Dietary Curcumin Prevented Astrocytosis, Microgliosis, and Apoptosis Caused by Acute and Chronic Exposure to Ozone
Abstract
:1. Introduction
2. Results
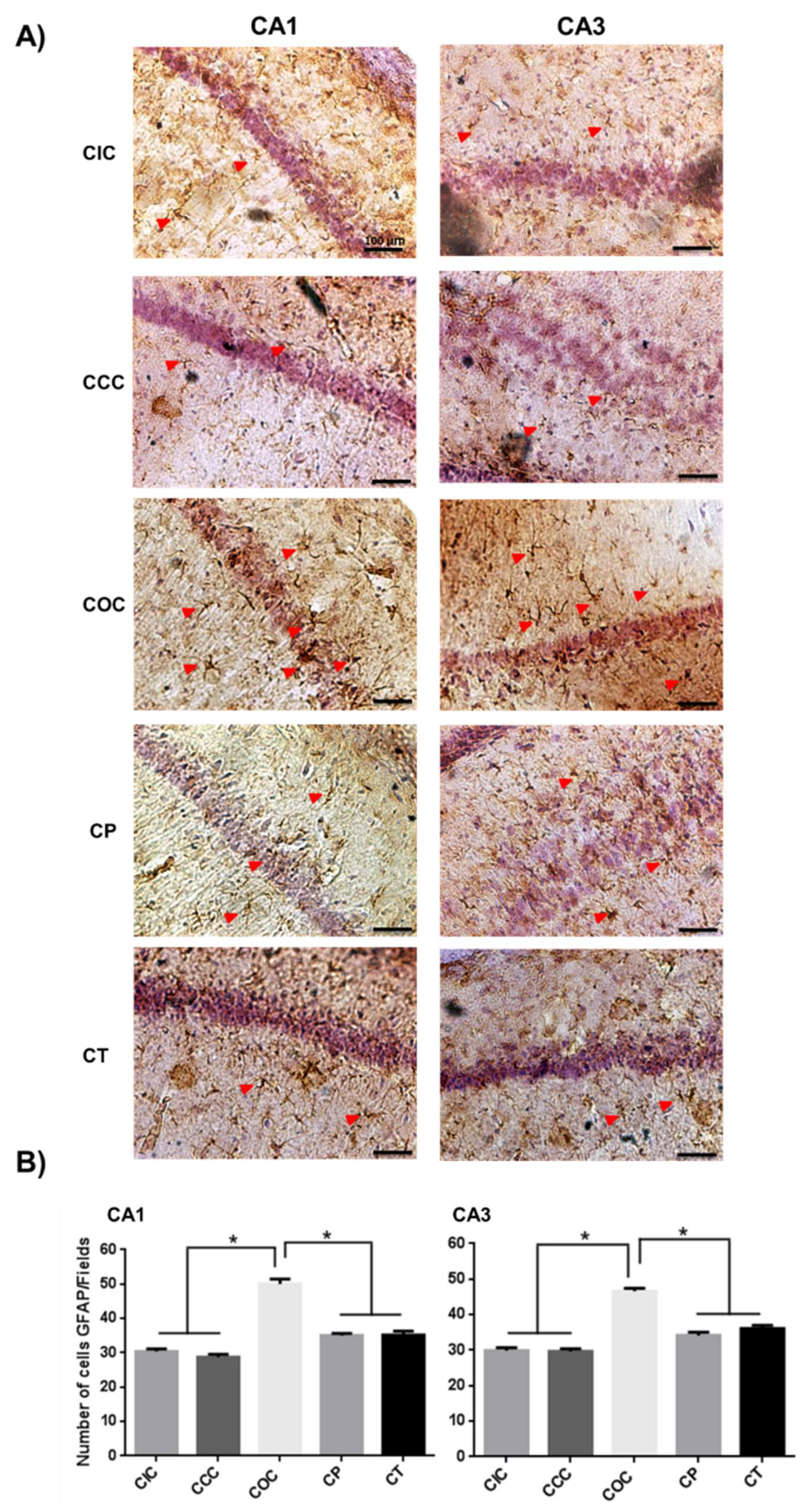
2.1. Curcumin Reduced Activation of Astrocytes in the Rat Hippocampus
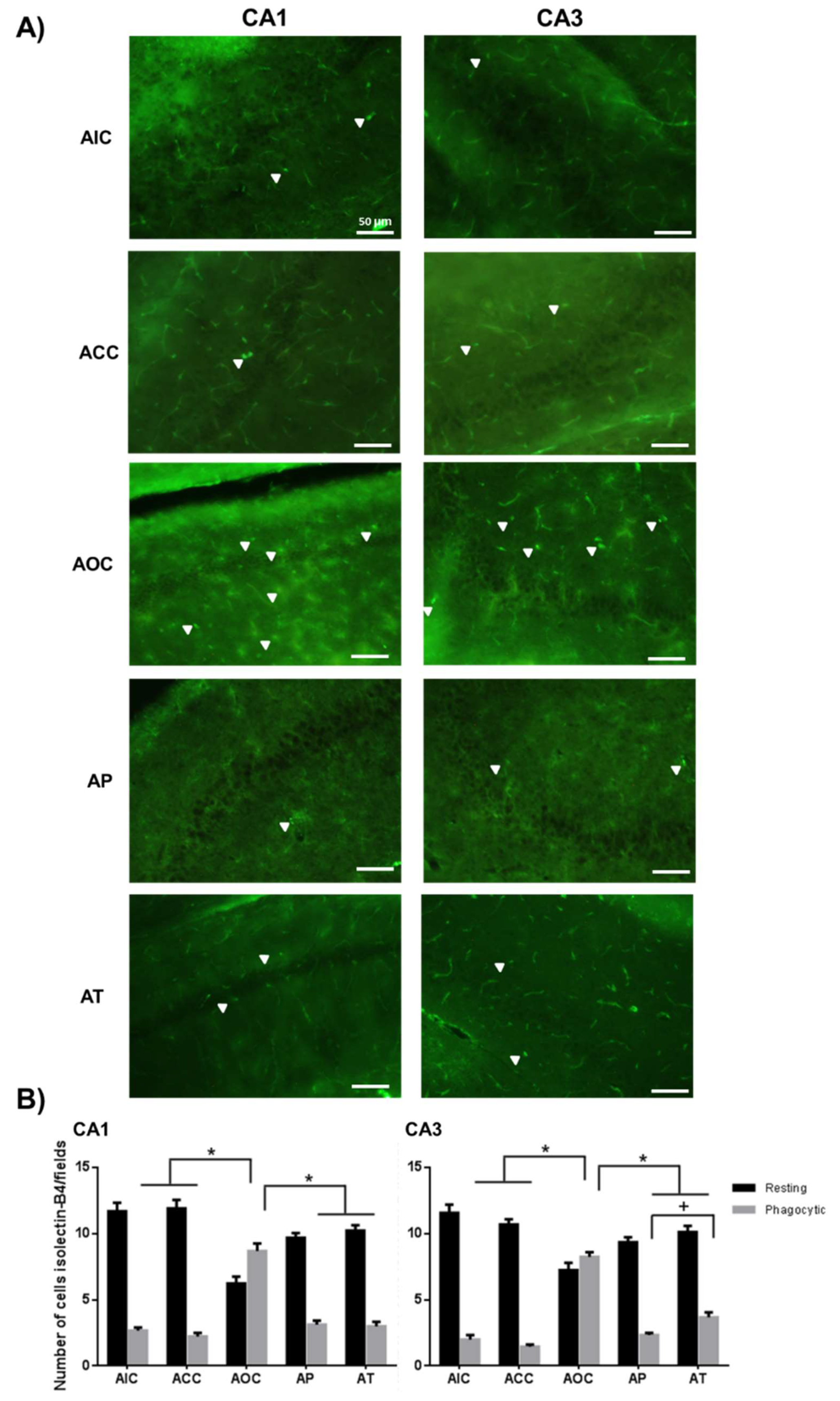
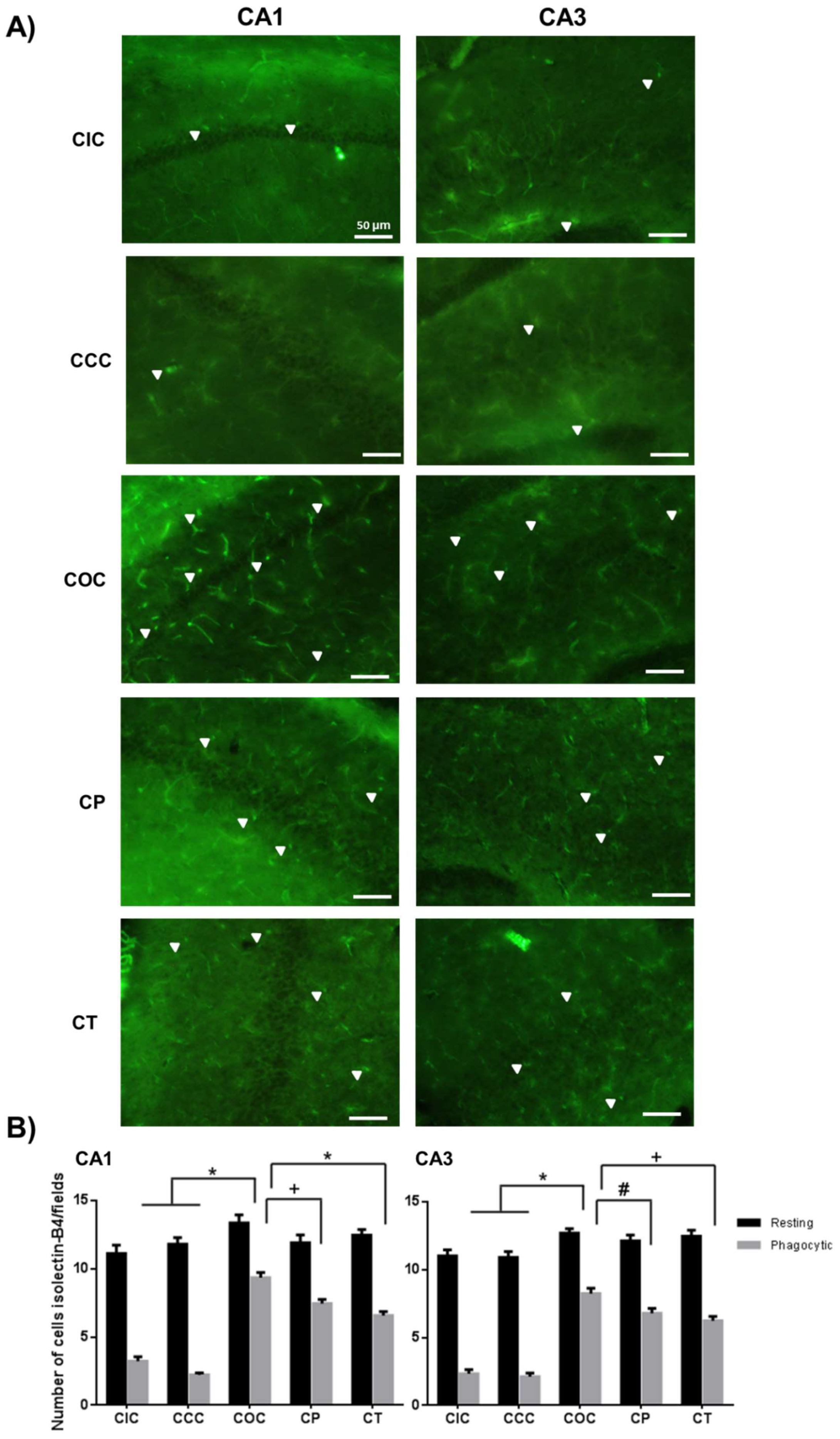
2.2. Curcumin Decreased the Microgliosis in the Rat Hippocampus
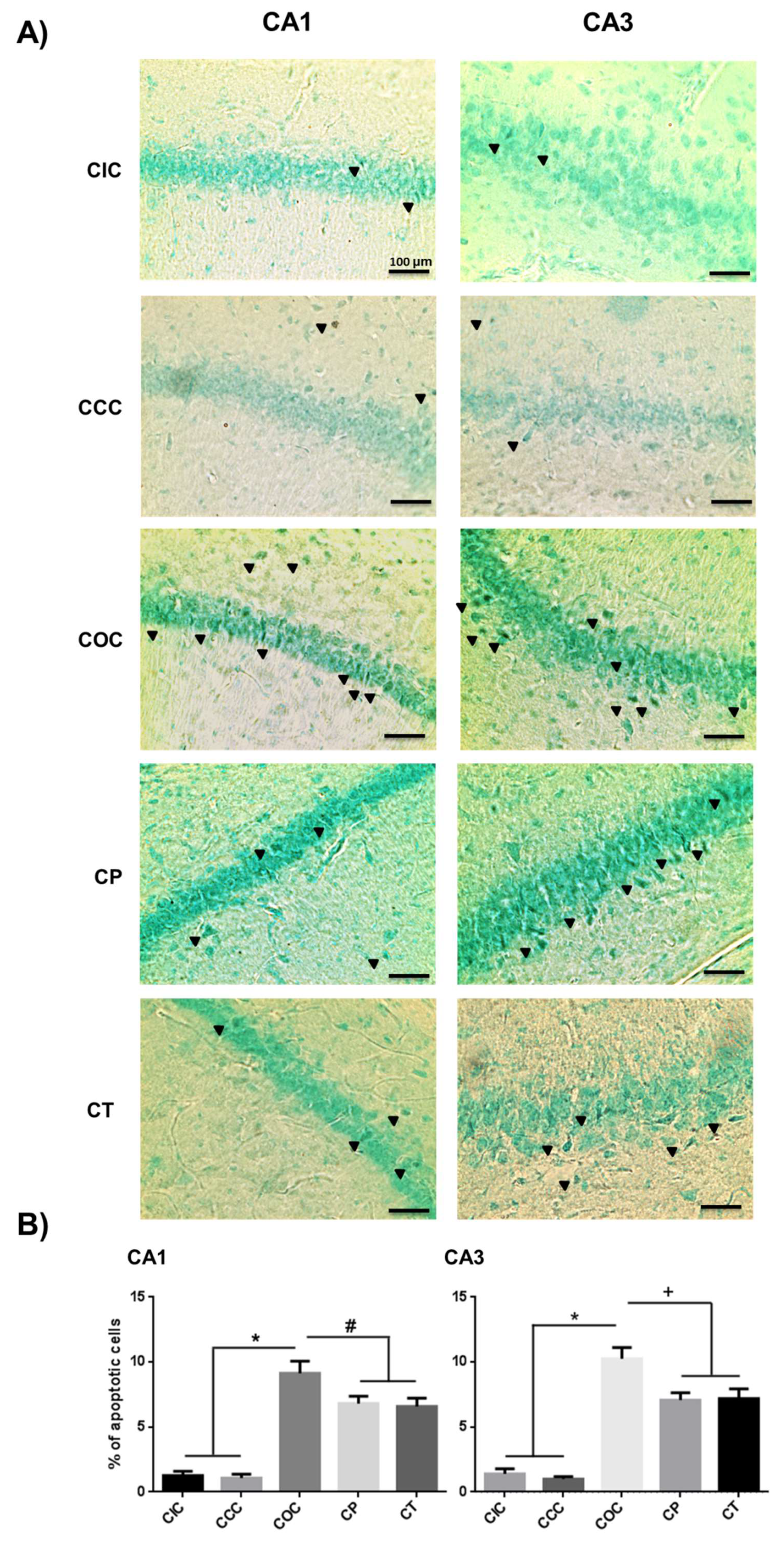
2.3. Curcumin Decreased Apoptosis Levels in Rat Hippocampus
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Diet
4.3. Experimental Design
4.4. Ozone Exposure
4.5. Tissue Samples
4.6. Immunohistochemistry for Astrocytes and Microglia
4.7. Fragment End Labeling of DNA (FragEL)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurt, O.K.; Zhang, J.; Pinkerton, K.E. Pulmonary health effects of air pollution. Curr. Opin. Pulm. Med. 2016, 22, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, P.A. Mechanisms of the acute effects of inhaled ozone in humans. Biochim. Biophys. Acta 2016, 1860, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lazcano, J.C.; Gonzalez-Guevara, E.; del Carmen Rubio, M.; Franco-Perez, J.; Custodio, V.; Hernandez-Ceron, M.; Livera, C.; Paz, C. The effects of ozone exposure and associated injury mechanisms on the central nervous system. Rev. Neurosci. 2013, 24, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Arancibia, S.; Guevara-Guzmán, R.; López-Vidal, Y.; Rodríguez-Martínez, E.; Zanardo-Gomes, M.; Angoa-Pérez, M.; Raisman-Vozari, R. Oxidative Stress Caused by Ozone Exposure Induces Loss of Brain Repair in the Hippocampus of Adult Rats. Toxicol. Sci. 2010, 113, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tighe, R.M.; Feng, F.; Ledford, J.G.; Hollingsworth, J.W. Genes of innate immunity and the biological response to inhaled ozone. J. Biochem. Mol. Toxicol. 2013, 27, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, E.; Nava-Ruiz, C.; Escamilla-Chimal, E.; Borgonio-Perez, G.; Rivas-Arancibia, S. The Effect of Chronic Ozone Exposure on the Activation of Endoplasmic Reticulum Stress and Apoptosis in Rat Hippocampus. Front. Aging Neurosci. 2016, 8, 245. [Google Scholar] [CrossRef]
- Gandhi, S.; Abramov, A.Y. Mechanism of Oxidative Stress in Neurodegeneration. Oxidative Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef]
- Niño-Cabrera, H.G.; Colin-Barenque, L.; Avila-Costa, M.R.; Espinosa-Villanueva, J.; Fortoul, T.I.; Rivas-Arancibia, S. Differences between Hippocampus and Cerebral Cortex in Aged Rats in an Oxidative Stress Model. Int. J. Neurosci. 2002, 112, 373–381. [Google Scholar] [CrossRef]
- Bartsch, T.; Wulff, P. The hippocampus in aging and disease: From plasticity to vulnerability. Neuroscience 2015, 309, 1–16. [Google Scholar] [CrossRef]
- Dorado-Martínez, C.; Paredes-Carbajal, C.; Mascher, D.; Borgonio-Pérez, G.; Rivas-Arancibia, S. Effects of different ozone doses on memory, motor activity and lipid peroxidation levels, in rats. Int. J. Neurosci. 2001, 108, 149–161. [Google Scholar] [CrossRef]
- Wang, X.; Pal, R.; Chen, X.W.; Limpeanchob, N.; Kumar, K.N.; Michaelis, E.K. High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Research. Mol. Brain Res. 2005, 140, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Leuner, B.; Gould, E. Structural plasticity and hippocampal function. Annu. Rev. Psychol. 2010, 61, 111–140. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Siddiqui, W.A.; Khandelwal, S. Differential susceptibility of brain regions to tributyltin chloride toxicity. Environ. Toxicol. 2015, 30, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.D.; Harry, G.J. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int. J. Environ. Res. Public Health 2011, 8, 2980–3018. [Google Scholar] [CrossRef] [PubMed]
- Lee, M. Neurotransmitters and microglial-mediated neuroinflammation. Curr. Protein Pept. Sci. 2013, 14, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Hol, E.M.; Pekny, M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef]
- Middeldorp, J.; Hol, E.M. GFAP in health and disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef]
- Sasaki, A. Microglia and brain macrophages: An update. Neuropathol. Off. J. Jpn. Soc. Neuropathol. 2016. [Google Scholar] [CrossRef]
- Kabba, J.A.; Xu, Y.; Christian, H.; Ruan, W.; Chenai, K.; Xiang, Y.; Zhang, L.; Saavedra, J.M.; Pang, T. Microglia: Housekeeper of the Central Nervous System. Cell. Mol. Neurobiol. 2017. [Google Scholar] [CrossRef]
- Fernandes, A.; Miller-Fleming, L.; Pais, T.F. Microglia and inflammation: Conspiracy, controversy or control? Cell. Mol. Life Sci. Cmls 2014, 71, 3969–3985. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; Lopez, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid. Redox. Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Honig, L.S.; Rosenberg, R.N. Apoptosis and neurologic disease. Am. J. Med. 2000, 108, 317–330. [Google Scholar] [CrossRef]
- Rivas-Arancibia, S.; Dorado-Martínez, C.; Borgonio-Pérez, G.; Hiriart-Urdanivia, M.; Verdugo-Díaz, L.; Durán-Vázquez, A.; Colin-Baranque, L.; Avila-Costa, M.R. Effects of Taurine on Ozone-Induced Memory Deficits and Lipid Peroxidation Levels in Brains of Young, Mature, and Old Rats. Environ. Res. 2000, 82, 7–17. [Google Scholar] [CrossRef]
- Farfán-García, E.D.; Castillo-Hernández, M.C.; Pinto-Almazán, R.; Rivas-Arancibia, S.; Gallardo, J.M.; Guerra-Araiza, C. Tibolone Prevents Oxidation and Ameliorates Cholinergic Deficit Induced by Ozone Exposure in the Male Rat Hippocampus. Neurochem. Res. 2014, 39, 1776–1786. [Google Scholar] [CrossRef]
- Mokoena, M.L.; Harvey, B.H.; Oliver, D.W.; Brink, C.B. Ozone modulates the effects of imipramine on immobility in the forced swim test, and nonspecific parameters of hippocampal oxidative stress in the rat. Metab. Brain Dis. 2010, 25, 125–133. [Google Scholar] [CrossRef]
- Nery-Flores, S.D.; Mendoza-Magaña, M.L.; Ramírez-Herrera, M.A.; Ramírez-Vázquez, J.d.J.; Romero-Prado, M.M.d.J.; Cortez-Álvarez, C.R.; Ramírez-Mendoza, A.A. Curcumin Exerted Neuroprotection against Ozone-Induced Oxidative Damage and Decreased NF-κB Activation in Rat Hippocampus and Serum Levels of Inflammatory Cytokines. Oxidative Med. Cell. Longev. 2018, 2018, 9620684. [Google Scholar] [CrossRef]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-inflammatory Agent, Against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Goel, A.; Jhurani, S.; Aggarwal, B.B. Multi-targeted therapy by curcumin: How spicy is it? Mol. Nutr. Food Res. 2008, 52, 1010–1030. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. Aaps J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.R.; Jardim, F.R.; Setzer, W.N.; Nabavi, S.M.; Nabavi, S.F. Curcumin, mitochondrial biogenesis, and mitophagy: Exploring recent data and indicating future needs. Biotechnol. Adv. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Yang, B.; Wang, L.; Li, B.; Guo, X.; Zhang, M.; Jiang, Z.; Fu, J.; Pi, J.; Guan, D.; et al. Curcumin plays neuroprotective roles against traumatic brain injury partly via Nrf2 signaling. Toxicol. Appl. Pharmacol. 2018, 346, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Rashid, K.; Sil, P.C. Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and endoplasmic reticulum-dependent apoptotic death. Biochim. Biophys. Acta 2015, 1852, 70–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyanka, A.; Anusree, S.S.; Nisha, V.M.; Raghu, K.G. Curcumin improves hypoxia induced dysfunctions in 3T3-L1 adipocytes by protecting mitochondria and down regulating inflammation. BioFactors 2014, 40, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Bigagli, E. Oxidative Stress and Air Pollution Exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Almazán, R.; Rivas-Arancibia, S.; Farfán-García, E.D.; Rodríguez-Martínez, E.; Guerra-Araiza, C. Neuroprotective effects of tibolone against oxidative stress induced by ozone exposure. Rev. Neurol. 2014, 58, 441–449. [Google Scholar] [PubMed]
- Bondan, E.; Cardoso, C.; Martins, M.F. Curcumin decreases astrocytic reaction after gliotoxic injury in the rat brainstem. Arq. De Neuro-Psiquiatr. 2017, 75, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Arancibia, S.; Zimbrón, L.F.H.; Rodríguez-Martínez, E.; Maldonado, P.D.; Borgonio Pérez, G.; Sepúlveda-Parada, M. Oxidative stress-dependent changes in immune responses and cell death in the substantia nigra after ozone exposure in rat. Front. Aging Neurosci. 2015, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.B.; Fu, Z.J.; Sun, T. Effects of different concentrations of oxygen-ozone on rats’ astrocytes in vitro. Neurosci. Lett. 2008, 441, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem. -Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Daverey, A.; Agrawal, S.K. Curcumin alleviates oxidative stress and mitochondrial dysfunction in astrocytes. Neuroscience 2016, 333, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Daverey, A.; Agrawal, S.K. Pre and Post treatment with curcumin and resveratrol protects astrocytes after oxidative stress. Brain Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nedzvetsky, V.S.; Agca, C.A.; Kyrychenko, S.V. Neuroprotective Effect of Curcumin on LPS-activated Astrocytes Is Related to the Prevention of GFAP and NF-κB Upregulation. Neurophysiology 2017, 49, 305–307. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, W.; Zhu, H.; Chen, Y.; Zhang, X.; Li, L.; Chu, W.; Wen, Z.; Feng, H.; Lin, J. Curcumin inhibits glial scar formation by suppressing astrocyte-induced inflammation and fibrosis in vitro and in vivo. Brain Res. 2017, 1655, 90–103. [Google Scholar] [CrossRef]
- Vilhardt, F.; Haslund-Vinding, J.; Jaquet, V.; McBean, G. Microglia antioxidant systems and redox signaling. Br. J. Pharmacol. 2016. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2015, 53. [Google Scholar] [CrossRef]
- Mumaw, C.L.; Levesque, S.; McGraw, C.; Robertson, S.; Lucas, S.; Stafflinger, J.E.; Campen, M.J.; Hall, P.; Norenberg, J.P.; Anderson, T.; et al. Microglial priming through the lung-brain axis: The role of air pollution-induced circulating factors. Faseb J. 2016, 30, 1880–1891. [Google Scholar] [CrossRef]
- Amici, S.A.; Dong, J.; Guerau-de-Arellano, M. Molecular Mechanisms Modulating the Phenotype of Macrophages and Microglia. Front. Immunol. 2017, 8, 1520. [Google Scholar] [CrossRef]
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory Effects of Curcumin in Microglial Cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Karlstetter, M.; Lippe, E.; Walczak, Y.; Moehle, C.; Aslanidis, A.; Mirza, M.; Langmann, T. Curcumin is a potent modulator of microglial gene expression and migration. J. Neuroinflammation 2011, 8, 125. [Google Scholar] [CrossRef]
- Jung, K.K.; Lee, H.S.; Cho, J.Y.; Shin, W.C.; Rhee, M.H.; Kim, T.G.; Kang, J.H.; Kim, S.H.; Hong, S.; Kang, S.Y. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006, 79, 2022–2031. [Google Scholar] [CrossRef]
- Liu, Z.; Ran, Y.; Huang, S.; Wen, S.; Zhang, W.; Liu, X.; Ji, Z.; Geng, X.; Ji, X.; Du, H.; et al. Curcumin Protects against Ischemic Stroke by Titrating Microglia/Macrophage Polarization. Front. Aging Neurosci. 2017, 9, 233. [Google Scholar] [CrossRef]
- Sorrenti, V.; Contarini, G.; Sut, S.; Dall’Acqua, S.; Confortin, F.; Pagetta, A.; Giusti, P.; Zusso, M. Curcumin Prevents Acute Neuroinflammation and Long-Term Memory Impairment Induced by Systemic Lipopolysaccharide in Mice. Front. Pharmacol. 2018, 9, 183. [Google Scholar] [CrossRef] [Green Version]
- Parada, E.; Buendia, I.; Navarro, E.; Avendano, C.; Egea, J.; Lopez, M.G. Microglial HO-1 induction by curcumin provides antioxidant, antineuroinflammatory, and glioprotective effects. Mol. Nutr. Food Res. 2015, 59, 1690–1700. [Google Scholar] [CrossRef]
- Akhter, H.; Ballinger, C.; Liu, N.; van Groen, T.; Postlethwait, E.M.; Liu, R.M. Cyclic Ozone Exposure Induces Gender-Dependent Neuropathology and Memory Decline in an Animal Model of Alzheimer’s Disease. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 147, 222–234. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Xing, S.S.; Zhang, W. Neuroprotective effect of curcumin on spinal cord in rabbit model with ischemia/reperfusion. J. Spinal Cord Med. 2013, 36, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, P.; Yadav, R.S.; Chandravanshi, L.P.; Shukla, R.K.; Dhuriya, Y.K.; Chauhan, L.K.S.; Dwivedi, H.N.; Pant, A.B.; Khanna, V.K. Unraveling the mechanism of neuroprotection of curcumin in arsenic induced cholinergic dysfunctions in rats. Toxicol. Appl. Pharmacol. 2014, 279, 428–440. [Google Scholar] [CrossRef]
- Uguz, A.C.; Oz, A.; Naziroglu, M. Curcumin inhibits apoptosis by regulating intracellular calcium release, reactive oxygen species and mitochondrial depolarization levels in SH-SY5Y neuronal cells. J. Recept. Signal Transduct. Res. 2016, 36, 395–401. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, A.Y.; Simonyi, A.; Jensen, M.D.; Shelat, P.B.; Rottinghaus, G.E.; MacDonald, R.S.; Miller, D.K.; Lubahn, D.E.; Weisman, G.A.; et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J. Neurosci. Res. 2005, 82, 138–148. [Google Scholar] [CrossRef]
- Motaghinejad, M.; Motevalian, M.; Fatima, S.; Faraji, F.; Mozaffari, S. The Neuroprotective Effect of Curcumin Against Nicotine-Induced Neurotoxicity is Mediated by CREB-BDNF Signaling Pathway. Neurochem. Res. 2017, 42, 2921–2932. [Google Scholar] [CrossRef]
- Motaghinejad, M.; Karimian, M.; Motaghinejad, O.; Shabab, B.; Yazdani, I.; Fatima, S. Protective effects of various dosage of Curcumin against morphine induced apoptosis and oxidative stress in rat isolated hippocampus. Pharmacol. Rep. 2015, 67, 230–235. [Google Scholar] [CrossRef]
- Feng, J.; Tao, T.; Yan, W.; Chen, C.S.; Qin, X. Curcumin inhibits mitochondrial injury and apoptosis from the early stage in EAE mice. Oxidative Med. Cell. Longev. 2014, 2014, 728751. [Google Scholar] [CrossRef]
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Yu, S.Y. Neuroprotective Effects of Curcumin on IL-1beta-Induced Neuronal Apoptosis and Depression-Like Behaviors Caused by Chronic Stress in Rats. Front. Cell. Neurosci. 2018, 12, 516. [Google Scholar] [CrossRef]
- Huang, T.; Zhao, J.; Guo, D.; Pang, H.; Zhao, Y.; Song, J. Curcumin mitigates axonal injury and neuronal cell apoptosis through the PERK/Nrf2 signaling pathway following diffuse axonal injury. Neuroreport 2018, 29, 661–677. [Google Scholar] [CrossRef]
- Canales-Aguirre, A.A.; Gomez-Pinedo, U.A.; Luquin, S.; Ramírez-Herrera, M.A.; Mendoza-Magaña, M.L.; Feria-Velasco, A. Curcumin protects against the oxidative damage induced by the pesticide parathion in the hippocampus of the rat brain. Nutr. Neurosci. 2012, 15, 62–69. [Google Scholar] [CrossRef]
- Pinto-Almazán, R.; Segura-Uribe, J.J.; Soriano-Ursúa, M.A.; Farfán-García, E.D.; Gallardo, J.M.; Guerra-Araiza, C. Effect of tibolone pretreatment on kinases and phosphatases that regulate the expression and phosphorylation of Tau in the hippocampus of rats exposed to ozone. Neural Regen. Res. 2018, 13, 440–448. [Google Scholar] [CrossRef]
- Kaneko, T.; Ikeda, H.; Fu, L.; Nishiyama, H.; Matsuoka, M.; Yamakawa, H.O.; Okubo, T. Capsaicin reduces ozone-induced airway inflammation in guinea pigs. Am. J. Respir. Crit. Care Med. 1994, 150, 724–728. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nery-Flores, S.D.; Ramírez-Herrera, M.A.; Mendoza-Magaña, M.L.; Romero-Prado, M.M.d.J.; Ramírez-Vázquez, J.d.J.; Bañuelos-Pineda, J.; Espinoza-Gutiérrez, H.A.; Ramírez-Mendoza, A.A.; Tostado, M.C. Dietary Curcumin Prevented Astrocytosis, Microgliosis, and Apoptosis Caused by Acute and Chronic Exposure to Ozone. Molecules 2019, 24, 2839. https://doi.org/10.3390/molecules24152839
Nery-Flores SD, Ramírez-Herrera MA, Mendoza-Magaña ML, Romero-Prado MMdJ, Ramírez-Vázquez JdJ, Bañuelos-Pineda J, Espinoza-Gutiérrez HA, Ramírez-Mendoza AA, Tostado MC. Dietary Curcumin Prevented Astrocytosis, Microgliosis, and Apoptosis Caused by Acute and Chronic Exposure to Ozone. Molecules. 2019; 24(15):2839. https://doi.org/10.3390/molecules24152839
Chicago/Turabian StyleNery-Flores, Sendar Daniel, Mario Alberto Ramírez-Herrera, María Luisa Mendoza-Magaña, Marina María de Jesús Romero-Prado, José de Jesús Ramírez-Vázquez, Jacinto Bañuelos-Pineda, Hugo Alejandro Espinoza-Gutiérrez, Abraham Alberto Ramírez-Mendoza, and Mariana Chávez Tostado. 2019. "Dietary Curcumin Prevented Astrocytosis, Microgliosis, and Apoptosis Caused by Acute and Chronic Exposure to Ozone" Molecules 24, no. 15: 2839. https://doi.org/10.3390/molecules24152839




