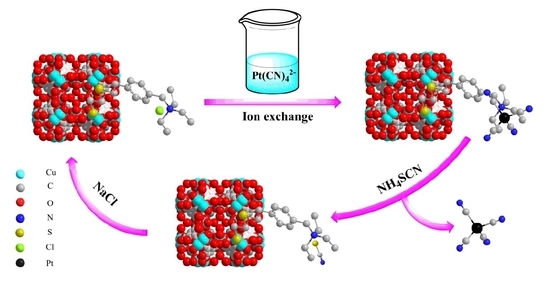
Two-Step Elution Recovery of Cyanide Platinum Using Functional Metal Organic Resin
Abstract
:1. Introduction
2. Results
2.1. Characterization
2.1.1. FTIR Spectra
2.1.2. XRD Spectra
2.1.3. SEM Analysis
2.1.4. TGA
2.1.5. N2 Adsorption–Desorption Isotherms
2.1.6. XPS
2.2. Effects of pH
2.3. Maximum Adsorption Capacities
2.4. Adsorption Kinetics
2.5. Sorption Isotherms
2.6. Thermodynamic Parameters
2.7. Removal of Metal Cyanide Complexes and Recovery of Pt(II)
2.8. Regeneration Experiment
3. Materials and Methods
3.1. Materials and Reagents
3.2. Apparatus
3.3. Preparation of TEBAC-HKUST-1 MOR
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jaszczak, E.; Polkowska, Z.; Narkowicz, S.; Namiesnik, J. Cyanides in the environment-analysis-problems and challenges. Environ. Sci. Pollut. Res. 2017, 24, 15929–15948. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Tae, J. Acridinium salt based fluorescent and colorimetric chemosensor for the detection of cyanide in water. Org. Lett. 2006, 8, 5721–5723. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Valinezhad, R.; Khani, H. A novel kinetic spectrophotometric method for the determination of ultra trace amount of cyanide. Spectrochim. Acta A 2010, 77, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, K. A new technique for extraction of platinum group metals by pressure cyanidation. Hydrometallurgy 2006, 82, 164–171. [Google Scholar] [CrossRef]
- Yu, B.; Li, C.Y.; Sun, Y.X.; Jia, H.R.; Guo, J.Q.; Li, J. A new azine derivative colorimetric and fluorescent dual-channel probe for cyanide detection. Spectrochim. Acta A 2017, 184, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.R.; Gaur, A.; Balomajumder, C. Cyanide in industrial wastewaters and its removal: A review on biotreatment. J. Hazard. Mater. 2009, 163, 1–11. [Google Scholar] [CrossRef]
- Dash, R.R.; Balomajumder, C.; Kumar, A. Treatment of metal cyanide bearing wastewater by simultaneous adsorption and biodegradation (SAB). J. Hazard. Mater. 2008, 152, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Yeddou, A.R.; Chergui, S.; Chergui, A.; Halet, F.; Hamza, A.; Nadjemi, B.; Ould-Dris, A.; Belkouch, J. Belkouch, Removal of cyanide in aqueous solution by oxidation with hydrogen peroxide in presence of copper-impregnated activated carbon. Miner. Eng. 2011, 24, 788–793. [Google Scholar] [CrossRef]
- Kim, S.J.; Lim, K.H.; Joo, K.H.; Lee, M.J.; Kil, S.G.; Cho, S.Y. Removal of heavy metal-cyanide complexes by ion exchange. Korean J. Chem. Eng. 2002, 19, 1078–1084. [Google Scholar] [CrossRef]
- Vidal, L.; Riekkola, M.L.; Canals, A. Ionic liquid-modified materials for solid-phase extraction and separation: A review. Anal. Chim. Acta 2012, 715, 19–41. [Google Scholar] [CrossRef]
- Schoeman, E.; Bradshaw, S.M.; Akdogan, G.; Snyders, C.A.; Eksteen, J.J. The extraction of platinum and palladium from a synthetic cyanide heap leach solution with strong base anion exchange resins. Int. J. Miner. Process. 2017, 162, 27–35. [Google Scholar] [CrossRef]
- Desai, A.V.; Manna, B.; Karmakar, A.; Sahu, A.; Ghosh, S.K. A water-stable cationic metal-organic framework as a dual adsorbent of oxoanion pollutants. Angew. Chem. Int. Ed. 2016, 55, 7811–7815. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Pournara, A.; Kim, K.H.; Bansal, V.; Rapti, S.; Manos, M.J. Metal-organic frameworks: Challenges and opportunities for ion-exchange/sorption applications. Prog. Mater. Sci. 2017, 86, 25–74. [Google Scholar] [CrossRef]
- Shi, P.F.; Zhao, B.; Xiong, G.; Hou, Y.L.; Cheng, P. Fast capture and separation of, and luminescent probe for, pollutant chromate using a multi-functional cationic heterometal-organic framework. Chem. Commun. 2012, 48, 8231–8233. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Katz, M.J.; Wang, T.C.; Platero-Prats, A.E.; Chapman, K.W.; Hupp, J.T.; Farha, O.K. High efficiency adsorption and removal of selenate and selenite from water using metal-organic frameworks. J. Am. Chem. Soc. 2015, 137, 7488–7494. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.A.; Chen, S.Y.; Jochems, A.P. Highly selective adsorbents for removal of phosphate from water and urine. Mater. Chem. Phys. 2015, 160, 168–176. [Google Scholar] [CrossRef]
- Karmakar, S.; Dechnik, J.; Janiak, C.; De, S. Aluminium fumarate metal-organic framework: A super adsorbent for fluoride from water. J. Hazard. Mater. 2016, 303, 10–20. [Google Scholar] [CrossRef]
- Li, T.; Yang, Z.Q.; Zhang, X.P.; Zhu, N.W.; Niu, X.J. Perchlorate removal from aqueous solution with a novel cationic metal-organic frameworks based on amino sulfonic acid ligand linking with Cu-4,4′-bipyridyl chains. Chem. Eng. J. 2015, 281, 1008–1016. [Google Scholar] [CrossRef]
- Chen, S.S.; Wang, P.; Takamizawa, S.; Okamura, T.; Chen, M.; Sun, W.Y. Zinc(II) and cadmium(II) metal-organic frameworks with 4-imidazole containing tripodal ligand: Sorption and anion exchange properties. Dalton Trans. 2014, 43, 6012–6020. [Google Scholar] [CrossRef]
- Li, Z.Q.; Yang, J.C.; Sui, K.W.; Yin, N. Facile synthesis of metal-organic framework MOF-808 for arsenic removal. Mater. Lett. 2015, 160, 412–414. [Google Scholar] [CrossRef]
- Liu, T.; Che, J.X.; Hu, Y.Z.; Dong, X.W.; Liu, X.Y.; Che, C.M. Alkenyl/thiol-derived metal-organic frameworks (MOFs) by means of postsynthetic modification for effective mercury adsorption. Chem. Eur. J. 2014, 20, 14090–14095. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.B.; Ding, L.; Luo, J.M. Adsorptive removal of Pb(II) ions from aqueous samples with amino-functionalization of metal-organic frameworks MIL-101(Cr). J. Chem. Eng. Data 2015, 60, 1732–1743. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, G.Q.; Chen, H.H.; Hu, X.Y.; Niu, Z.; Ma, S.Q. Functionalized metal-organic framework as a new platform for efficient and selective removal of cadmium(II) from aqueous solution. J. Mater. Chem. A 2015, 3, 15292–15298. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.J.; Li, J.F.; Chen, J. Solvent extraction of palladium(II) from alkaline cyanide solution by the dodecyl dimethhy-2-phenoxyethyl ammonium bromide. J. Chil. Chem. Soc. 2016, 61, 2864–2869. [Google Scholar] [CrossRef]
- Chen, M.H.; Wu, S.J.; Huang, Z.J.; Chen, J.; Chen, M.J. Separation and recovery of Pd(II) and Pt(II) from cyanide liquors of Pd-Pt flotation concentrate via solvent extraction. J. Chem. Technol. Biotechnol. 2017, 92, 1699–1709. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Zhou, W.J.; Gao, H.C.; Wu, J.G.; Xu, G.X. Solvent extraction and stripping of gold(I) cyanide in the tetradecyldimethylbenzylammonium chloride system. Hydrometallurgy 2003, 70, 73–81. [Google Scholar] [CrossRef]
- Zhang, T.X.; Huang, B.G.; Zhou, W.J.; Gao, H.C.; Chen, J.; Wu, H.S.; Wu, J.G. Extraction and recovery of gold from KAu(CN)2 using cetyltrimethylammonium bromide microemulsions. J. Chem. Technol. Biotechnol. 2001, 76, 1107–1111. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, M.; Zhong, L.; Ye, Q.; Jiang, S.; Huang, Z. Highly effective removal of metal cyanide complexes and recovery of palladium using quaternary-ammonium-functionalized MOFs. Molecules 2018, 23, 2086. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Yang, H.T.; Petit, C.; Hsu, F.K. Removing oil droplets from water using a copper-based metal organic frameworks. Chem. Eng. J. 2014, 249, 293–301. [Google Scholar] [CrossRef]
- Azhar, M.R.; Abid, H.R.; Sun, H.Q.; Periasamy, V.; Tade, M.O.; Wang, S. Excellent performance of copper based metal organic framework in adsorptive removal of toxic sulfonamide antibiotics from wastewater. J. Colloid Interface Sci. 2016, 478, 344–352. [Google Scholar] [CrossRef]
- Maleki, A.; Hayati, B.; Naghizadeh, M.; Joo, S.W. Adsorption of hexavalent chromium by metal organic frameworks from aqueous solution. J. Ind. Eng. Chem. 2015, 28, 211–216. [Google Scholar] [CrossRef]
- Ke, F.; Qiu, L.G.; Yuan, Y.P.; Peng, F.M.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J. Hazard. Mater. 2011, 196, 36–43. [Google Scholar] [CrossRef]
- Alemdar, A.; Atici, O.; Güngör, N. The influence of cationic surfactants on rheological properties of bentonite-water systems. Mater. Lett. 2000, 43, 57–61. [Google Scholar] [CrossRef]
- Hibble, S.J.; Chippindale, A.M.; Bilbe, E.J.; Marelli, E.; Harris, P.J.F.; Hannon, A.C. Structures of Pd(CN)2 and Pt(CN)2: Intrinsically Nanocrystalline Materials? Inorg. Chem. 2011, 50, 104–113. [Google Scholar] [CrossRef]
- Wee, L.H.; Lohe, M.R.; Janssens, N.; Kaskel, S.; Martens, J.A. Fine tuning of the metal-organic framework Cu3(BTC)2 HKUST-1 crystal size in the 100 nm to 5 micron range. J. Mater. Chem. 2012, 22, 13742–13746. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Z.W.; Que, Y.G.; Li, B.X.; Guo, Q.R.; Li, Z.; Wang, L.; Wan, H.; Guan, G.F. Immobilization of a thiol-functionalized ionic liquid onto HKUST-1 through thiol compounds as the chemical bridge. RSC Adv. 2016, 6, 54119–54128. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J. Nanoporous metal-organic framework (MOF-199): Synthesis, characterization and photocatalytic degradation of Basic Blue 41. Microchem. J. 2019, 144, 436–442. [Google Scholar] [CrossRef]
- Abid, H.R.; Pham, G.H.; Ang, H.M.; Tade, M.O.; Wang, S. Adsorption of CH4 and CO2 on Zr-metal organic frameworks. J. Colloid Interface Sci. 2012, 366, 120–124. [Google Scholar] [CrossRef]
- Senkovska, I.; Kaskel, S. High pressure methane adsorption in the metal-organic frameworks Cu3(btc)2, Zn2(bdc)2dabco, and Cr3F(H2O)2O(bdc)3. Microporous Mesoporous Mater. 2008, 112, 108–115. [Google Scholar] [CrossRef]
- Alavi, M.A.; Morsali, A. Synthesis and characterization of different nanostructured copper(II) metal-organic frameworks by a ligand functionalization and modulation method. Crystengcomm 2014, 16, 2246–2250. [Google Scholar] [CrossRef]
- Laiho, T.; Leiro, J.A.; Heinonen, M.H.; Mattila, S.S.; Lukkari, J. Photoelectron spectroscopy study of irradiation damage and metal-sulfur bonds of thiol on silver and copper surfaces. J. Electron Spectrosc. 2005, 142, 105–112. [Google Scholar] [CrossRef]
- Muench, F.; Fuchs, A.; Mankel, E.; Rauber, M.; Lauterbach, S.; Kleebe, H.J.; Ensinger, W. Synthesis of nanoparticle/ligand composite thin films by sequential ligand self assembly and surface complex reduction. J. Colloid Interface Sci. 2013, 389, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wang, Z.Q.; Zeng, Q.L.; Shen, C.H. 13C NMR and XPS characterization of anion adsorbent with quaternary ammonium groups prepared from rice straw, corn stalk and sugarcane bagasse. Appl. Surf. Sci. 2016, 389, 404–410. [Google Scholar] [CrossRef]
- Beck, M.T.; Bertóti, I.; Mohai, M.; Németh, P.; Jakab, E.; Szabó, L.; Szépvölgyi, J. Gold nano-particle formation from crystalline AuCN: Comparison of thermal, plasma- and ion-beam activated decomposition. J. Solid State Chem. 2017, 246, 65–74. [Google Scholar] [CrossRef]
- Azhar, M.R.; Abid, H.R.; Sun, H.; Periasamy, V.; Tade, M.O.; Wang, S. One-pot synthesis of binary metal organic frameworks (HKUST-1 and UiO-66) for enhanced adsorptive removal of water contaminants. J. Colloid Interface Sci. 2017, 490, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Lukey, G.C.; van Deventer, J.S.J.; Shallcross, D.C. The effect of functional group structure on the elution of metal cyanide complexes from ion exchange resins. Sep. Sci. Technol. 2000, 35, 2393–2413. [Google Scholar] [CrossRef]
- Mpinga, C.N.; Bradshaw, S.M.; Akdogan, G.; Snyders, C.A.; Eksteen, J.J. The extraction of Pt, Pd and Au from an alkaline cyanide simulated heap leachate by granular activated carbon. Miner. Eng. 2014, 55, 11–17. [Google Scholar] [CrossRef]
- Snyders, C.A.; Bradshaw, S.M.; Akdogan, G.; Eksteen, J.J. Factors affecting the elution of Pt, Pd and Au cyanide from activated carbon. Miner. Eng. 2015, 80, 14–24. [Google Scholar] [CrossRef]
- Taguchi, S.; Takayoshi, K.; Yotsu, Y.; Kasahara, I. Ion-pair solid-phase extraction with membranes: Group contributions and steric effects of ions on the extraction behaviour. Analyst 1996, 121, 1621–1625. [Google Scholar] [CrossRef]
- Lin, L.C.; Juang, R.S. Ion-exchange equilibria of Cu(II) and Zn(II) from aqueous solutions with Chelex 100 and Amberlite IRC 748 resins. Chem. Eng. J. 2005, 112, 211–218. [Google Scholar] [CrossRef]
- Lukey, G.C.; van Deventer, J.S.J.; Shallcross, D.C. Selective elution of copper and iron cyanide complexes from ion exchange resins using saline solutions. Hydrometallurgy 2000, 56, 217–236. [Google Scholar] [CrossRef]
- Kumar, P.; Pandey, R.K.; Hegde, V.R. Anti-markovnikov addition of thiols across double bonds catalyzed by H-Rho-zeolite. Synlett 1999, 12, 1921–1922. [Google Scholar] [CrossRef]
- Silveira, C.C.; Mendes, S.R.; Líbero, F.M. Solvent-free anti-markovnikov addition of thiols to alkenes using anhydrous cerium(III) chloride as catalyst. Synlett 2010, 2010, 790–792. [Google Scholar] [CrossRef]
- Marino, M.G.; Kreuer, K.D. Alkaline stability of quaternary ammonium cations for alkaline fuel cell Membranes and Ionic Liquids. Chemsuschem 2015, 8, 513–523. [Google Scholar] [CrossRef]
- Wang, M.L.; Hsieh, Y.M. Kinetic study of dichlorocyclopropanation of 4-vinyl-1-cyclohexene by a novel multisite phase transfer catalyst. J. Mol. Catal. A Chem. 2004, 210, 59–68. [Google Scholar] [CrossRef]
- Chen, J.Z.; Li, K.G.; Chen, L.M.; Liu, R.L.; Huang, X.; Ye, D. Conversion of fructose into 5-hydroxymethylfurfural catalyzed by recyclable sulfonic acid-functionalized metal–Organic frameworks. Green Chem. 2014, 16, 2490–2499. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |












| Sample | BET Specific Surface Area m2 g−1 | Pore Volume cm3 g−1 |
|---|---|---|
| as-prepared HKUST-1 | 1651.896 | 0.738 |
| TEBAC-HKUST-1 | 523.703 | 0.260 |
| Pseudo-First-Order Model | Pseudo-Second-Order Model | Intra-Particle Diffusion Model | |||
|---|---|---|---|---|---|
| qe,exp. (mg g−1) | 235.5 | qe,exp. (mg g−1) | 235.5 | Kp (mg g−1 min−0.5) | 5.533 |
| qe,cal. (mg g−1) | 61.19 | qe,cal. (mg g−1) | 238.1 | C | 208.2 |
| k1 (min−1) | 0.1820 | k2 (g mg−1 min−1) | 0.0123 | R2 | 0.8530 |
| R2 | 0.8470 | R2 | 0.9994 | ||
| Metal | qma (mg g-1) | Langmuir isotherm | Freundlich isotherm | ||||
|---|---|---|---|---|---|---|---|
| qmb (mg g−1) | b (L mg−1) | R2 | Kf (L g−1) | n | R2 | ||
| Pt | 290.2 | 289.9 | 10.17 | 0.9998 | 206.25 | 10.64 | 0.9535 |
| Adsorbent | Capacity (mg g−1) | Adsorption Time (h) | Optimum pH | Ref. |
|---|---|---|---|---|
| Polymer resins | 10–80 | 6–48 h | 10.0 | [11,46] |
| Activated carbon | 4–25 mg g−1 | 3–12 h | 10.5 | [47,48] |
| TEBAC-HKUST-1 | 290.2 mg g−1 | 0.5 | 8 | Present work |
| Temperature (K) | ∆G (kJ mol−1) | ∆S (J mol−1 K−1) | ∆H (kJ mol−1) |
|---|---|---|---|
| 293.15 | −23.13 | −61526.9 | −41.17 |
| 303.15 | −22.51 | ||
| 313.15 | −21.90 | ||
| 323.15 | −21.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Ye, Q.; Jiang, S.; Shao, M.; Jin, C.; Huang, Z. Two-Step Elution Recovery of Cyanide Platinum Using Functional Metal Organic Resin. Molecules 2019, 24, 2779. https://doi.org/10.3390/molecules24152779
Chen M, Ye Q, Jiang S, Shao M, Jin C, Huang Z. Two-Step Elution Recovery of Cyanide Platinum Using Functional Metal Organic Resin. Molecules. 2019; 24(15):2779. https://doi.org/10.3390/molecules24152779
Chicago/Turabian StyleChen, Muhan, Qun Ye, Shaosong Jiang, Min Shao, Ci Jin, and Zhangjie Huang. 2019. "Two-Step Elution Recovery of Cyanide Platinum Using Functional Metal Organic Resin" Molecules 24, no. 15: 2779. https://doi.org/10.3390/molecules24152779
APA StyleChen, M., Ye, Q., Jiang, S., Shao, M., Jin, C., & Huang, Z. (2019). Two-Step Elution Recovery of Cyanide Platinum Using Functional Metal Organic Resin. Molecules, 24(15), 2779. https://doi.org/10.3390/molecules24152779