S.; Piga, I.; Biagini, D.; Onor, M.; Fuoco, R.; Di Francesco, F. Influence of sampling on the determination of warfarin and warfarin alcohols in oral fluid. PLoS ONE 2014, 9, e114430. [Google Scholar] [CrossRef] [PubMed]
- Bellagambi, F.G.; Degano, I.; Ghimenti, S.; Lomonaco, T.; Dini, V.; Romanelli, M.; Mastorci, F.; Gemignani, A.; Salvo, P.; Fuoco, R.; et al. Determination of salivary α-amylase and cortisol in psoriatic subjects undergoing the Trier Social Stress Test. Microchem. J. 2018, 136, 177–184. [Google Scholar] [CrossRef]
- Martin, H.J.; Riazanskaia, S.; Thomas, C.L.P. Sampling and characterisation of volatile organic compound profiles in human saliva using a polydimethylsiloxane coupon placed within the oral cavity. Analyst 2012, 137, 3627–3634. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Not available. |


| Volatile Sulfur Compound | Group of Patients | ||
|---|---|---|---|
| HE | AB | HA | |
| methyl thiolacetate | X | ||
| dimethyl disulfide | X | X | X |
| dimethyl trisulfide | X | X | X |
| dimethyl tetrasulfide | X | X | |
| dimethyl pentasulfide | X | ||
| dimethyl sulfone | X | X | X |
| allyl thiocyanate | X | ||
| allyl isothiocyanate | X | ||
| S-methyl pentanethioate | X | ||
| thiolan-2-one | X | ||
| TOTAL | 3 | 4 | 10 |
| Class | Control | Submandibular Abscess | Halitosis |
|---|---|---|---|
| Sensitivity | 66.7% | 100.0% | 100.0% |
| Specificity | 100% | 81.8% | 92.3% |
| Balanced accuracy | 83.3% | 90.9% | 96.1% |
| AUC | 0.927 | 0.950 | 0.992 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monedeiro, F.; Milanowski, M.; Ratiu, I.-A.; Zmysłowski, H.; Ligor, T.; Buszewski, B. VOC Profiles of Saliva in Assessment of Halitosis and Submandibular Abscesses Using HS-SPME-GC/MS Technique. Molecules 2019, 24, 2977. https://doi.org/10.3390/molecules24162977
Monedeiro F, Milanowski M, Ratiu I-A, Zmysłowski H, Ligor T, Buszewski B. VOC Profiles of Saliva in Assessment of Halitosis and Submandibular Abscesses Using HS-SPME-GC/MS Technique. Molecules. 2019; 24(16):2977. https://doi.org/10.3390/molecules24162977
Chicago/Turabian StyleMonedeiro, Fernanda, Maciej Milanowski, Ileana-Andreea Ratiu, Hubert Zmysłowski, Tomasz Ligor, and Bogusław Buszewski. 2019. "VOC Profiles of Saliva in Assessment of Halitosis and Submandibular Abscesses Using HS-SPME-GC/MS Technique" Molecules 24, no. 16: 2977. https://doi.org/10.3390/molecules24162977
APA StyleMonedeiro, F., Milanowski, M., Ratiu, I.-A., Zmysłowski, H., Ligor, T., & Buszewski, B. (2019). VOC Profiles of Saliva in Assessment of Halitosis and Submandibular Abscesses Using HS-SPME-GC/MS Technique. Molecules, 24(16), 2977. https://doi.org/10.3390/molecules24162977








