Electrochemical Performance of Nitrogen-Doped TiO2 Nanotubes as Electrode Material for Supercapacitor and Li-Ion Battery
Abstract
:1. Introduction
2. Results and Discussions
2.1. Structural Analysis
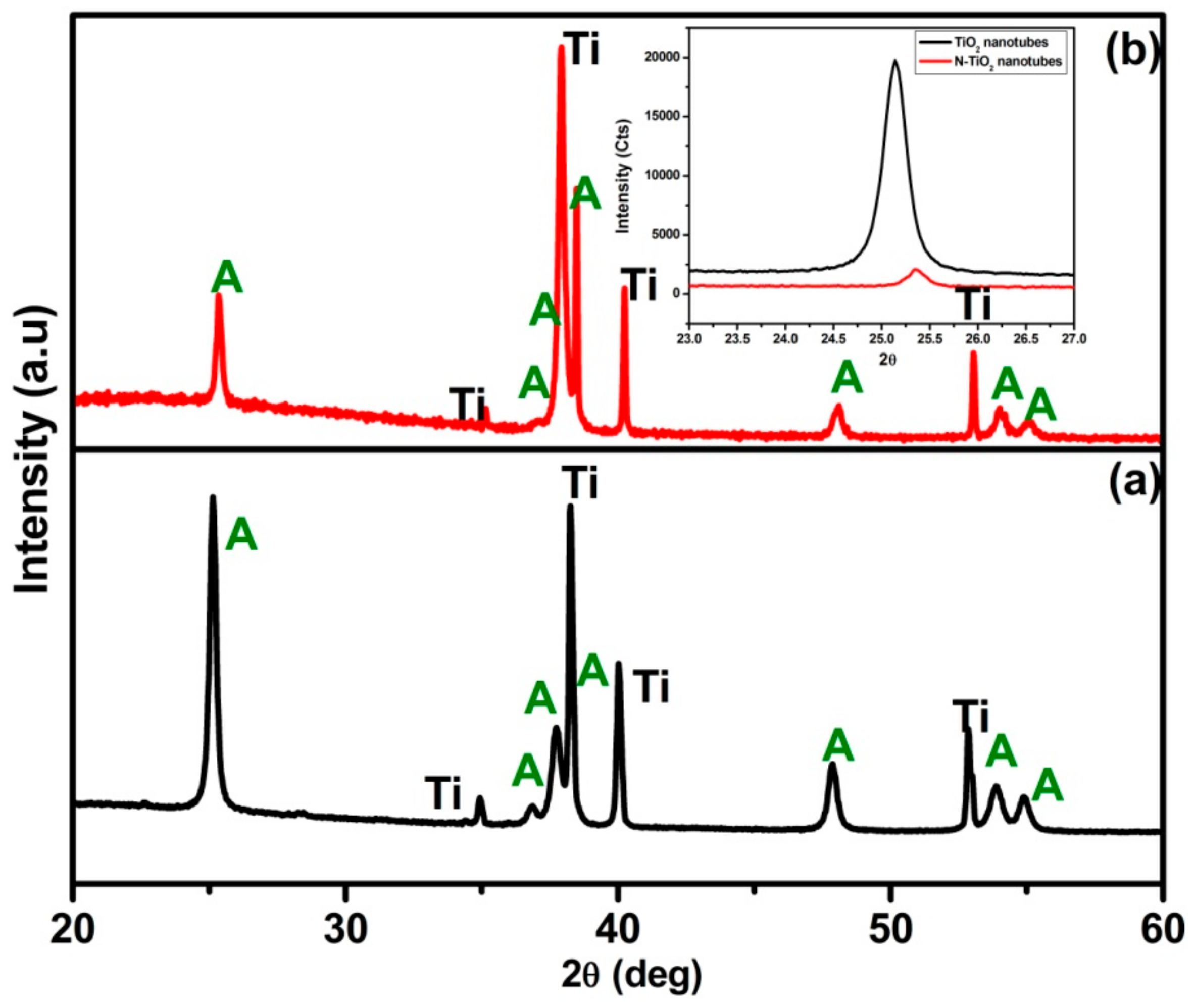
2.1.1. X-ray Diffraction (XRD)
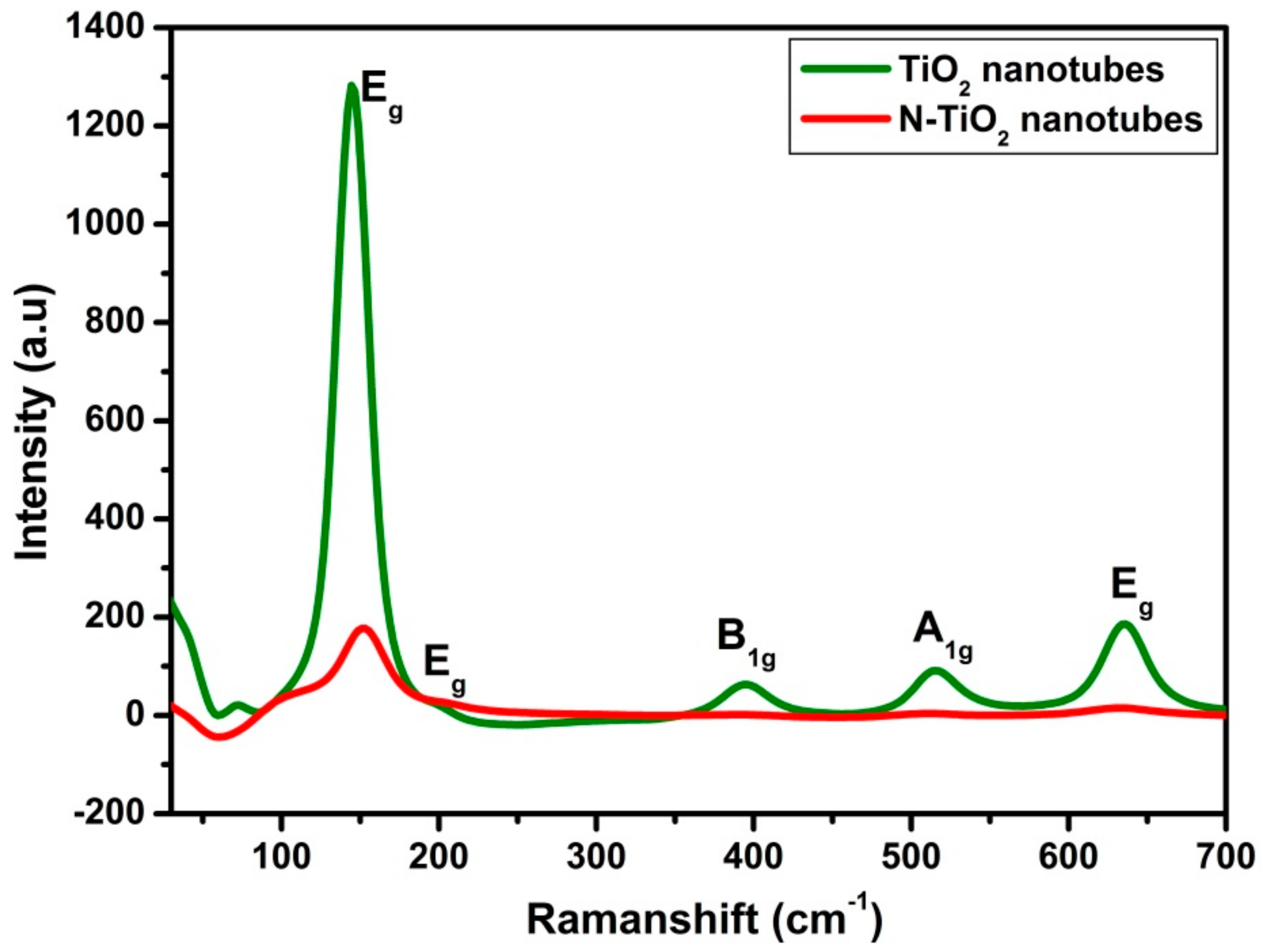
2.1.2. Raman Spectroscopy
2.2. Morphological and Compositional Analysis: Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive Spectroscopy (EDS)
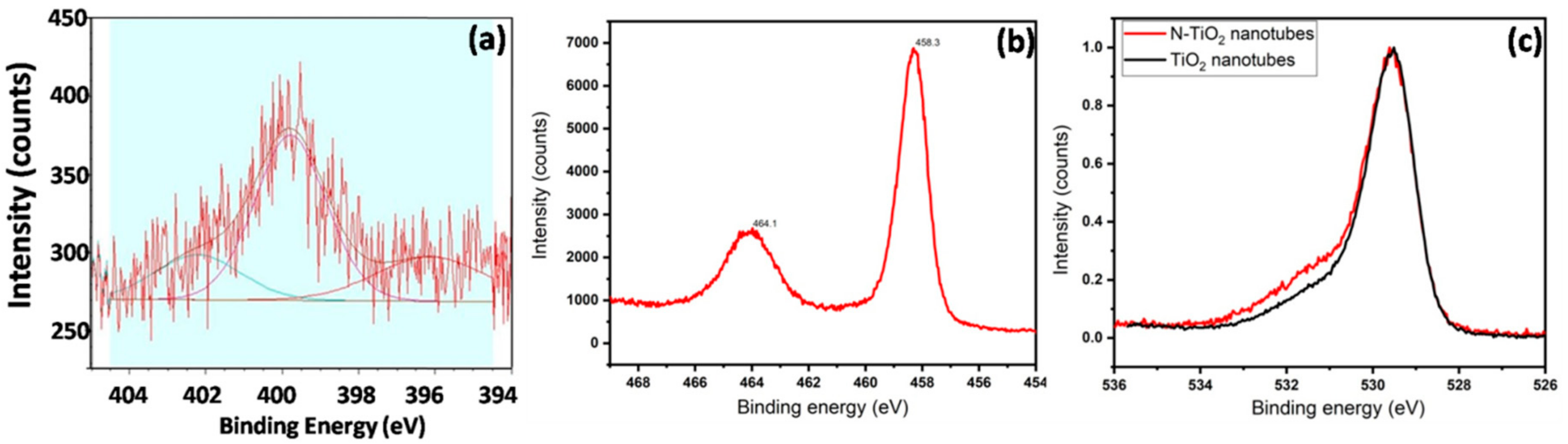
2.3. Chemical Analysis: X-ray Photoelectron Spectroscopy (XPS)
2.4. Supercapacitor Application
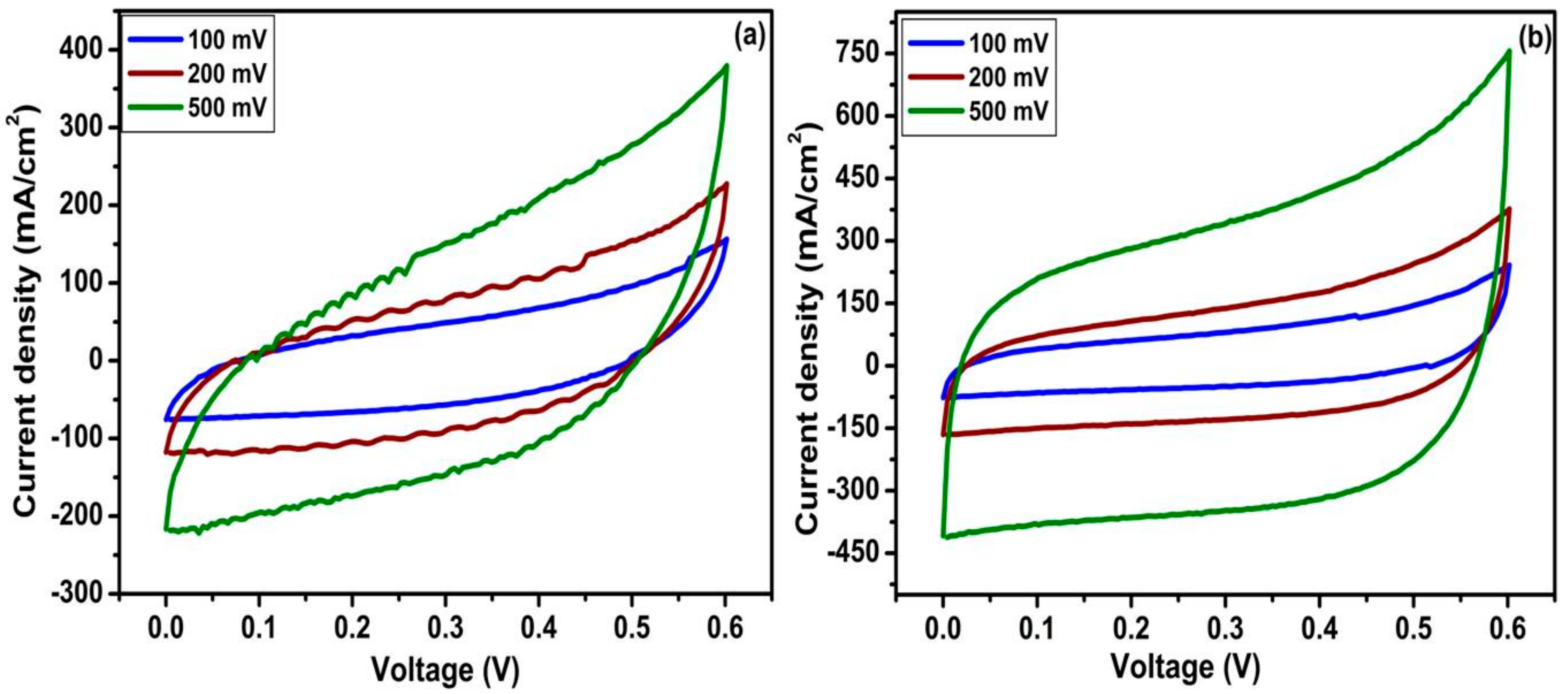
2.4.1. Cyclic Voltammetry (CV)
2.4.2. Electrochemical Impedance Spectroscopy
2.5. Lithium-Ion Battery Application
3. Materials and Methods
3.1. Synthesis of TiO2 Nanotubes and N-Doped TiO2 (N-TiO2) Nanotubes
3.2. Material Characterizations
3.3. Electrochemical Characterization
3.3.1. Supercapacitor
3.3.2. Li-Ion Battery Anode
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alireza, K.; Li, Z. Battery, Ultracapacitor, Fuel Cell, and Hybrid Energy Storage Systems for Electric, Hybrid Electric, Fuel Cell, and Plug-In Hybrid Electric Vehicles: State of the Art. IEEE Trans. Veh. Technol. 2010, 59, 2806–2814. [Google Scholar]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Muralidharan, P.; Lee, H.-W.; Ruffo, R.Y.; Yang, C.K.; Chan, H.; Peng, R.A.; Huggins, Y. Spinel LiMn2O4 Nanorods as Lithium Ion Battery Cathodes. Nano Lett. 2008, 8, 3948–3952. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chockla, A.M.; Harris, J.T.; Akhavan, V.A.; Bogart, T.D.; Holmberg, V.C.; Steinhagen, C.; Mullins, C.B.; Stevenson, K.J.; Korgel, B.A. Silicon Nanowire Fabric as a Lithium Ion Battery Electrode Material. J. Amer. Chem. Soc. 2011, 133, 20914–20921. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Z.; Jiang, R.; Bian, C.; Huang, T.; Yu, A. TiO2 nanotube array film prepared by anodization as anode material for lithium ion batteries. J. Solid State Electrochem. 2010, 14, 1045–1050. [Google Scholar]
- Wang, Y.; Liu, S.; Huang, K.; Fang, D.; Zhuang, S. Electrochemical properties of freestanding TiO2 nanotube membranes annealed in Ar for lithium anode material. J. Solid State Electrochem. 2012, 16, 723–729. [Google Scholar]
- Mancini, M.; Nobili, F.; Tossici, R.; Wohlfahrt-Mehrens, M.; Marassi, R. High performance, environmentally friendly and low cost anodes for lithium-ion battery based on TiO2 anatase and water soluble binder carboxymethyl cellulose. J. Power Sources 2011, 196, 9665–9671. [Google Scholar] [CrossRef]
- Yang, Z.; Choi, D.; Kerisit, S.; Rosso, K.M.; Wang, D.; Zhang, J.; Graff, G.; Liu, J. Nanostructures and lithium electrochemical reactivity of lithium titanites and titanium oxides: A review. J. Power Sources 2009, 192, 588–598. [Google Scholar] [CrossRef]
- Pei, Z.; Zhu, M.; Huang, Y.; Huang, Y.; Xue, Q.; Geng, H.; Zhi, C. Dramatically improved energy conversion and storage efficiencies by simultaneously enhancing charge transfer and creating active sites in MnOx/TiO2 nanotube composite electrodes. Nano Energy 2016, 20, 254–263. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Zhu, M.; Huang, Yan.; Tang, Z.; Pei, Z.; Wang, Z.; Shi, Z.; Liu, J.; Huang, Y.; et al. Towards wearable electronic devices: A quasi-solid-state aqueous lithium-ion battery with outstanding stability, flexibility, safety and breathability. Nano Energy 2018, 44, 164–173. [Google Scholar]
- Pan, D.; Huang, H.; Wang, X.; Wang, L.; Liao, H.; Li, Z.; Wu, M. C-axis preferentially oriented and fully activated TiO2 nanotube arrays for lithium ion batteries and supercapacitors. J. Mater. Chem. A 2014, 2, 11454–11464. [Google Scholar] [CrossRef]
- Bresser, D.; Oschmann, B.; Muhammad, N.; Tahir, F.M.; Ingo, L.; Wolfgang, T.; Rudolf, Z.; Stefano, P. Carbon-Coated Anatase TiO2 Nanotubes for Li- and Na-Ion Anodes. J. Electrochem. Soc. 2015, 162, A3013–3020. [Google Scholar] [CrossRef]
- Madian, M.; Giebeler, L.; Klose, M.; Jaumann, T.; Uhlemann, M.; Gebert, A.; Oswald, S.; Ismail, N.; Eychmüller, A.; Eckert, J. Self-Organized TiO2/CoO Nanotubes as Potential Anode Materials for Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2015, 3, 909–919. [Google Scholar] [CrossRef]
- Liu, D.; Xiao, P.; Zhang, Y.; Betzaida, B.; Garcia, Q.; Zhang, F.; Guo, Q.; Champion, R.; Cao, G. TiO2 Nanotube Arrays Annealed in N2 for Efficient Lithium-Ion Intercalation. J. Phys. Chem. C 2008, 112, 11175–11180. [Google Scholar] [CrossRef]
- Palgrave, R.G.; Payne, D.G.; Egdell, R.G. Nitrogen diffusion in doped TiO2 (110) single crystals: A combined XPS and SIMS study. J. Mater. Chem. 2009, 19, 8418–8425. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, X.; Lin, B.; Gao, B. Origin of the visible-light photoactivity of NH3-treated TiO2: Effect of nitrogen doping and oxygen vacancies. Appl. Surf. Sci. 2013, 264, 845–852. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-Dimensional Titanium Dioxide Nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Song, T.; Bae, J.-Y.; Nazar, L.F.; Kim, H.; Paik, U. Nitridated TiO2 hollow nanofibers as an anode material for high power lithium ion batteries. Energy Environ. Sci. 2011, 4, 4532–4536. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Lv, X.-J. N-doped TiO2 nanotubes/N-doped graphene nanosheets composites as high performance anode materials in lithium-ion battery. J. Mater. Chem. A 2014, 2, 15473–15479. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Q.; Xu, Q.; Yan, X.; Zhang, R.; Guo, Z.; Du, F.; Wei, Y.; Zhang, D.; Chen, G. Improved electrochemical performance of nitrogen doped TiO2-B nanowires as anode materials for Li-ion batteries. Nanoscale 2015, 7, 12215–12224. [Google Scholar] [CrossRef]
- Pan, X.; Xu, Y.-J. Defect-Mediated Growth of Noble-Metal (Ag, Pt, and Pd) Nanoparticles on TiO2 with Oxygen Vacancies for Photocatalytic Redox Reactions under Visible Light. J. Phys. Chem. C 2013, 117, 17996–18005. [Google Scholar] [CrossRef]
- Sun, H.; Bai, Y.; Jin, W.; Xu, N. Visible-light-driven TiO2 catalysts doped with low-concentration nitrogen species. Solar Energy Mater. Solar Cells 2008, 92, 76–83. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Ovenstone, J. Crystallization of Anatase from Amorphous Titania Using the Hydrothermal Technique: Effects of Starting Material and Temperature. J. Phys. Chem. B 1999, 103, 7781–7787. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.; Zhang, Y.; Liu, S. An Efficient Two-Step Technique for Nitrogen-Doped Titanium Dioxide Synthesizing: Visible-Light-Induced Photodecomposition of Methylene Blue. J. Phys. Chem. C 2007, 111, 1010–1014. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwak, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Burda, C. Photoelectron Spectroscopic Investigation of Nitrogen-Doped Titania Nanoparticles. J. Phys. Chem. B 2004, 108, 15446–15449. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R.P.; Gopinath, C.S. Synthesis, Characterization, Electronic Structure, and Photocatalytic Activity of Nitrogen-Doped TiO2. Nanocatalyst. Chem. Mater. 2005, 17, 6349–6353. [Google Scholar] [CrossRef]
- Emerson, C.; Kohlrausch, M.; Zapata, J.M.; Renato, V.; Gonçalves, S.K.; de O. Vaz, M.; Sérgio, J.D.; Marcos, R.T.; Santos, J.L. Polymorphic phase study on nitrogen-doped TiO2 nanoparticles: Effect on oxygen site occupancy dye sensitized solar cells efficiency and hydrogen production. RSC Adv. 2015, 5, 101276–101286. [Google Scholar]
- Tamilselvan, A.; Balakumar, S. Anatase TiO2 nanotube by electrochemical anodization method: Effect of tubes dimension on the supercapacitor application. Ionics 2016, 22, 99–105. [Google Scholar] [CrossRef]
- Ratha, S.; Rout, C.S. Supercapacitor Electrodes Based on Layered Tungsten Disulfide-Reduced Graphene Oxide Hybrids Synthesized by a Facile Hydrothermal Method. ACS Appl. Mater. Interfaces 2013, 5, 11427–11433. [Google Scholar] [CrossRef]
- Salari, M.; Aboutalebi, S.H.; Konstantinov, K.; Liu, H.K. A highly ordered titania nanotube array as a supercapacitor electrode. Phys. Chem. Chem. Phys. 2011, 13, 5038–5041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.G.; Lee, Y.-G.; Song, S.-W.; Kim, K.M. Lithium-ion battery anode properties of TiO2 nanotubes prepared by the hydrothermal synthesis of mixed (anatase and rutile) particle. Electrochim. Acta 2010, 55, 5975–5983. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Xiao, P.; Garcia, B.B.; Zhang, Q.; Zhou, X.; Jeong, Y.-H.; Cao, G. TiO2 nanotube arrays annealed in CO exhibiting high performance for lithium ion intercalation. Electrochim. Acta 2009, 54, 6816–6820. [Google Scholar] [CrossRef]
- Zakharova, G.S.; Jähne A Popa, C.; Täschner, Ch.; Gemming, Th.; Leonhardt, A.; Büchner, B.; Klingeler, R. Anatase Nanotubes as an Electrode Material for Lithium-Ion Batteries. J. Phys. Chem. C 2012, 116, 8714–8720. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available with author-T.A. and B.S. |


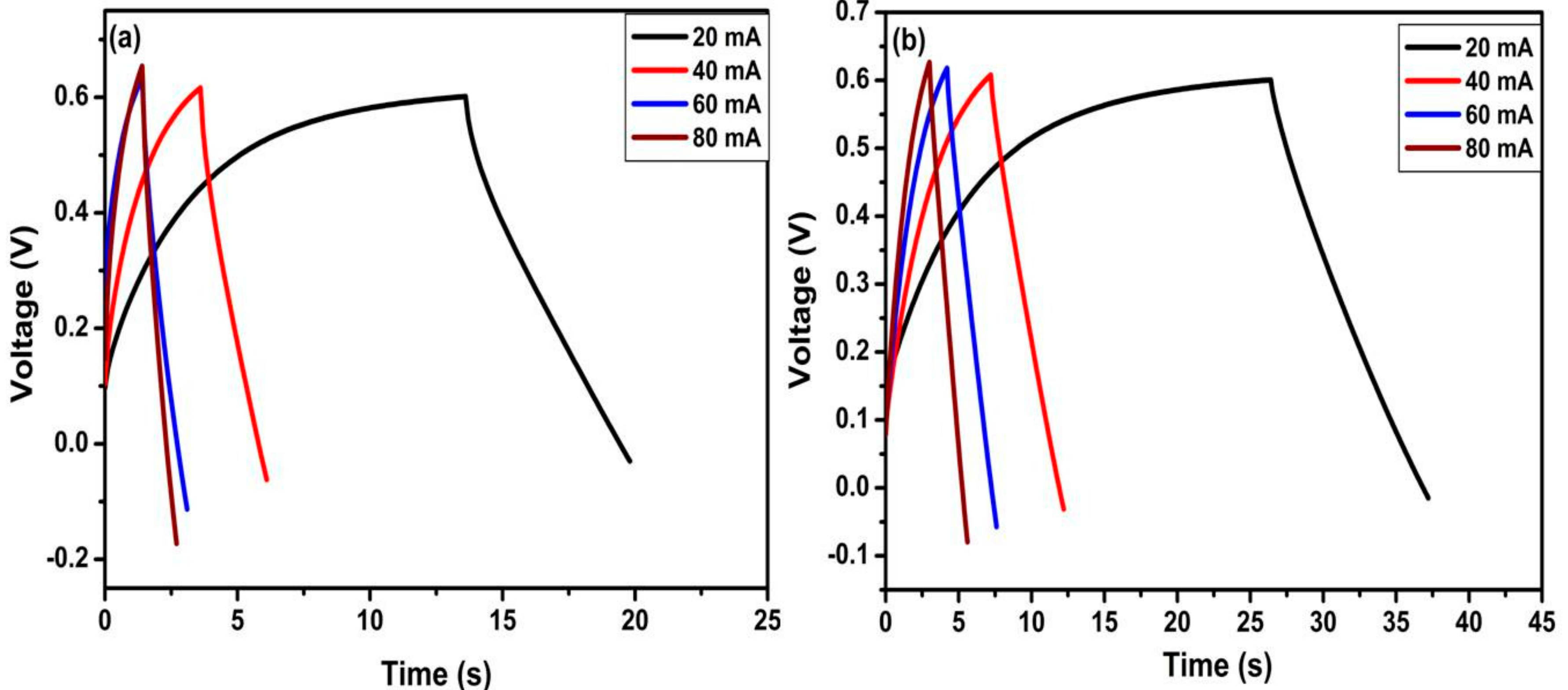



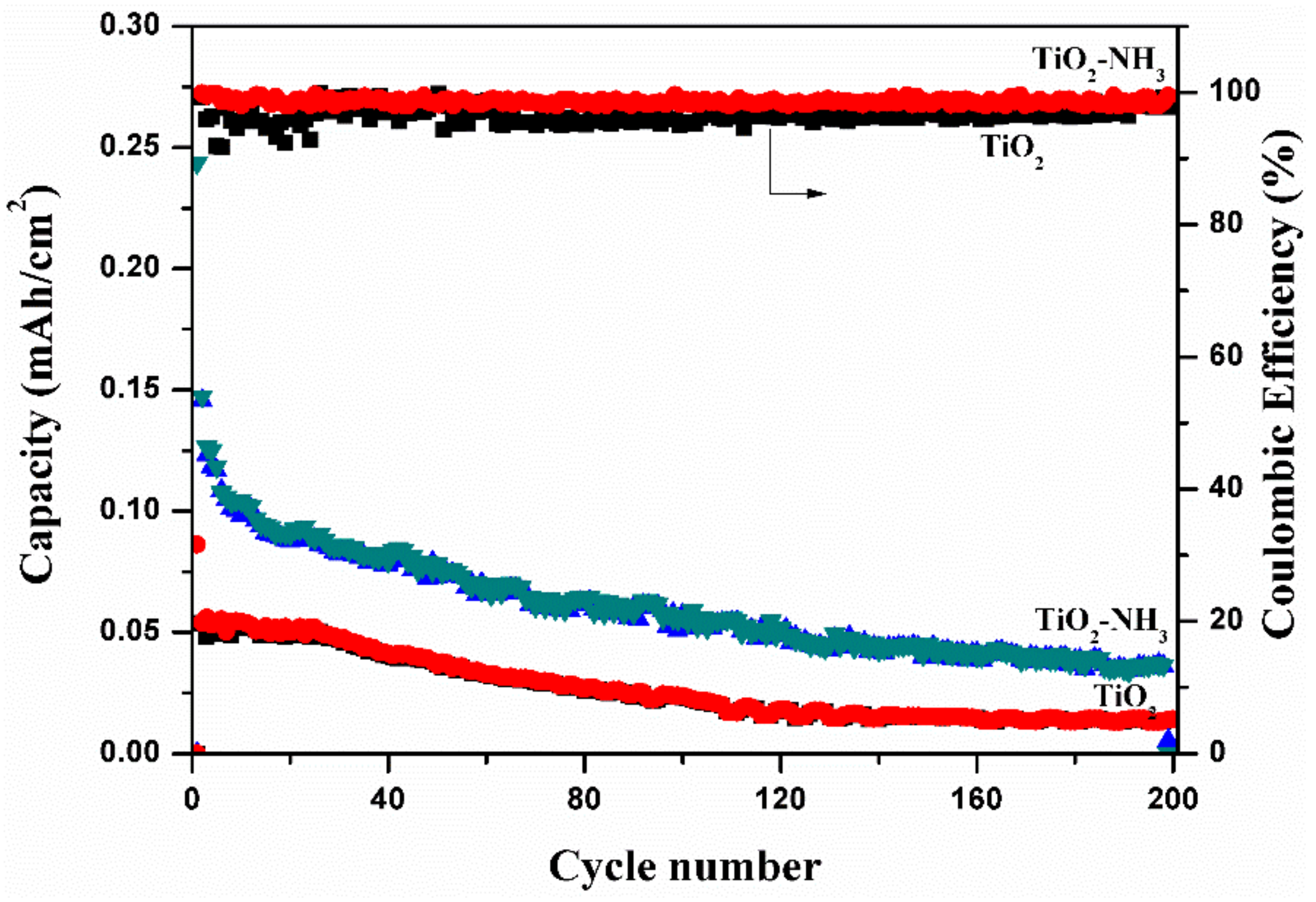
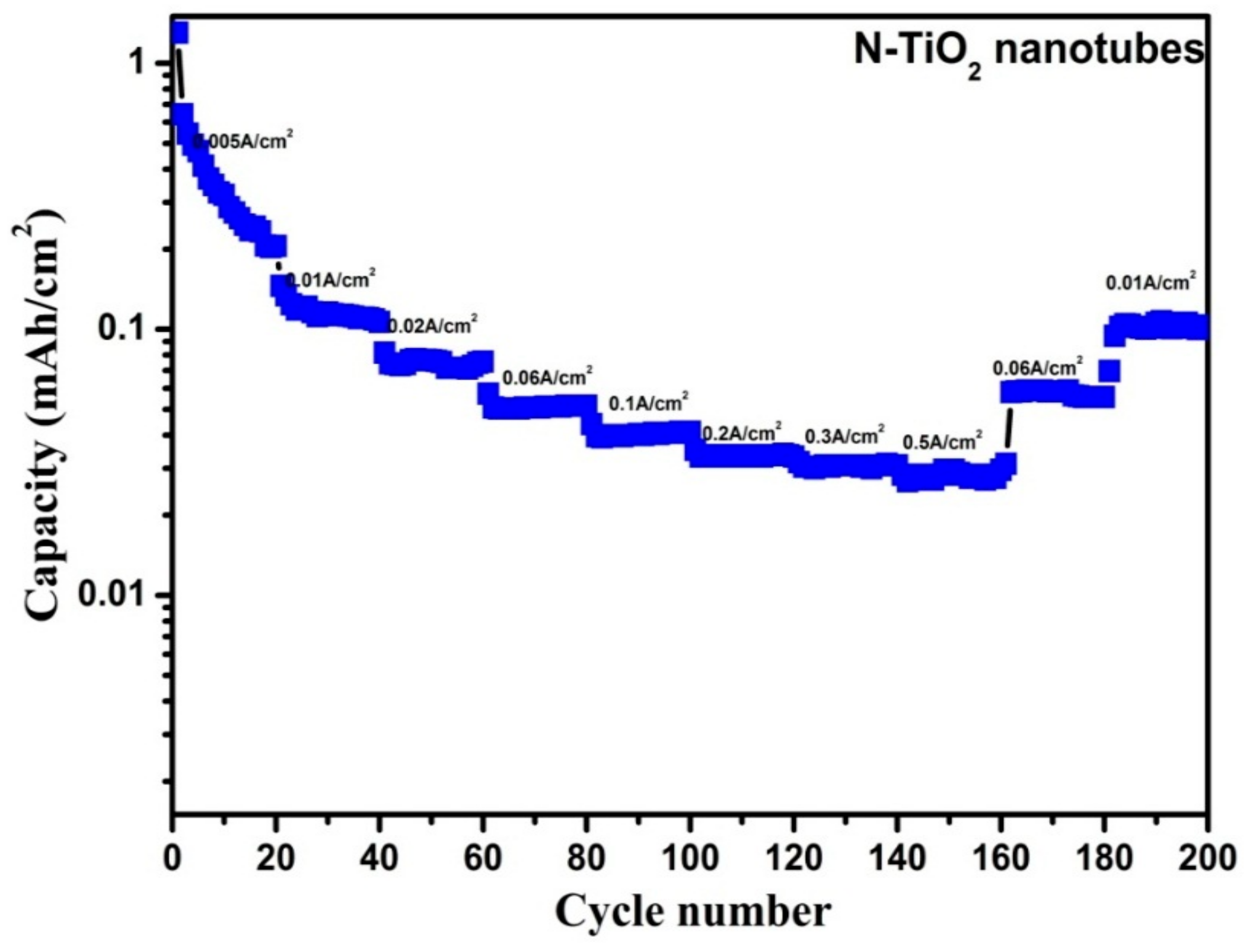
| S.No. | Scan Rate (mV/s) | TiO2 Nanotubes | N-TiO2 Nanotubes | ||
|---|---|---|---|---|---|
| Current Density (µA cm−2) | Specific Capacitance (µF cm−2) | Current Density (µA cm−2) | Specific Capacitance (µF cm−2) | ||
| 1 2 3 | 100 200 500 | 50.456 84.854 150.590 | 504.56 424.27 301.18 | 83.576 139.780 348.483 | 835.760 698.900 696.966 |
| S.NO | Current Density (µAcm−2) | TiO2 Nanotubes | N Doped TiO2 Nanotubes | ||||
|---|---|---|---|---|---|---|---|
| Time (s) | Voltage (mV) | Specific Capacitance (µF cm−2) | Time (s) | Voltage (mV) | Specific Capacitance (µF cm−2) | ||
| 1 | 80 | 19.6 | 0.606 | 2587.4 | 36.91 | 0.600 | 4921.3 |
| 2 | 160 | 5.74 | 0.609 | 1508.0 | 11.92 | 0.611 | 3121.4 |
| 3 | 240 | 2.70 | 0.649 | 998.4 | 7.17 | 0.614 | 2802.6 |
| 4 | 320 | 2.29 | 0.649 | 1129.1 | 5.22 | 0.627 | 2664.0 |
| S.No. | Cycle | TiO2 Nanotubes | N-Doped TiO2 Nanotubes | ||||
|---|---|---|---|---|---|---|---|
| Cathodic Peak (V) | Anodic Peak (V) | Potential Difference (V) | Cathodic Peak (V) | Anodic Peak (V) | Potential Difference (V) | ||
| 1 | 1 | 1.58 | 2.150 | 0.580 | 1.49 | 2.17 | 0.680 |
| 2 | 5 | 1.69 | 2.117 | 0.437 | 1.70 | 2.09 | 0.390 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appadurai, T.; Subramaniyam, C.M.; Kuppusamy, R.; Karazhanov, S.; Subramanian, B. Electrochemical Performance of Nitrogen-Doped TiO2 Nanotubes as Electrode Material for Supercapacitor and Li-Ion Battery. Molecules 2019, 24, 2952. https://doi.org/10.3390/molecules24162952
Appadurai T, Subramaniyam CM, Kuppusamy R, Karazhanov S, Subramanian B. Electrochemical Performance of Nitrogen-Doped TiO2 Nanotubes as Electrode Material for Supercapacitor and Li-Ion Battery. Molecules. 2019; 24(16):2952. https://doi.org/10.3390/molecules24162952
Chicago/Turabian StyleAppadurai, Tamilselvan, Chandrasekar M Subramaniyam, Rajesh Kuppusamy, Smagul Karazhanov, and Balakumar Subramanian. 2019. "Electrochemical Performance of Nitrogen-Doped TiO2 Nanotubes as Electrode Material for Supercapacitor and Li-Ion Battery" Molecules 24, no. 16: 2952. https://doi.org/10.3390/molecules24162952





