Potentiating Antigen-Specific Antibody Production with Peptides Obtained from In Silico Screening for High-Affinity against MHC-II
Abstract
1. Introduction
2. Results
2.1. Screening the Agretope Peptides
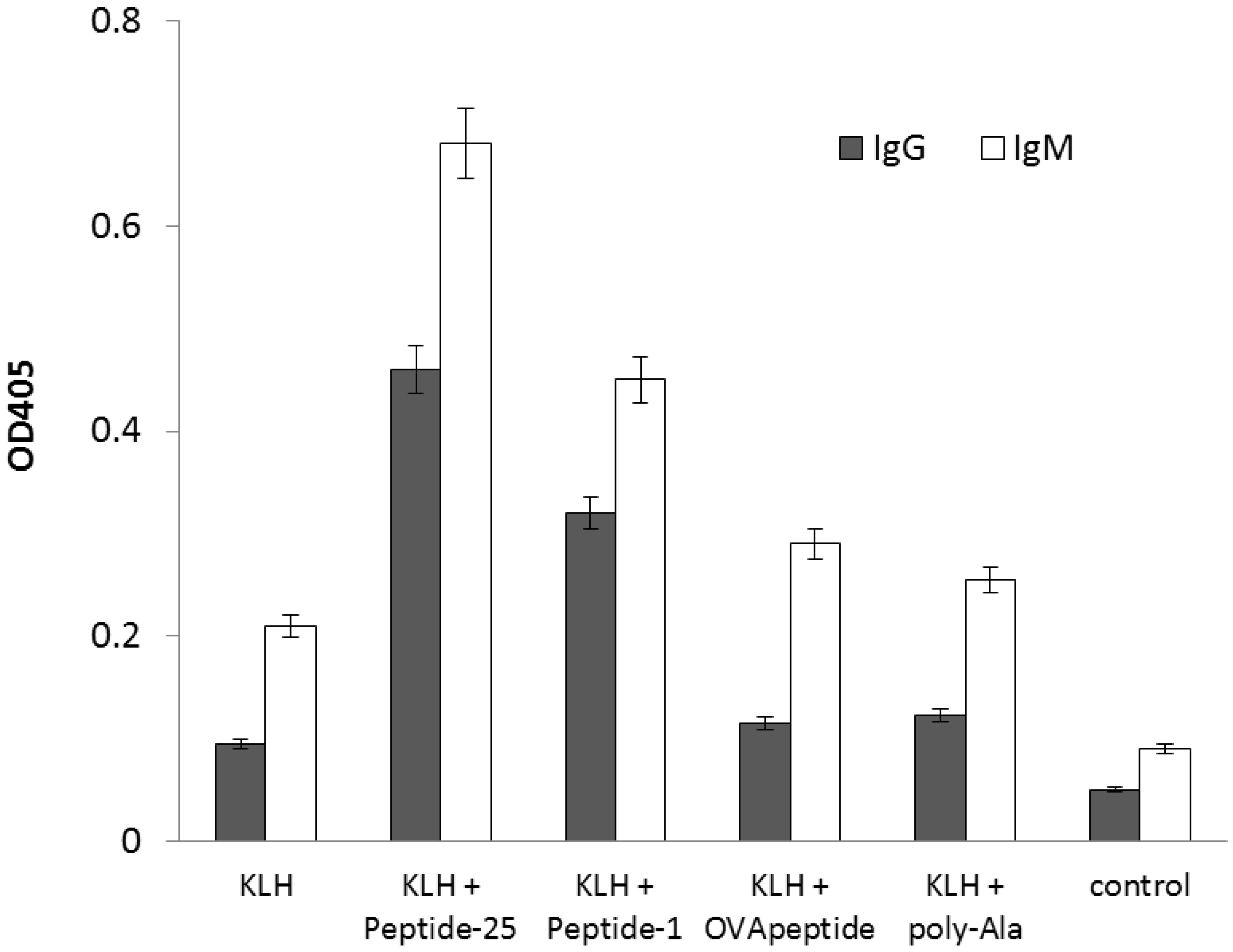
2.2. Enhancement of Antigen-Specific Antibody Production by the Peptide Obtained from In Silico Screening
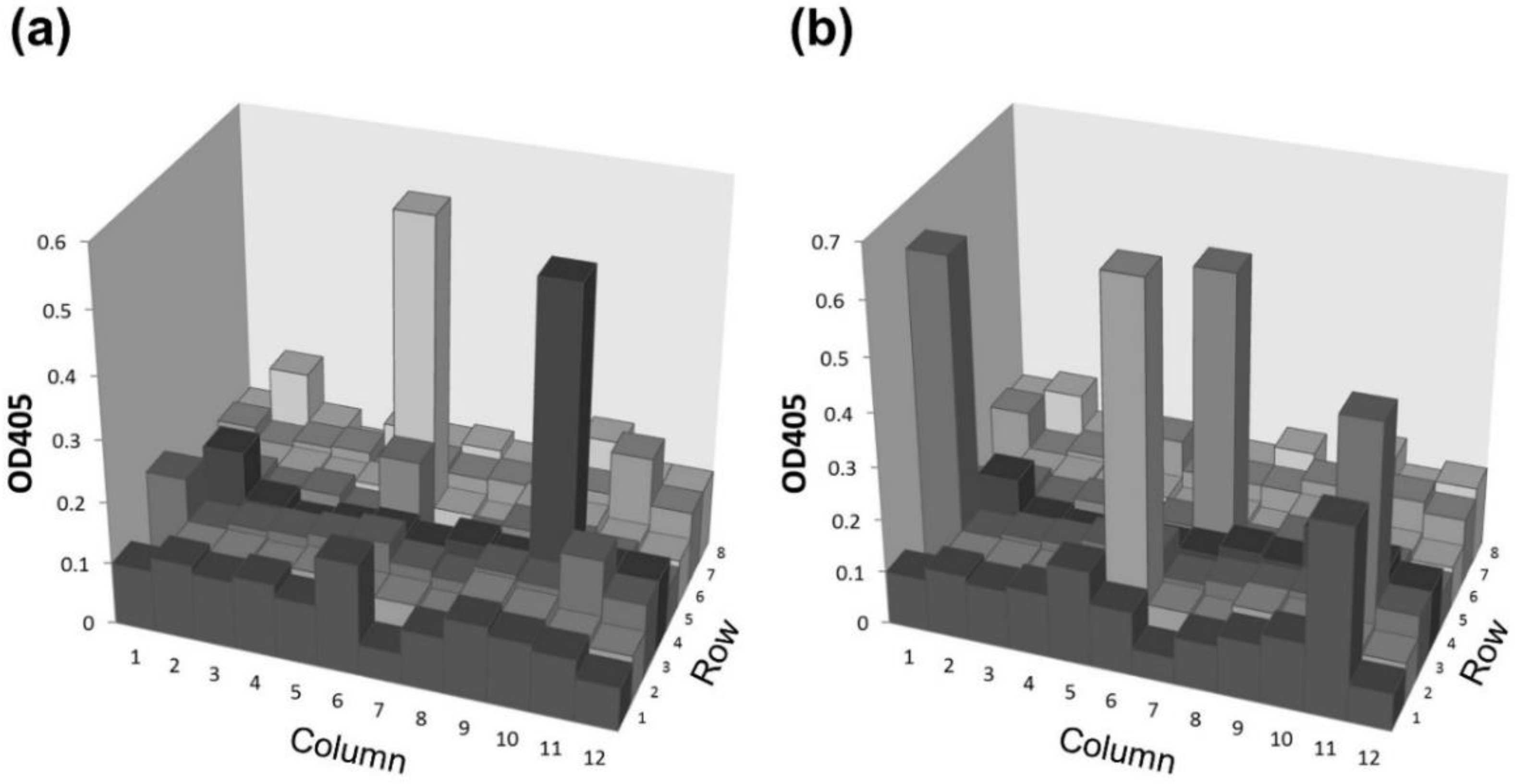
2.3. Application of the Agretope Peptide to Establish Monoclonal Antibodies by In Vitro Immunization (IVI)
3. Discussion
4. Materials and Methods
4.1. Mice and Immunization
4.2. In Silico Screening
4.3. Synthetic Peptides
4.4. IVI
4.5. ELISA
4.6. Hybridoma Formation and Quantification of Positive Clones by IVI
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stills, H.F. Adjuvants and antibody production: Dispelling the myths associated with Freund’s complete and other adjuvants. Ilar J. Natl. Res. Counc. Inst. Lab. Anim. Resour. 2005, 46, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.R.; Fox, J.G. Institutional Policies and Guidelines on Adjuvants and Antibody Production. Ilar J. Natl. Res. Counc. Inst. Lab. Anim. Resour. 1995, 37, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Haensler, J. Liposomal adjuvants: Preparation and formulation with antigens. Methods Mol. Biol. (Cliftonnj) 2010, 626, 73–90. [Google Scholar] [CrossRef]
- Malyala, P.; Singh, M. Micro/nanoparticle adjuvants: Preparation and formulation with antigens. Methods Mol. Biol. (Cliftonnj) 2010, 626, 91–101. [Google Scholar] [CrossRef]
- Gupta, R.K.; Chang, A.C.; Siber, G.R. Biodegradable polymer microspheres as vaccine adjuvants and delivery systems. Dev. Biol. Stand. 1998, 92, 63–78. [Google Scholar] [PubMed]
- Weeratna, R.D.; McCluskie, M.J.; Xu, Y.; Davis, H.L. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine 2000, 18, 1755–1762. [Google Scholar] [CrossRef]
- de Titta, A.; Ballester, M.; Julier, Z.; Nembrini, C.; Jeanbart, L.; van der Vlies, A.J.; Swartz, M.A.; Hubbell, J.A. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl. Acad. Sci. USA 2013, 110, 19902–19907. [Google Scholar] [CrossRef]
- Kato, M.; Sasamori, E.; Chiba, T.; Hanyu, Y. Cell activation by CpG ODN leads to improved electrofusion in hybridoma production. J. Immunol. Methods 2011, 373, 102–110. [Google Scholar] [CrossRef]
- Takatsu, K.; Kariyone, A. The immunogenic peptide for Th1 development. Int. Immunopharmacol. 2003, 3, 783–800. [Google Scholar] [CrossRef]
- Kariyone, A.; Higuchi, K.; Yamamoto, S.; Nagasaka-Kametaka, A.; Harada, M.; Takahashi, A.; Harada, N.; Ogasawara, K.; Takatsu, K. Identification of amino acid residues of the T-cell epitope of Mycobacterium tuberculosis alpha antigen critical for Vbeta11(+) Th1 cells. Infect. Immun. 1999, 67, 4312–4319. [Google Scholar]
- Bold, T.D.; Banaei, N.; Wolf, A.J.; Ernst, J.D. Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog. 2011, 7, e1002063. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, A.; Takeda, Y.; Furuichi, Y.; Kurachi, M.; Kimura, K.; Maekawa, R.; Takatsu, K.; Kakimi, K. Memory Th1 cells augment tumor-specific CTL following transcutaneous peptide immunization. Cancer Res. 2008, 68, 3941–3949. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Hanyu, Y. Potentiation of antigen-specific antibody production by peptides derived from Ag85B of Mycobacterium tuberculosis. J. Immunol. Methods 2015, 417. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.; Peterson, P.; Teyton, L.; Wilson, I. Crystal Structures of Two I-Ad-Peptide Complexes Reveal That High Affinity Can Be Achieved without Large Anchor Residues. Immunity 1998, 8, 319–329. [Google Scholar] [CrossRef]
- Zafiropoulos, A.; Andersson, E.; Krambovitis, E.; Borrebaeck, C.A. Induction of antigen-specific isotype switching by in vitro immunization of human naive B lymphocytes. J. Immunol. Methods 1997, 200, 181–190. [Google Scholar] [CrossRef]
- Udaka, K.; Mamitsuka, H.; Nakaseko, Y.; Abe, N. Prediction of MHC class I binding peptides by a query learning algorithm based on hidden markov models. J. Biol. Phys. 2002, 28, 183–194. [Google Scholar] [CrossRef]
- Udaka, K.; Mamitsuka, H.; Nakaseko, Y.; Abe, N. Empirical Evaluation of a Dynamic Experiment Design Method for Prediction of MHC Class I-Binding Peptides. J. Immunol. 2002, 169, 5744–5753. [Google Scholar] [CrossRef]
- Rammensee, H.-G.; Friede, T.; Stevanović, S. MHC ligands and peptide motifs: First listing. Immunogenetics 1995, 41, 178–228. [Google Scholar] [CrossRef]
- Matsumoto, S.E.; Yamashita, M.; Katakura, Y.; Aiba, Y.; Tomimatsu, K.; Kabayama, S.; Teruya, K.; Shirahata, S. A rapid and efficient strategy to generate antigen-specific human monoclonal antibody by in vitro immunization and the phage display method. J. Immunol. Methods 2008, 332, 2–9. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yamashita, M.; Katakura, Y.; Noguchi, E.; Aiba, Y.; Ichikawa, A.; Teruya, K.; Shirahata, S. In vitro immunization can elicit the expansion of diverse repertoire of B cells from peripheral blood mononuclear cells. Cytotechnology 2007, 52, 227–233. [Google Scholar] [CrossRef][Green Version]
- Kettleborough, C.A.; Saldanha, J.; Heath, V.J.; Morrison, C.J.; Bendig, M.M. Humanization of a mouse monoclonal antibody by CDR-grafting: The importance of framework residues on loop conformation. Protein Eng. 1991, 4, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Yan, H.; Tsuji, N.M.; Chiba, T.; Hanyu, Y. A method for inducing antigen-specific IgG production by in vitro immunization. J. Immunol. Methods 2012, 386, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Geha, R.S.; Jabara, H.H.; Brodeur, S.R. The regulation of immunoglobulin E class-switch recombination. Nat. Rev. Immunol. 2003, 3, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Peled, J.U.; Kuang, F.L.; Iglesias-Ussel, M.D.; Roa, S.; Kalis, S.L.; Goodman, M.F.; Scharff, M.D. The Biochemistry of Somatic Hypermutation. Annu. Rev. Immunol. 2008, 26, 481–511. [Google Scholar] [CrossRef] [PubMed]
- Pavri, R.; Nussenzweig, M.C. Chapter 1—AID Targeting in Antibody Diversity. Adv. Immunol. 2011, 110, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Böhm, H.J. The computer program LUDI: A new method for the de novo design of enzyme inhibitors. J. Comput.-Aided Mol. Des. 1992, 6, 61–78. [Google Scholar]
- Böhm, H.J. The development of a simple empirical scoring function to estimate the binding constant for a protein-ligand complex of known three-dimensional structure. J. Comput.-Aided Mol. Des. 1994, 8, 243–256. [Google Scholar]
- Böhm, H.J. Prediction of binding constants of protein ligands: A fast method for the prioritization of hits obtained from de novo design or 3D database search programs. J. Comput.-Aided Mol. Des. 1998, 12, 309–323. [Google Scholar]
- Kato, M.; Hanyu, Y. Single-step colony assay for screening antibody libraries. J. Biotechnol. 2017, 255. [Google Scholar] [CrossRef]
- Kato, M.; Hanyu, Y. Single-step colony assay with autoinduction of scFv expression for the screening of antibody libraries. BioTechniques 2019, 66, 194–197. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
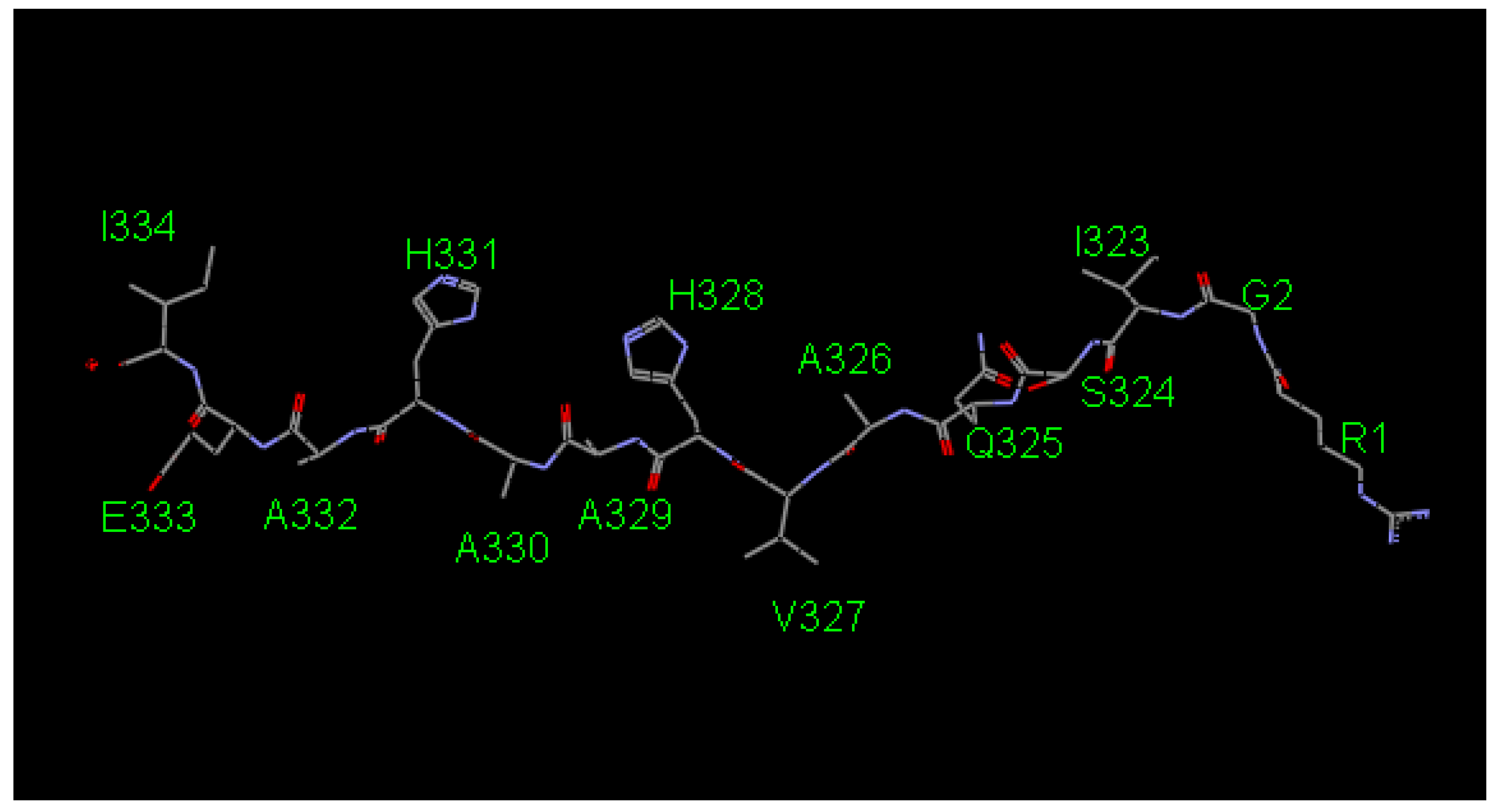

| Residue Position | Original | Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 | Rank 6 | Rank 7 |
|---|---|---|---|---|---|---|---|---|
| 1 | Arg * | Lys | Trp | Tyr | Met | |||
| 2 | Gly * | Tyr | Phe | Met | Ser | Thr | ||
| 3 | Ile * | Lys | Tyr | Phe | Thr | Ser | Ile | Met |
| 4 | Ser * | Phe * | Leu * | Ile * | Val | |||
| 5 | Gln * | Phe * | Lys * | Met | ||||
| 6 | Ala * | Tyr * | Gln * | Met | ||||
| 7 | Val * | Thr * | Ser | |||||
| 8 | His * | Phe * | Lys * | Glu | Trp | Met | Ile | Val |
| 9 | Ala * | (Gly *) | ||||||
| 10 | Ala * | (Gly *) | ||||||
| 11 | His * | Tyr * | Phe | Met | Ile | Leu | Val | Lys |
| 12 | Ala * | Lys * | ||||||
| 13 | Glu * | Met | Ile | Leu | Val | |||
| 14 | Ile * | Met |
| Rank | Score | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | R11 | R12 | R13 | R14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1649 | Arg | Gly | Ile | Phe | Phe | Tyr | Val | Phe | Ala | Ala | Tyr | Lys | Glu | Ile |
| 2 | 1620 | Arg | Gly | Ile | Phe | Phe | Tyr | Val | Phe | Gly | Ala | Tyr | Lys | Glu | Ile |
| 3 | 1620 | Arg | Gly | Ile | Phe | Phe | Gln | Val | Phe | Ala | Ala | Tyr | Lys | Glu | Ile |
| 4 | 1617 | Arg | Gly | Ile | Phe | Phe | Tyr | Thr | Phe | Ala | Ala | Tyr | Lys | Glu | Ile |
| 5 | 1613 | Arg | Gly | Ile | Phe | Phe | Tyr | Val | Phe | Ala | Gly | Tyr | Lys | Glu | Ile |
| 6 | 1610 | Arg | Gly | Ile | Phe | Phe | Tyr | Val | Phe | Ala | Ala | His | Lys | Glu | Ile |
| 7 | 1605 | Arg | Gly | Ile | Phe | Phe | Tyr | Val | His | Ala | Ala | Tyr | Lys | Glu | Ile |
| 8 | 1597 | Arg | Gly | Ile | Phe | Phe | Tyr | Val | Lys | Ala | Ala | Tyr | Lys | Glu | Ile |
| 9 | 1593 | Arg | Gly | Ile | Phe | Phe | Gln | Val | Phe | Gly | Ala | Tyr | Lys | Glu | Ile |
| 10 | 1588 | Arg | Gly | Ile | Phe | Phe | Tyr | Thr | Phe | Gly | Ala | Tyr | Lys | Glu | Ile |
| - | |||||||||||||||
| 3362 * | 968 | Arg | Gly | Ile | Ser | Gln | Ala | Val | His | Ala | Ala | His | Ala | Glu | Ile |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanyu, Y.; Komeiji, Y.; Kato, M. Potentiating Antigen-Specific Antibody Production with Peptides Obtained from In Silico Screening for High-Affinity against MHC-II. Molecules 2019, 24, 2949. https://doi.org/10.3390/molecules24162949
Hanyu Y, Komeiji Y, Kato M. Potentiating Antigen-Specific Antibody Production with Peptides Obtained from In Silico Screening for High-Affinity against MHC-II. Molecules. 2019; 24(16):2949. https://doi.org/10.3390/molecules24162949
Chicago/Turabian StyleHanyu, Yoshiro, Yuto Komeiji, and Mieko Kato. 2019. "Potentiating Antigen-Specific Antibody Production with Peptides Obtained from In Silico Screening for High-Affinity against MHC-II" Molecules 24, no. 16: 2949. https://doi.org/10.3390/molecules24162949
APA StyleHanyu, Y., Komeiji, Y., & Kato, M. (2019). Potentiating Antigen-Specific Antibody Production with Peptides Obtained from In Silico Screening for High-Affinity against MHC-II. Molecules, 24(16), 2949. https://doi.org/10.3390/molecules24162949





