Aquaphotomics—From Innovative Knowledge to Integrative Platform in Science and Technology
Abstract
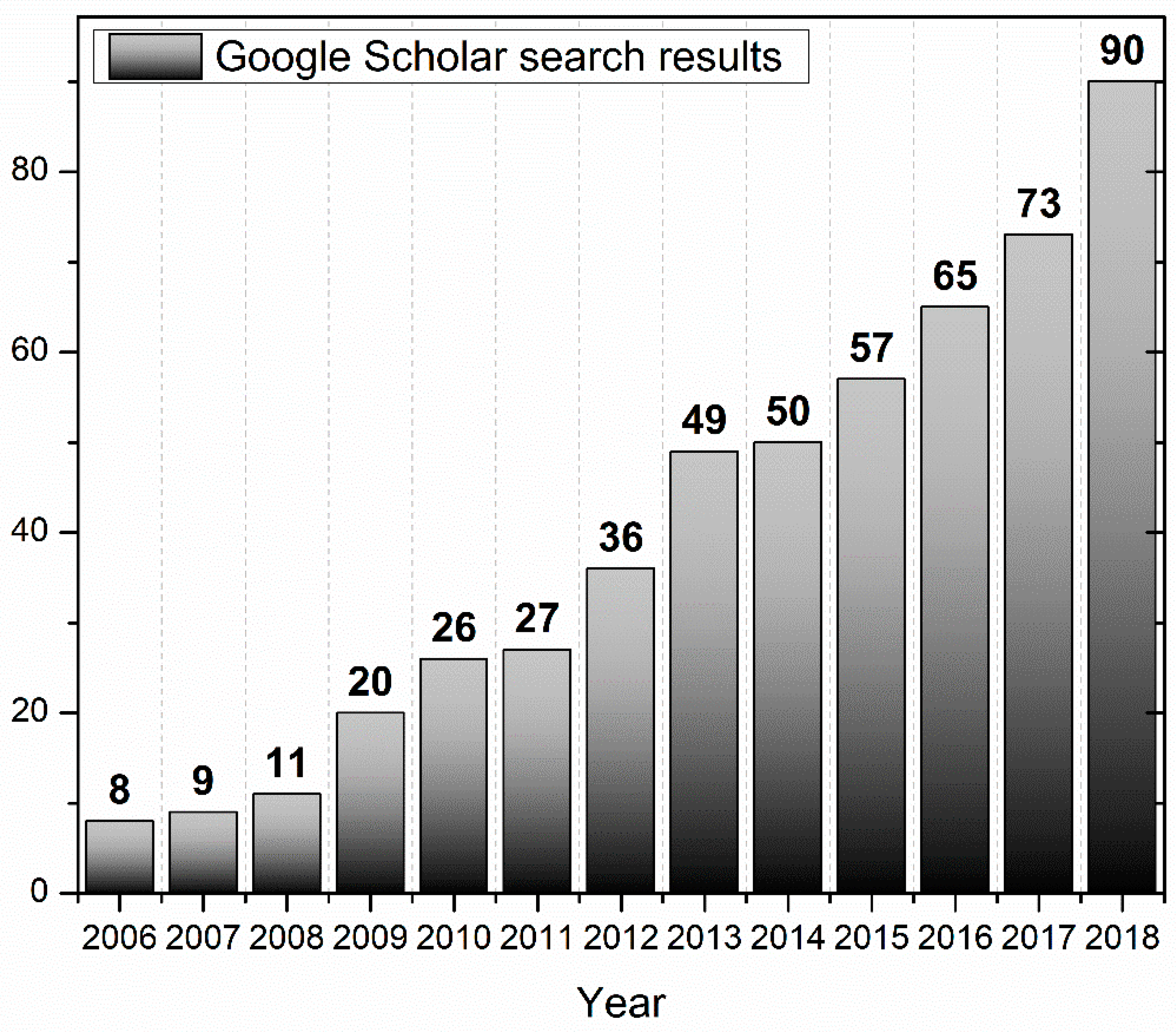
:1. Introduction to Aquaphotomics
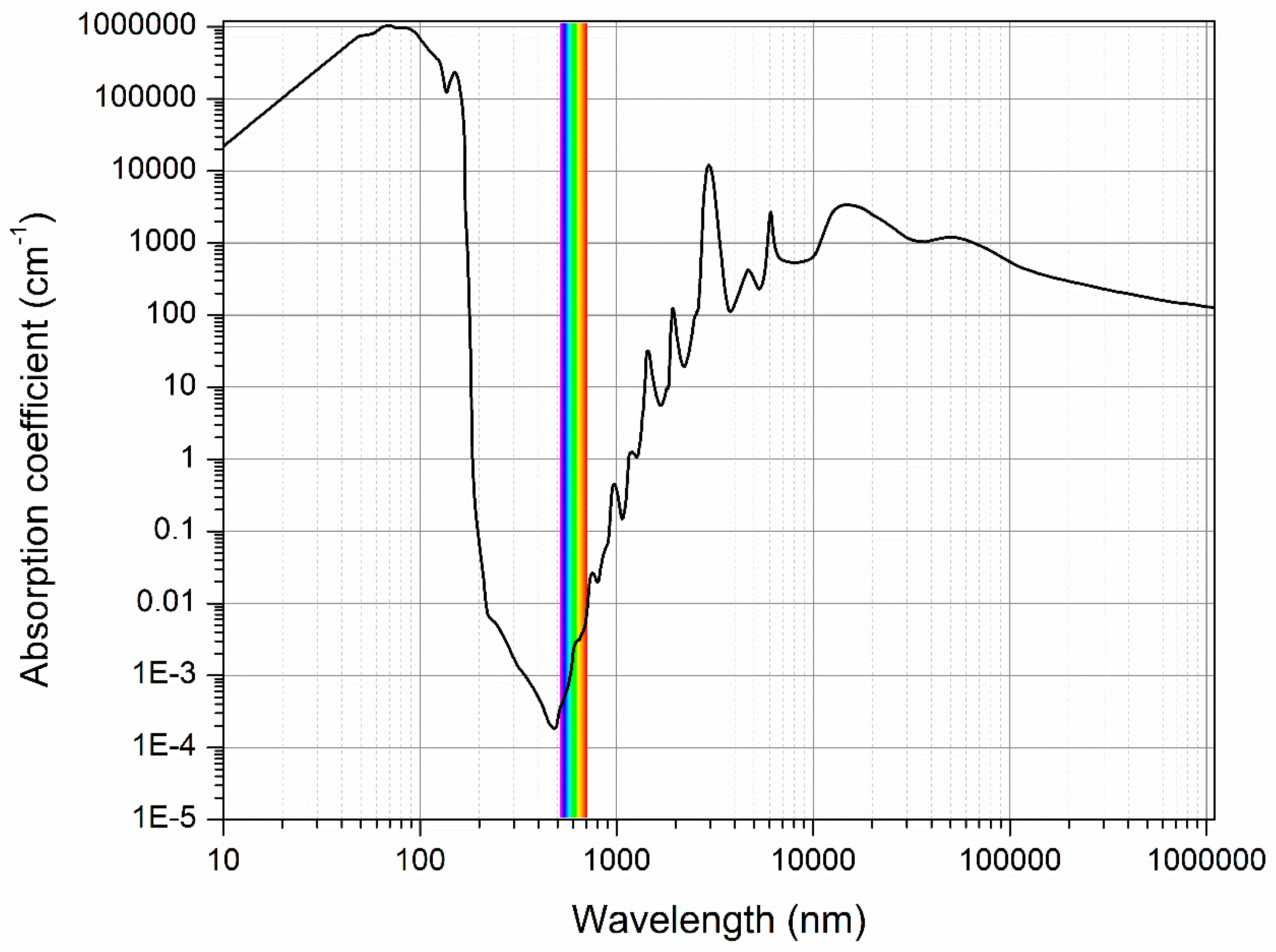
2. Water Spectrum as a Source of Information
2.1. Water as a Sensor and an Amplifier: The Water-mirror Approach
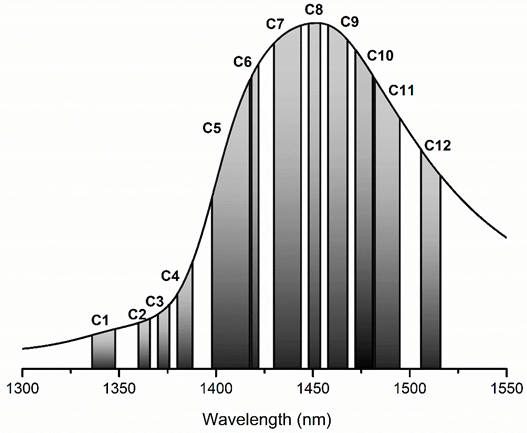
2.2. Water Matrix Coordinates (WAMACS) and Water Spectral Pattern (WASP)
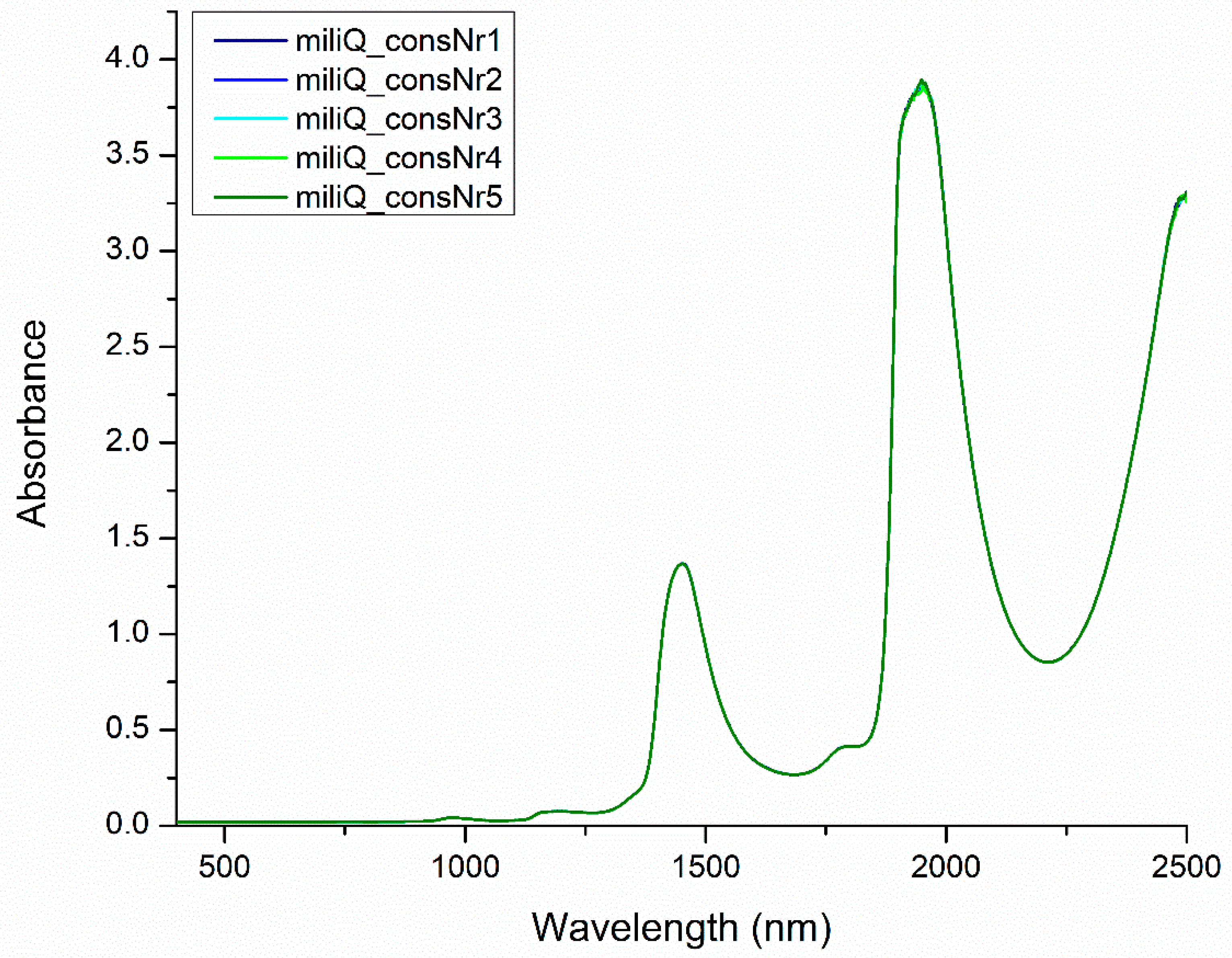
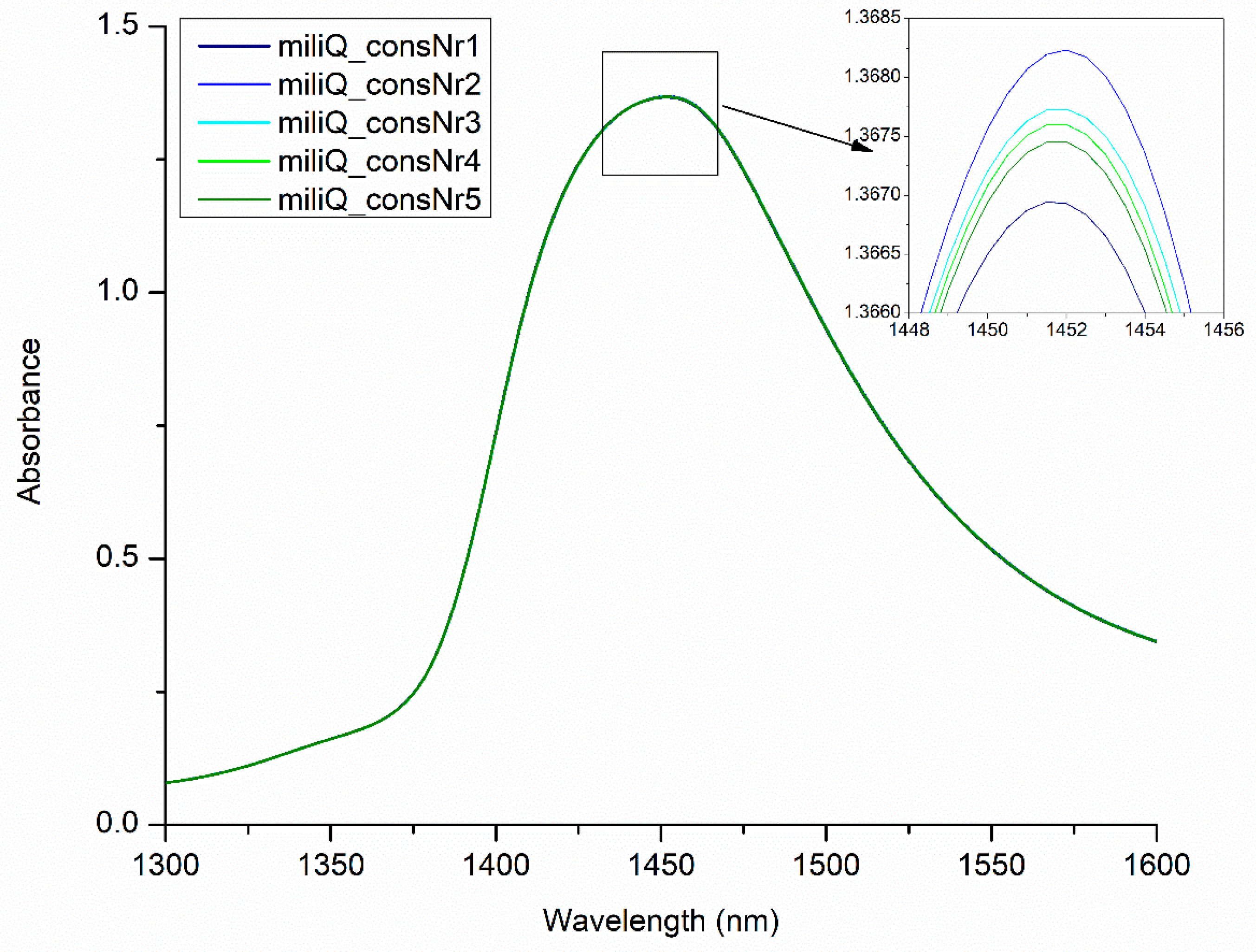
2.3. Using Perturbation to Elicit Information
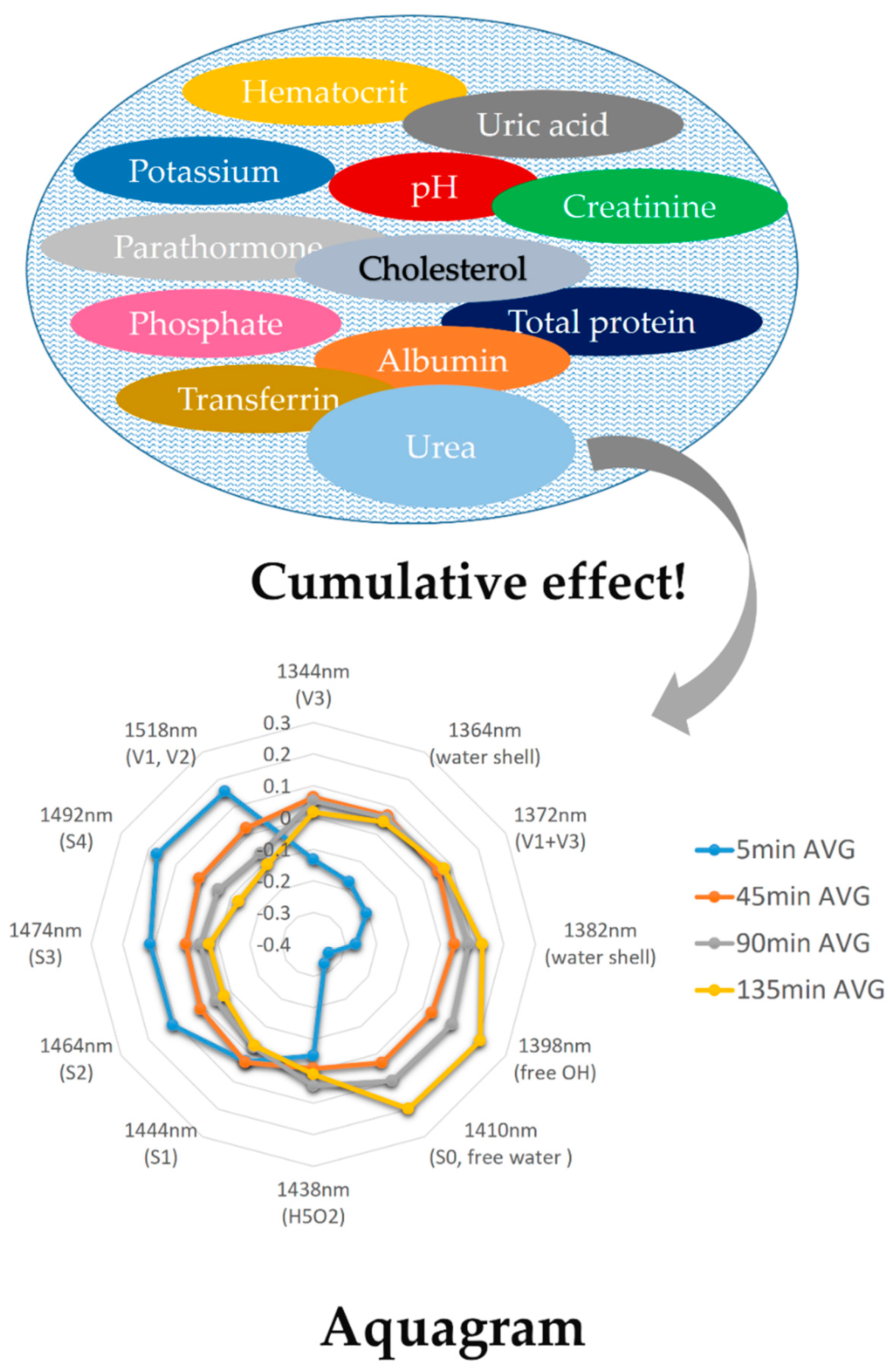
2.4. Water as a Biomolecule and Water Spectral Pattern as a Collective Biomarker
3. Aquaphotomics—Innovative Knowledge Leads to Innovative Applications
4. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsenkova, R. Visible-Near Infrared perturbation spectroscopy: Water in action seen as a source of information. In Proceedings of the 12th International Conference on Near-infrared Spectroscopy, Auckland, New Zealand, 9–15 April 2005; pp. 514–519. [Google Scholar]
- Tsenkova, R. Aquaphotomics: Aquaphotomics and Chambersburg. NIR News 2006, 17, 12–14. [Google Scholar] [CrossRef]
- Tsenkova, R. Aquaphotomics: Exploring water-light interactions for a better understanding of the biological world. Part1: Good yogurt comes from good milk and healthy cows. NIR News 2006, 17, 10–11. [Google Scholar] [CrossRef]
- Tsenkova, R. AquaPhotomics: Water absorbance pattern as a biological marker. NIR News 2006, 17, 13–20. [Google Scholar] [CrossRef]
- Tsenkova, R. Aquaphotomics: Dynamic spectroscopy of aqueous and biological systems describes peculiarities of water. J. Near Infrared Spectrosc. 2009, 17, 303–313. [Google Scholar] [CrossRef]
- Masato, T.; Gianmario, M.; Salvatore, C.; Masakazu, A. Investigations of the structure of H2O clusters adsorbed on TiO2 surfaces by near-infrared absorption spectroscopy. J. Phys. Chem. B 2005, 109, 7387–7391. [Google Scholar]
- Tanaka, M.; Hayashi, T.; Morita, S. The roles of water molecules at the biointerface of medical polymers. Polym. J. 2013, 45, 701–710. [Google Scholar] [CrossRef]
- Morita, S.; Tanaka, M.; Kitagawa, K.; Ozaki, Y. Hydration structure of poly(2-methoxyethyl acrylate): Comparison with a 2-methoxyethyl acetate model monomer. J. Biomater. Sci. Polym. Ed. 2010, 21, 1925–1935. [Google Scholar] [CrossRef]
- Morita, S.; Tanaka, M.; Ozaki, Y. Time-resolved in situ ATR-IR observations of the process of sorption of water into a poly(2-methoxyethyl acrylate) film. Langmuir 2007, 23, 3750–3761. [Google Scholar] [CrossRef]
- Chaplin, M. Do we underestimate the importance of water in cell biology? Nat. Rev. Mol. Cell Biol. 2006, 7, 861–866. [Google Scholar] [CrossRef]
- Khesbak, H.; Savchuk, O.; Tsushima, S.; Fahmy, K. The role of water H-bond imbalances in B-DNA substate transitions and peptide recognition revealed by time-resolved FTIR spectroscopy. J. Am. Chem. Soc. 2011, 133, 5834–5842. [Google Scholar] [CrossRef]
- Espinosa-Marzal, R.M.; Fontani, G.; Reusch, F.B.; Roba, M.; Spencer, N.D.; Crockett, R. Sugars communicate through water: Oriented glycans induce water structuring. Biophys. J. 2013, 104, 2686–2694. [Google Scholar] [CrossRef]
- De Simone, A.; Dodson, G.G.; Verma, C.S.; Zagari, A.; Fraternali, F. Prion and water: Tight and dynamical hydration sites have a key role in structural stability. Proc. Natl. Acad. Sci. USA 2005, 102, 7535–7540. [Google Scholar] [CrossRef] [Green Version]
- Erkut, C.; Penkov, S.; Fahmy, K.; Kurzchalia, T.V. How worms survive desiccation. Worm 2012, 1, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Hong, B.H.; Rubenthaler, G.L.; Allan, R.E. Wheat pentosans. II. Estimating kernel hardness and pentosans in water extracts by near-infrared reflectance. Cereal Chem. 1989, 66, 374–377. [Google Scholar]
- Rafsanjani, A.; Brulé, V.; Western, T.L.; Pasini, D. Hydro-responsive curling of the resurrection plant Selaginella lepidophylla. Sci. Rep. 2015, 5, 8064. [Google Scholar] [CrossRef]
- Segelstein, D.J. The Complex Refractive Index of Water. Master’s Thesis, University of Missouri, Kansas, MO, USA, 1981. [Google Scholar]
- Tsenkova, R.; Atanassova, S.; Toyoda, K. Near infrared spectroscopy for diagnosis: Influence of mammary gland inflammation on cow’s milk composition measurement. Near Infrared Anal. 2001, 2, 59–66. [Google Scholar]
- Tsenkova, R.; Atanassova, S.; Ozaki, Y.; Toyoda, K.; Itoh, K. Near-infrared spectroscopy for biomonitoring: Influence of somatic cell count on cow’s milk composition analysis. Int. Dairy J. 2001, 11, 779–783. [Google Scholar] [CrossRef]
- Pravdova, V.; Walczak, B.; Massart, D.; Kawano, S.; Toyoda, K.; Tsenkova, R. Calibration of somatic cell count in milk based on near-infrared spectroscopy. Anal. Chim. Acta 2001, 450, 131–141. [Google Scholar] [CrossRef]
- Tsenkova, R.; Atanassova, S.; Kawano, S.; Toyoda, K. Somatic cell count determination in cow’s milk by near-infrared spectroscopy: A new diagnostic tool. J. Anim. Sci. 2001, 79, 2550–2557. [Google Scholar] [CrossRef]
- Tsenkova, R. Aquaphotomics: Extended water mirror approach reveals peculiarities of prion protein alloforms. NIR News 2007, 18, 14–17. [Google Scholar] [CrossRef]
- Bazar, G.; Kovacs, Z.; Tanaka, M.; Tsenkova, R. Aquaphotomics and its extended water mirror concept explain why NIRS can measure low concentration aqueous solutions. In Proceedings of the Aquaphotomics, “Understanding Water in Biological World”, The 5th Kobe University Brussels European Centre Symposium Innovation, Environment, and Globalisation, Brussels, Belgium, 14 October 2014. [Google Scholar]
- Biedermannová, L.; Schneider, B. Hydration of proteins and nucleic acids: Advances in experiment and theory. A review. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 1821–1835. [Google Scholar] [CrossRef]
- Bázár, G.; Kovacs, Z.; Tanaka, M.; Furukawa, A.; Nagai, A.; Osawa, M.; Itakura, Y.; Sugiyama, H.; Tsenkova, R. Water revealed as molecular mirror when measuring low concentrations of sugar with near infrared light. Anal. Chim. Acta 2015, 896, 52–62. [Google Scholar] [CrossRef]
- Tsenkova, R.; Munćan, J.; Pollner, B.; Kovacs, Z. Essentials of aquaphotomics and its chemometrics approaches. Front. Chem. 2018, 6, 363. [Google Scholar] [CrossRef]
- Tsenkova, R. Aquaphotomics: The extended water mirror effect explains why small concentrations of protein in solution can be measured with near infrared light. NIR News 2008, 19, 13–14. [Google Scholar] [CrossRef]
- Hirschfeld, T. Salinity determination using NIRA. Appl. Spectrosc. 1985, 39, 740–741. [Google Scholar] [CrossRef]
- Iwamoto, M.; Uozumi, J.; Nishinari, K. Preliminary investigation of the state of water in foods by near infrared spectroscopy. In Proceedings of the International Near Infrared Spectroscopy/Near Infrared Technology Conference, Budapest, Hungary, 12–16 May 1986; Hello, J., Kaffka, K., Gonczy, J., Eds.; Akademiai Kiado: Budapest, Hungary, 1987; pp. 3–12. [Google Scholar]
- Grant, A.; Davies, A.M.C.; Bilverstone, T. Simultaneous determination of sodium hydroxide, sodium carbonate and sodium chloride concentrations in aqueous solutions by near-infrared spectrometry. Analyst 1989, 114, 819. [Google Scholar] [CrossRef]
- Tanaka, M.; Shibata, A.; Hayashi, N.; Kojima, T.; Maeda, H.; Ozaki, Y. Discrimination of commercial natural mineral waters using near infrared spectroscopy and principal component analysis. J. Near Infrared Spectrosc. 1995, 3, 203–210. [Google Scholar] [CrossRef]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives—A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Tsenkova, R.; Iso, E.; Parker, M.; Fockenberg, C.; Okubo, M. Aqua-Photomics: A NIRS investigation into the perturbation of water spectrum in an aqueous suspension of mesoscopic scale polystyrene spheres. In Proceedings of the 13th International Conference on Near Infrared Spectroscopy, Umea-Vasa, Sweden, 15–21 June 2007. [Google Scholar]
- Workman, J. Handbook of Organic Compounds: NIR, IR, Raman, and UV Spectra Featuring Polymers and Surfaces; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Gowen, A.A.; Marini, F.; Tsuchisaka, Y.; De Luca, S.; Bevilacqua, M.; O’Donnell, C.; Downey, G.; Tsenkova, R. On the feasibility of near infrared spectroscopy to detect contaminants in water using single salt solutions as model systems. Talanta 2015, 131, 609–618. [Google Scholar] [CrossRef]
- Tsenkova, R. Near Infrared Spectroscopy of Raw Milk for Cow’s Biomonitoring. Ph.D. Thesis, Hokkaido University, Sapporo, Japan, 2004. [Google Scholar]
- Luck, W.A. Structure of water and aqueous solutions. In Proceedings of the International Symposium, Marburg, Germany, 18–28 July 1973; Luck, W.A.P., Ed.; Verlag Chemie: Weinheim, Germany, 1974; pp. 248–284. [Google Scholar]
- Tsenkova, R.; Kovacs, Z.; Kubota, Y. Aquaphotomics: Near infrared spectroscopy and water states in biological systems. In Membrane Hydration; DiSalvo, E.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 189–211. [Google Scholar]
- Ozaki, Y. Applications in Chemistry. In Near-Infrared Spectroscopy: Principles, Instruments, Applications; Siesler, H., Ozaki, Y., Kawata, S., Heise, H., Eds.; Wiley: Chichester, UK, 2002; pp. 179–211. [Google Scholar]
- Osborne, B.G.; Fearn, T.; Hindle, P.H. Practical NIR Spectroscopy with Applications in Food and Beverage Analysis; Browning, D., Ed.; Longman Singapore Publ. Ltd.: Singapore, 1993. [Google Scholar]
- Pollner, B.; Kovacs, Z. Multivariate Data Analysis Tools for R Including Aquaphotomics Methods-Aquap2. Available online: https://www.aquaphotomics.com/resources/aquap2/ (accessed on 28 July 2016).
- Cui, X.; Zhang, J.; Cai, W.; Shao, X. Chemometric algorithms for analyzing high dimensional temperature dependent near infrared spectra. Chemom. Intell. Lab. Syst. 2017, 170, 109–117. [Google Scholar] [CrossRef]
- Shao, X.; Cui, X.; Yu, X.; Cai, W. Mutual factor analysis for quantitative analysis by temperature dependent near infrared spectra. Talanta 2018, 183, 142–148. [Google Scholar] [CrossRef]
- Kojić, D.; Tsenkova, R.; Tomobe, K.; Yasuoka, K.; Yasui, M. Water confined in the local field of ions. ChemPhysChem 2014, 15, 4077–4086. [Google Scholar] [CrossRef]
- Gowen, A.A.; Esquerre, C.; O’Donnell, C.P.; Downey, G.; Tsenkova, R. Use of near infrared hyperspectral imaging to identify water matrix co-ordinates in mushrooms (Agaricus bisporus) subjected to mechanical vibration. J. Near Infrared Spectrosc. 2009, 17, 363–371. [Google Scholar] [CrossRef]
- Tsenkova, R. Aquaphotomics: Water in the biological and aqueous world scrutinised with invisible light. Spectrosc. Eur. 2010, 22, 6–10. [Google Scholar]
- Tsenkova, R.N.; Iordanova, I.K.; Toyoda, K.; Brown, D.R. Prion protein fate governed by metal binding. Biochem. Biophys. Res. Commun. 2004, 325, 1005–1012. [Google Scholar] [CrossRef]
- Goto, N.; Bazar, G.; Kovacs, Z.; Kunisada, M.; Morita, H.; Kizaki, S.; Sugiyama, H.; Tsenkova, R.; Nishigori, C. Detection of UV-induced cyclobutane pyrimidine dimers by near-infrared spectroscopy and aquaphotomics. Sci. Rep. 2015, 5, 11808. [Google Scholar] [CrossRef]
- Cui, X.; Cai, W.; Shao, X. Glucose induced variation of water structure from temperature dependent near infrared spectra. RSC Adv. 2016, 6, 105729–105736. [Google Scholar] [CrossRef]
- Cui, X.; Sun, Y.; Cai, W.; Shao, X. Chemometric methods for extracting information from temperature-dependent near-infrared spectra. Sci. China Chem. 2019, 62, 583–591. [Google Scholar] [CrossRef]
- Ma, L.; Cui, X.; Cai, W.; Shao, X. Understanding the function of water during the gelation of globular proteins by temperature-dependent near infrared spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 20132–20140. [Google Scholar] [CrossRef]
- Cui, X.; Liu, X.; Yu, X.; Cai, W.; Shao, X. Water can be a probe for sensing glucose in aqueous solutions by temperature dependent near infrared spectra. Anal. Chim. Acta 2017, 957, 47–54. [Google Scholar] [CrossRef]
- Ball, P. Water as a Biomolecule. ChemPhysChem 2008, 9, 2677–2685. [Google Scholar] [CrossRef]
- Ball, P. Life’s matrix: Water in the cell. Cell. Mol. Biol. 2001, 47, 717–720. [Google Scholar]
- Raschke, T.M. Water structure and interactions with protein surfaces. Curr. Opin. Struct. Biol. 2006, 16, 152–159. [Google Scholar] [CrossRef]
- Ball, P. Water as an active constituent in cell biology. Chem. Rev. 2007, 108, 74–108. [Google Scholar] [CrossRef]
- Bella, J.; Brodsky, B.; Berman, H.M. Hydration structure of a collagen peptide. Structure 1995, 3, 893–906. [Google Scholar] [CrossRef] [Green Version]
- Büning-Pfaue, H. Analysis of water in food by near infrared spectroscopy. Food Chem. 2003, 82, 107–115. [Google Scholar] [CrossRef]
- Sakudo, A.; Tsenkova, R.; Tei, K.; Onozuka, T.; Ikuta, K.; Yoshimura, E.; Onodera, T. Comparison of the vibration mode of metals in HNO3 by a partial least-squares regression analysis of near-infrared spectra. Biosci. Biotechnol. Biochem. 2006, 70, 1578–1583. [Google Scholar] [CrossRef]
- Sakudo, A.; Suganuma, Y.; Sakima, R.; Ikuta, K. Diagnosis of HIV-1 infection by near-infrared spectroscopy: Analysis using molecular clones of various HIV-1 subtypes. Clin. Chim. Acta 2012, 413, 467–472. [Google Scholar] [CrossRef]
- Gowen, A.A.; Amigo, J.M.; Tsenkova, R. Characterisation of hydrogen bond perturbations in aqueous systems using aquaphotomics and multivariate curve resolution-alternating least squares. Anal. Chim. Acta 2013, 759, 8–20. [Google Scholar] [CrossRef]
- Gowen, A.A.; Tsuchisaka, Y.; O’Donnell, C.; Tsenkova, R. Investigation of the potential of near infrared spectroscopy for the detection and quantification of pesticides in aqueous solution. Am. J. Anal. Chem. 2011, 2, 53–62. [Google Scholar] [CrossRef]
- Omar, A.F.; Atan, H.; MatJafri, M.Z. NIR spectroscopic properties of aqueous acids solutions. Molecules 2012, 17, 7440–7450. [Google Scholar] [CrossRef]
- Cattaneo, T.M.P.; Vero, S.; Napoli, E.; Elia, V. Influence of filtration processes on aqueous nanostructures by NIR spectroscopy. J. Chem. Chem. Eng. 2011, 5, 1046–1052. [Google Scholar]
- Kovacs, Z.; Bázár, G.; Oshima, M.; Shigeoka, S.; Tanaka, M.; Furukawa, A.; Nagai, A.; Osawa, M.; Itakura, Y.; Tsenkova, R. Water spectral pattern as holistic marker for water quality monitoring. Talanta 2016, 147, 598–608. [Google Scholar] [CrossRef]
- Vanoli, M.; Lovati, F.; Grassi, M.; Buccheri, M.; Zanella, A.; Cattaneo, T.M.P.; Rizzolo, A. Water spectral pattern as a marker for studying apple sensory texture. Adv. Hortic. Sci. 2018, 32, 343–351. [Google Scholar]
- Muncan, J.; Mileusnic, I.; Matovic, V.; Sakota Rosic, J.; Matija, L. The prospects of aquaphotomics in biomedical science and engineering. Presented at the 2nd International Aquaphotomics Symposium, Kobe, Japan, 26–29 November 2016. [Google Scholar]
- Kinoshita, K.; Miyazaki, M.; Morita, H.; Vassileva, M.; Tang, C.; Li, D.; Ishikawa, O.; Kusunoki, H.; Tsenkova, R. Spectral pattern of urinary water as a biomarker of estrus in the giant panda. Sci. Rep. 2012, 2, 856. [Google Scholar] [CrossRef] [Green Version]
- Jinendra, B. Near infrared Spectroscopy and Aquaphotomics: Novel Tool for Biotic and Abiotic Stress Diagnosis of Soybean. Ph.D. Thesis, Kobe University, Kobe, Japan, 2011. [Google Scholar]
- Jinendra, B.; Tamaki, K.; Kuroki, S.; Vassileva, M.; Yoshida, S.; Tsenkova, R. Near infrared spectroscopy and aquaphotomics: Novel approach for rapid in vivo diagnosis of virus infected soybean. Biochem. Biophys. Res. Commun. 2010, 397, 685–690. [Google Scholar] [CrossRef]
- Haq, Q.M.I.; Mabood, F.; Naureen, Z.; Al-Harrasi, A.; Gilani, S.A.; Hussain, J.; Jabeen, F.; Khan, A.; Al-Sabari, R.S.M.; Al-khanbashi, F.H.S.; et al. Application of reflectance spectroscopies (FTIR-ATR & FT-NIR) coupled with multivariate methods for robust in vivo detection of begomovirus infection in papaya leaves. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 198, 27–32. [Google Scholar]
- Kuroki, S.; Tsenkova, R.; Moyankova, D.P.; Muncan, J.; Morita, H.; Atanassova, S.; Djilianov, D. Water molecular structure underpins extreme desiccation tolerance of the resurrection plant Haberlea rhodopensis. Sci. Rep. 2019, 9, 3049. [Google Scholar] [CrossRef]
- Matija, L.; Tsenkova, R.; Munćan, J.; Miyazaki, M.; Banba, K.; Tomić, M.; Jeftić, B. Fullerene based nanomaterials for biomedical applications: Engineering, functionalization and characterization. Adv. Mater. Res. 2013, 633, 224–238. [Google Scholar] [CrossRef]
- Matija, L.; Muncan, J.; Mileusnic, I.; Koruga, D. Fibonacci nanostructures for novel nanotherapeutical approach. In Nano-and Microscale Drug Delivery Systems: Design and Fabrication; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 49–74. [Google Scholar]
- Cui, X.; Yu, X.; Cai, W.; Shao, X. Water as a probe for serum–based diagnosis by temperature–dependent near–infrared spectroscopy. Talanta 2019, 204, 359–366. [Google Scholar] [CrossRef]
- Azar, A.T. Modeling and Control of Dialysis Systems, Volume 2: Biofeedback Systems and Soft Computing Techniques of Dialysis; Springer-Verlag: Berlin, Germany, 2013. [Google Scholar]
- Tsenkova, R.; Atanassova, S.; Toyoda, K.; Ozaki, Y.; Itoh, K. Near infrared spectroscopy for biomonitoring. J. Jpn. Soc. Agric. Mach. 1999, 61, 529–530. [Google Scholar]
- Tsenkova, R.; Murayama, K.; Kawamura, S.; Itoh, K.; Toyoda, K. Near infrared mastitis diagnosis in the process of milking. In Robotic Milking; Hogeveen, H., Meijering, A., Eds.; Wageningen Pers: Wageningen, The Netherlands, 2000; p. 127. [Google Scholar]
- Tsenkova, R.; Murayama, K.; Kawano, S.; Wu, Y.; Toyoda, K.; Ozaki, Y. Near infrared spectroscopy for mastitis diagnosis: Two-dimensional correlation study in short wavelength region. In Two-Dimensional Correlation Spectroscopy; Ozaki, Y., Noda, I., Eds.; AIP: Melville, NY, USA, 1999; pp. 307–311. [Google Scholar]
- Meilina, H.; Kuroki, S.; Jinendra, B.M.; Ikuta, K.; Tsenkova, R. Double threshold method for mastitis diagnosis based on NIR spectra of raw milk and chemometrics. Biosyst. Eng. 2009, 104, 243–249. [Google Scholar] [CrossRef]
- Tsenkova, R.; Atanassova, S. Mastitis diagnostics by near infrared spectra of cow’s milk, blood and urine using soft independent modelling of class analogy classification. In Proceedings of the Near Infrared Spectroscopy 10th International Conference, Kyongju, Korea, 10–15 June 2001; p. 123. [Google Scholar]
- Tsenkova, R.; Yordanova, K.I.; Itoh, K.; Shinde, Y.; Nishibu, J. Near-infrared spectroscopy of individual cow milk as a means for automated monitoring of udder health and milk quality. In Proceedings of the Dairy System for the 21st Century, the Third International Dairy Housing Conference, Orlando, FL, USA, 2–5 February 1994; pp. 82–91. [Google Scholar]
- Kinoshita, K.; Morita, H.; Miyazaki, M.; Hama, N.; Kanemitsu, H.; Kawakami, H.; Wang, P.; Ishikawa, O.; Kusunoki, H.; Tsenkova, R. Near infrared spectroscopy of urine proves useful for estimating ovulation in giant panda (Ailuropoda melanoleuca). Anal. Methods 2010, 2, 1671. [Google Scholar] [CrossRef]
- Kinoshita, K.; Kuze, N.; Kobayashi, T.; Miyakawa, E.; Narita, H.; Inoue-Murayama, M.; Idani, G.; Tsenkova, R. Detection of urinary estrogen conjugates and creatinine using near infrared spectroscopy in Bornean orangutans (Pongo Pygmaeus). Primates 2016, 57, 51–59. [Google Scholar] [CrossRef]
- Takemura, G.; Bázár, G.; Ikuta, K.; Yamaguchi, E.; Ishikawa, S.; Furukawa, A.; Kubota, Y.; Kovács, Z.; Tsenkova, R. Aquagrams of raw milk for oestrus detection in dairy cows. Reprod. Domest. Anim. 2015, 50, 522–525. [Google Scholar] [CrossRef]
- Agcanas, L.A.; Counsell, K.R.; Shappell, N.; Bowers, S.; Ryan, P.L.; Willard, S.T.; Vance, C.K. A novel approach to comparing reproductive stage serum profiles in mares using near-infrared spectroscopy and aquaphotomics. Reprod. Fertil. Dev. 2017, 29, 117. [Google Scholar] [CrossRef]
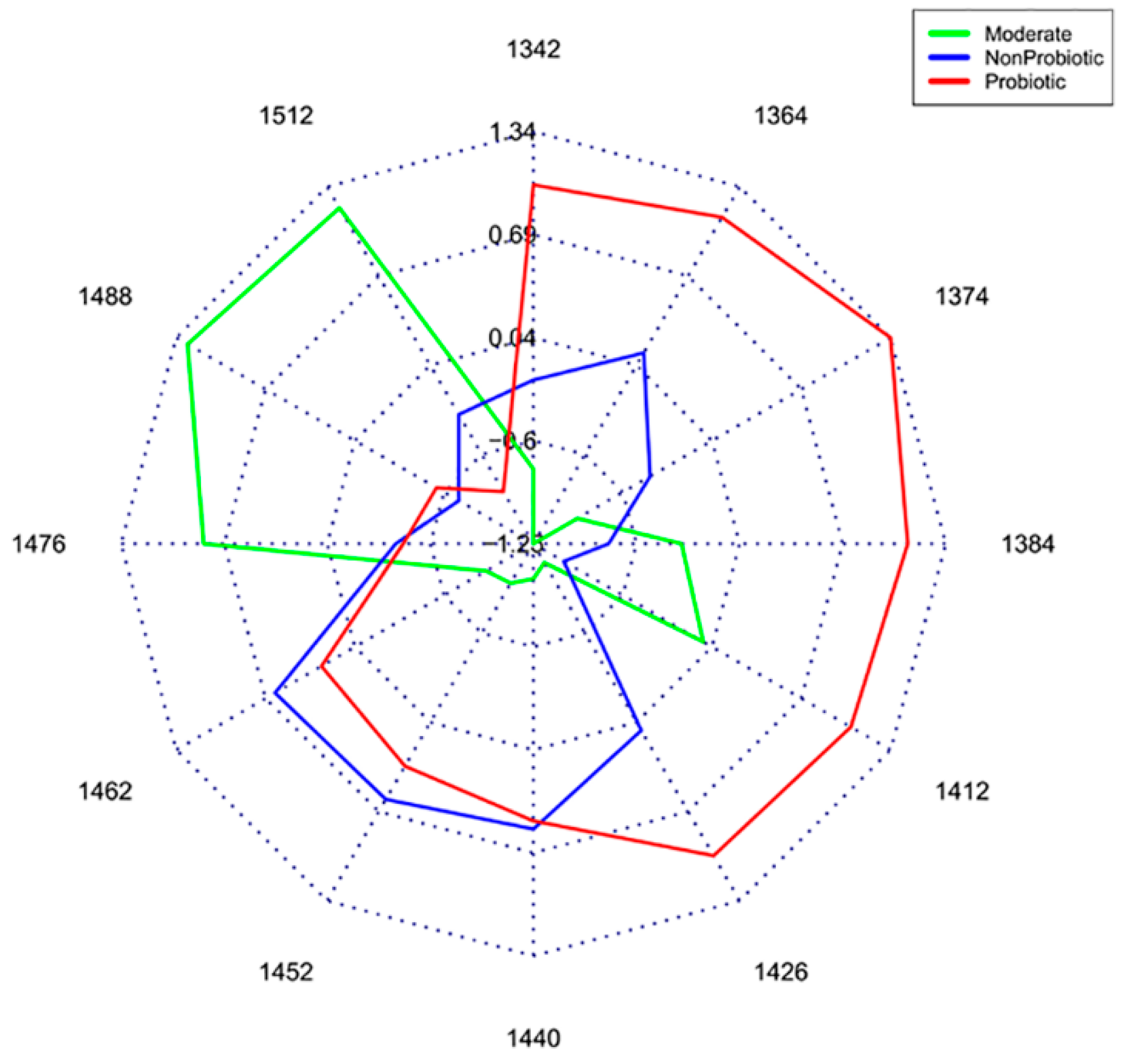
- Slavchev, A.; Kovacs, Z.; Koshiba, H.; Bazar, G.; Pollner, B.; Krastanov, A.; Tsenkova, R. Monitoring of water spectral patterns of lactobacilli development as a tool for rapid selection of probiotic candidates. J. Near Infrared Spectrosc. 2017, 25, 423–431. [Google Scholar] [CrossRef]
- Kovacs, Z.; Slavchev, A.; Bazar, G.; Pollner, B.; Tsenkova, R. Rapid bacteria selection using Aquaphotomics and near infrared spectroscopy. In Proceedings of the 18th International Conference on Near Infrared Spectroscopy, Copenhagen, Denmark, 11–15 June 2017; IM Publications Open LLP: Chichester, UK, 2019; pp. 65–69. [Google Scholar] [Green Version]
- Slavchev, A.; Kovacs, Z.; Koshiba, H.; Nagai, A.; Bázár, G.; Krastanov, A.; Kubota, Y.; Tsenkova, R. Monitoring of water spectral pattern reveals differences in probiotics growth when used for rapid bacteria selection. PLoS ONE 2015, 10, e0130698. [Google Scholar] [CrossRef]
- Remagni, M.C.; Morita, H.; Koshiba, H.; Cattaneo, T.M.P.; Tsenkova, R. Near infrared spectroscopy and aquaphotomics as tools for bacteria classification. In Proceedings of the NIR2013: Picking Up Good Vibrations, La Grande-Motte, France, 2–7 June 2013; p. 602. [Google Scholar]
- Tanaka, S.; Kojić, D.; Tsenkova, R.; Yasui, M. Quantification of anomeric structural changes of glucose solutions using near-infrared spectra. Carbohydr. Res. 2018, 463, 40–46. [Google Scholar] [CrossRef]
- Vaidyanathan, S.; Harvey, L.M.; McNeil, B. Deconvolution of near-infrared spectral information for monitoring mycelial biomass and other key analytes in a submerged fungal bioprocess. Anal. Chim. Acta 2001, 428, 41–59. [Google Scholar] [CrossRef]
- Yanmin, Y.; Na, W.; Youqi, C.; Yingbin, H.; Pengqin, T. Soil moisture monitoring using hyper-spectral remote sensing technology. In Proceedings of the 2010 Second IITA International Conference on Geoscience and Remote Sensing, Qingdao, China, 28–31 August 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 373–376. [Google Scholar]
- Šakota Rosić, J.; Munćan, J.; Mileusnić, I.; Kosić, B.; Matija, L. Detection of protein deposits using NIR spectroscopy. Soft Mater. 2016, 14, 264–271. [Google Scholar] [CrossRef]
- Munćan, J.; Rosić, J.; Mileusnić, I.; Matović, V.; Matija, L.; Tsenkova, R. The structure of water in soft contact lenses: Near infrared spectroscopy and Aquaphotomics study. In Proceedings of the 18th International Conference on Near Infrared Spectroscopy, Copenhagen, Denmark, 11–15 June 2017; IM Publications Open LLP: Chichester, UK, 2019; pp. 99–104. [Google Scholar]
- Muncan, J.; Mileusnic, I.; Sakota Rosic, J.; Vasic-Milovanovic, A.; Matija, L. Water Properties of Soft Contact Lenses: A Comparative Near-Infrared Study of Two Hydrogel Materials. Int. J. Polym. Sci. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Matija, L.R.; Tsenkova, R.N.; Miyazaki, M.; Banba, K.; Muncan, J.S. Aquagrams: Water spectral pattern as characterization of hydrogenated nanomaterial. FME Trans. 2012, 40, 51–56. [Google Scholar]
- Haghi, R.K.; Yang, J.; Tohidi, B. Integrated near infrared and ultraviolet spectroscopy techniques for determination of hydrate inhibitors in the presence of NaCl. Ind. Eng. Chem. Res. 2018, 57, 11728–11737. [Google Scholar] [CrossRef]
- Chang, K.; Shinzawa, H.; Chung, H. Concentration determination of inorganic acids that do not absorb near-infrared (NIR) radiation through recognizing perturbed NIR water bands by them and investigation of accuracy dependency on their acidities. Microchem. J. 2018, 139, 443–449. [Google Scholar] [CrossRef]
- Shao, X.; Cui, X.; Wang, M.; Cai, W. High order derivative to investigate the complexity of the near infrared spectra of aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 83–89. [Google Scholar] [CrossRef]
- Dong, Q.; Yu, C.; Li, L.; Nie, L.; Li, D.; Zang, H. Near-infrared spectroscopic study of molecular interaction in ethanol-water mixtures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117183. [Google Scholar] [CrossRef]
- Kang, J.; Cai, W.; Shao, X. Quantitative determination by temperature dependent near-infrared spectra: A further study. Talanta 2011, 85, 420–424. [Google Scholar] [CrossRef]
- Shao, X.; Cui, X.; Liu, Y.; Xia, Z.; Cai, W. Understanding the molecular interaction in solutions by chemometric resolution of near−infrared spectra. ChemistrySelect 2017, 2, 10027–10032. [Google Scholar] [CrossRef]
- Li, D.; Li, L.; Quan, S.; Dong, Q.; Liu, R.; Sun, Z.; Zang, H. A feasibility study on quantitative analysis of low concentration methanol by FT-NIR spectroscopy and aquaphotomics. J. Mol. Struct. 2019, 1182, 197–203. [Google Scholar] [CrossRef]
- Shan, R.; Zhao, Y.; Fan, M.; Liu, X.; Cai, W.; Shao, X. Multilevel analysis of temperature dependent near-infrared spectra. Talanta 2015, 131, 170–174. [Google Scholar] [CrossRef]
- Shao, X.; Kang, J.; Cai, W. Quantitative determination by temperature dependent near-infrared spectra. Talanta 2010, 82, 1017–1021. [Google Scholar] [CrossRef]
- Putra, A.; Faridah, F.; Inokuma, E.; Santo, R. Robust spectral model for low metal concentration measurement in aqueous solution reveals the importance of water absorbance bands. J. Sains dan Teknol. Reaksi 2010, 8. [Google Scholar] [CrossRef]
- Tsenkova, R.; Fockenberg, C.; Koseva, N.; Sakudo, A.; Parker, M. Aqua-Photomics: Water absorbance patterns in NIR range used for detection of metal ions reveal the importance of sample preparation. In Proceedings of the 13th International Conference on Near Infrared Spectroscopy, Umea-Vasa, Sweden, 15–21 June 2007. [Google Scholar]
- Putra, A.; Meilina, H.; Tsenkova, R. Use of near-infrared spectroscopy for determining the characterization metal ion in aqueous solution. In Proceedings of the Annual International Conference, Syiah Kuala University-Life Sciences & Engineering Chapter, Banda Aceh, Indonesia, 22–24 November 2012; Volume 2, pp. 154–158. [Google Scholar]
- Vero, S.; Tornielli, C.; Cattaneo, T.M.P. Aquaphotomics: Wavelengths involved in the study of the speciation of metal ions (Zn2+, Pb2+ and Ag+) in aqueous Solutions. NIR News 2010, 21, 11–13. [Google Scholar] [CrossRef]
- Wenz, J.J. Examining water in model membranes by near infrared spectroscopy and multivariate analysis. Biochim. Biophys. Acta Biomembr. 2018, 1860, 673–682. [Google Scholar] [CrossRef]
- Cheng, D.; Cai, W.; Shao, X. Understanding the interaction between oligopeptide and water in aqueous solution using temperature-dependent near-infrared spectroscopy. Appl. Spectrosc. 2018, 72, 1354–1361. [Google Scholar] [CrossRef]
- Chatani, E.; Tsuchisaka, Y.; Masuda, Y.; Tsenkova, R. Water molecular system dynamics associated with amyloidogenic nucleation as revealed by real time near infrared spectroscopy and aquaphotomics. PLoS ONE 2014, 9, e101997. [Google Scholar] [CrossRef]
- Yamada, K.; Murayama, K.; Tsenkova, R.; Wang, Y.; Ozaki, Y. Multivariate determination of human serum albumin and γ-globulin in a phosphate buffer solution by near infrared spectroscopy. J. Near Infrared Spectrosc. 1998, 6, 375–381. [Google Scholar]
- Yuan, B.; Murayama, K.; Wu, Y.; Tsenkova, R.; Dou, X.; Era, S.; Ozaki, Y. Temperature-dependent near-infrared spectra of bovine serum albumin in aqueous solutions: Spectral analysis by principal component analysis and evolving factor analysis. Appl. Spectrosc. 2003, 57, 1223–1229. [Google Scholar] [CrossRef]
- Munćan, J.S.; Matija, L.; Simić-Krstić, J.B.; Nijemčević, S.S.; Koruga, D.L. Discrimination of mineral waters using near-infrared spectroscopy and aquaphotomics. Hem. Ind. 2014, 68, 257–264. [Google Scholar] [CrossRef]
- Bázár, G.; Romvári, R.; Szabó, A.; Somogyi, T.; Éles, V.; Tsenkova, R. NIR detection of honey adulteration reveals differences in water spectral pattern. Food Chem. 2016, 194, 873–880. [Google Scholar] [CrossRef]
- Esquerre, C.; Gowen, A.; O’Donnell, C.P.; Downey, G. Water absorbance pattern of physical damage in mushrooms. In Proceedings of the Biosystems Engineering Research Review 14, Dublin, Ireland, 11 March 2009; Cummins, E., Curran, T., Eds.; University College Dublin: Dublin, Ireland, 2009; pp. 68–71. [Google Scholar]
- Gowen, A.A. Water and Food Quality. Contemp. Mater. 2012, 1, 31–37. [Google Scholar] [CrossRef]
- Achata, E.; Esquerre, C.; O’Donnell, C.; Gowen, A.; Achata, E.; Esquerre, C.; O’Donnell, C.; Gowen, A. A study on the application of near infrared hyperspectral chemical imaging for monitoring moisture content and water activity in low moisture systems. Molecules 2015, 20, 2611–2621. [Google Scholar] [CrossRef]
- Veleva-Doneva, P.; Atanassova, S.; Zhelyazkov, G. Innovative engineering methods for quality evaluation and food safety. Zesz. Nauk. Małopolskiej Wyższej Szkoły Ekon. Tarn. 2017, 36, 13–23. [Google Scholar]
- Atanassova, S. Near Infrared Spectroscopy and aquaphotomics for monitoring changes during yellow cheese ripening. Agric. Sci. Technol. 2015, 7, 269–272. [Google Scholar]
- Cattaneo, T.M.P.; Vanoli, M.; Grassi, M.; Rizzolo, A.; Barzaghi, S. The aquaphotomics approach as a tool for studying the influence of food coating materials on cheese and winter melon samples. J. Near Infrared Spectrosc. 2016, 24, 381–390. [Google Scholar] [CrossRef]
- Vanoli, M.; Grassi, M.; Lovati, F.; Barzaghi, S.; Cattaneo, T.M.P.; Rizzolo, A. Influence of innovative coatings on salami ripening assessed by near infrared spectroscopy and aquaphotomics. J. Near Infrared Spectrosc. 2019, 27, 54–64. [Google Scholar] [CrossRef]
- Barzaghi, S.; Cremonesi, K.; Cattaneo, T.M.P. Influence of the presence of bioactive compounds in smart-packaging materials on water absorption using NIR spectroscopy and aquaphotomics. NIR News 2017, 28, 21–24. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, C.; Cao, F.; Liu, F.; Luo, S.; Tang, Y.; He, Y.; Kong, W.; Zhang, C.; Cao, F.; et al. Detection of sclerotinia stem rot on oilseed rape (Brassica napus L.) leaves using hyperspectral imaging. Sensors 2018, 18, 1764. [Google Scholar] [CrossRef]
- He, X.; Feng, X.; Sun, D.; Liu, F.; Bao, Y.; He, Y.; He, X.; Feng, X.; Sun, D.; Liu, F.; et al. Rapid and nondestructive measurement of rice seed vitality of different years using near-infrared hyperspectral imaging. Molecules 2019, 24, 2227. [Google Scholar] [CrossRef]
- Chu, B.; Yu, K.; Zhao, Y.; He, Y.; Chu, B.; Yu, K.; Zhao, Y.; He, Y. Development of noninvasive classification methods for different roasting degrees of coffee beans using hyperspectral imaging. Sensors 2018, 18, 1259. [Google Scholar] [CrossRef]
- Mura, S.; Cappai, C.; Greppi, G.F.; Barzaghi, S.; Stellari, A.; Cattaneo, T.M.P. Vibrational spectroscopy and Aquaphotomics holistic approach to determine chemical compounds related to sustainability in soil profiles. Comput. Electron. Agric. 2019, 159, 92–96. [Google Scholar] [CrossRef]
- Bozhynov, V.; Soucek, P.; Barta, A.; Urbanova, P.; Bekkozhayeva, D. Visible aquaphotomics spectrophotometry for aquaculture systems. In Proceedings of the International Conference on Bioinformatics and Biomedical Engineering, Granada, Spain, 25–27 April 2018; Springer: Cham, Switzerland, 2018; pp. 107–117. [Google Scholar]
- Nakakimura, Y.; Vassileva, M.; Stoyanchev, T.; Nakai, K.; Osawa, R.; Kawano, J.; Tsenkova, R. Extracellular metabolites play a dominant role in near-infrared spectroscopic quantification of bacteria at food-safety level concentrations. Anal. Methods 2012, 4, 1389. [Google Scholar] [CrossRef]
- Sakudo, A.; Yoshimura, E.; Tsenkova, R.; Ikuta, K.; Onodera, T. Native state of metals in non-digested tissues by partial least squares regression analysis of visible and near-infrared spectra. J. Toxicol. Sci. 2007, 32, 135–141. [Google Scholar] [CrossRef]
- Sakudo, A.; Tsenkova, R.; Tei, K.; Morita, H.; Ikuta, K.; Onodera, T. Ex vivo tissue discrimination by visible and near-infrared spectra with chemometrics. J. Vet. Med. Sci. 2006, 68, 1375–1378. [Google Scholar] [CrossRef]
- Huck, C.W. Theoretical and technical advancements of near-infrared spectroscopy and its operational impact in industry. NIR News 2017, 28, 17–21. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
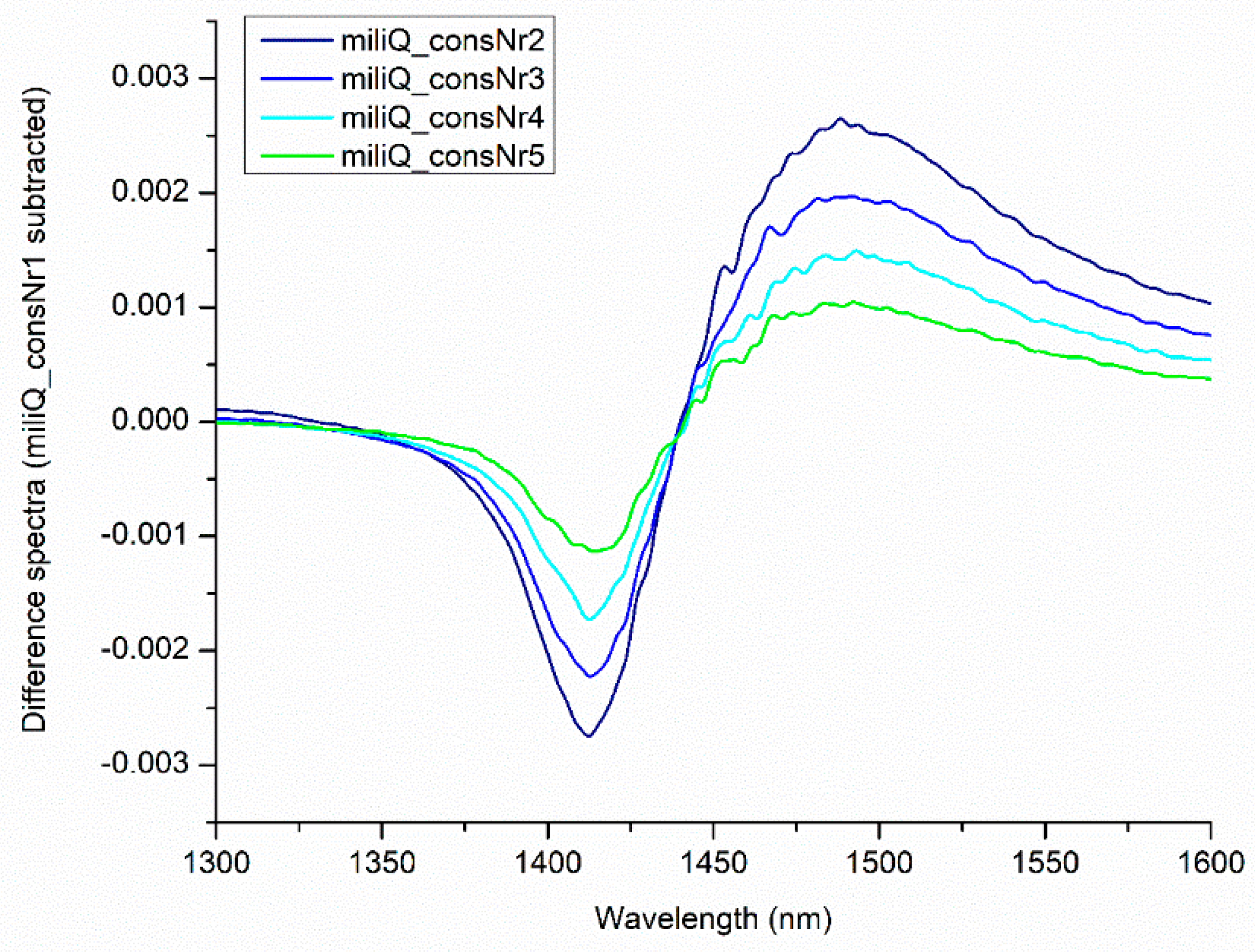
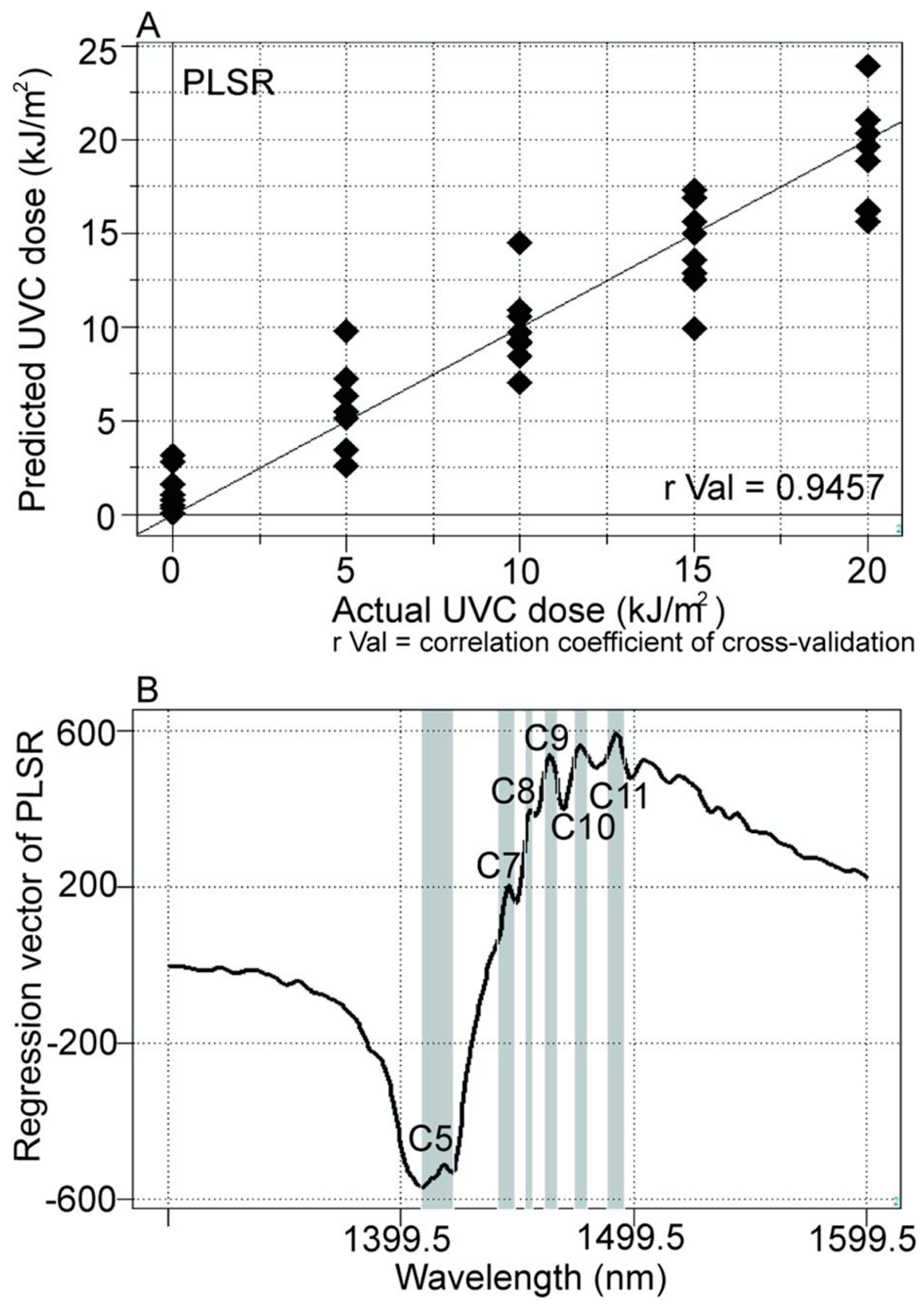
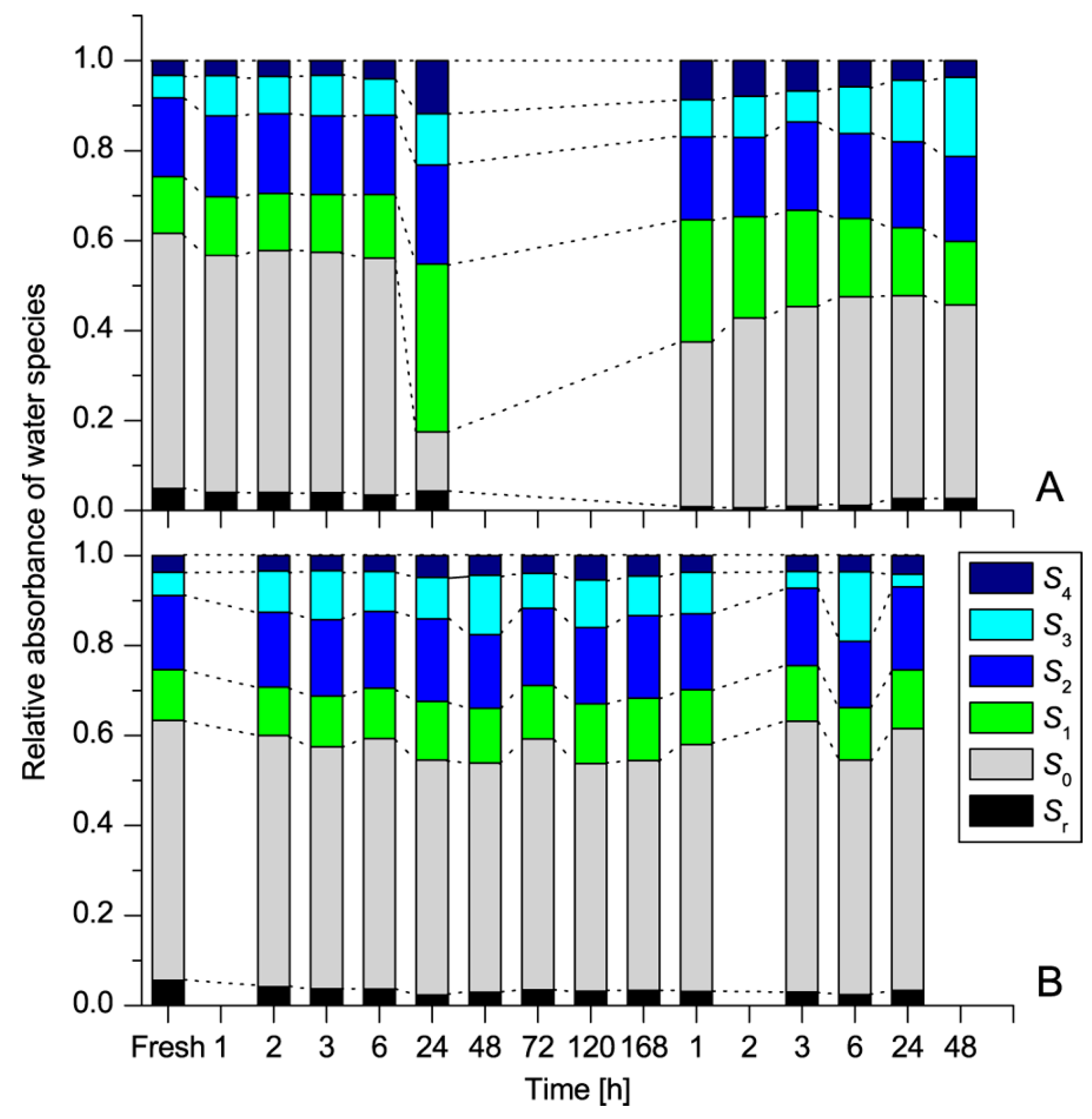
| WAMACS | Range (nm) | Assignment |
|---|---|---|
| C1 | 1336–1348 | 2ν3: H2O asymmetric stretching vibration |
| C2 | 1360–1366 | OH-·(H2O)1,2,4: Water solvation shell |
| C3 | 1370–1376 | ν1 + ν3: H2O symmetrical stretching vibration and H2O asymmetric stretching vibration |
| C4 | 1380–1388 | OH-·(H2O)1,4: Water solvation shell O2-·(H2O)4: Hydrated superoxide clusters 2ν1: H2O symmetrical stretching vibration |
| C5 | 1398–1418 | Water confined in a local field of ions (trapped water) S0: Free water Water with free OH- |
| C6 | 1421–1430 | Water hydration band H-OH bend and O-H…O |
| C7 | 1432–1444 | S1: Water molecules with 1 hydrogen bond |
| C8 | 1448–1454 | OH-·(H2O)4,5: Water solvation shell |
| C9 | 1458–1468 | S2: Water molecules with 2 hydrogen bonds 2ν2 + ν3: H2O bending and asymmetrical stretching vibration |
| C10 | 1472–1482 | S3: Water molecules with 3 hydrogen bonds |
| C11 | 1482–1495 | S4: Water molecules with 4 hydrogen bonds |
| C12 | 1506–1516 | ν1: H2O symmetrical stretching vibration ν2: H2O bending vibration Strongly bound water |
 | ||
| Application | Object of Study | Purpose | References |
|---|---|---|---|
| Fundamental research | Sugars | Quantification | [25,43,49,52] |
| Glucose | Distinguishing anomers | [91] | |
| Salts | Quantification and influence on water spectra | [26,35,44,61,98] | |
| Acids | Quantification, accuracy of prediction depending on acidity | [99] | |
| Acids and pH | Quantification | [63] | |
| Ethanol | Quantification, structural analysis | [42,100,101,102,103] | |
| Methanol | Quantification | [98,104] | |
| Water-ethanol-isopropanol mixture | Quantitative analysis and the effect of temperature | [105] | |
| Water, methanol, ethanol and ethylenediamine mixture | Quantitative analysis and the effect of temperature | [106] | |
| Monoethylene-glycol | Quantification | [98] | |
| Metal ions | Quantification | [107,108,109,110] | |
| Near infrared light | Influence of consecutive irradiation | [1] | |
| UV light | Measurement of irradiation dose | [48] | |
| Temperature | Influence of temperature on water spectra | [42,43,111] | |
| Biomolecules | Oligopeptides | Interaction with water – elucidating the structure, dynamics and function of proteins | [112] |
| Prion proteins | Stability of protein structure as a function of metal binding | [47] | |
| Insulin | Fibrillation phases | [113] | |
| Albumin and γ-globulin | Quantification | [114] | |
| Albumin | Structural analysis and hydration properties | [115] | |
| Ovalbumin | Gelation of globular proteins | [51] | |
| DNA | Quantification and detection of mutation products | [48] | |
| Phospholipids | Structural analysis and effect on water | [111] | |
| Water | Water contamination | Quantification of pesticides alachlor and atrazine | [62] |
| Water contamination | Detection of contaminants based on salts as model systems | [35] | |
| Commercial mineral waters | Discrimination | [116] | |
| Ground water quality | Continuous monitoring based on water spectral pattern as a holistic/integrative marker | [65] | |
| Pure water | Influence of filtration process | [64] | |
| Food | Honey | Adulteration | [117] |
| Mushrooms | Detection of physical damage | [45,118] | |
| Milk | Components | [36] | |
| Wafer, coffee, soybean | Water activity and moisture content | [119,120] | |
| Perches (fish) | Discriminating between wild fishes and raised in the recirculation system | [121] | |
| Pork loin | Discrimination between fresh and spoiled meat | [121] | |
| Porcine muscles | Discrimination between fresh and thawed meat | [121] | |
| Cheese | Ripening process | [122] | |
| Cheese and winter melon | Influence of packaging material on ripening | [123] | |
| Salami | Influence of coating on ripening | [124] | |
| Packaging material | Influence of bioactive compound - propolis | [125] | |
| Apples | Sensory texture - specific mechanical and structural properties related to water spectral pattern | [66] | |
| Oilseed Rape | Stem rot detection | [126] | |
| Rice | Seed vitality | [127] | |
| Coffee | Roasting degree | [128] | |
| Wheat kernels | Hardness | [15] | |
| Materials | Soft contact lenses: hydrogels | Discrimination of hydrogels with different water content | [95,96] |
| Soft contact lenses: hydrogels | Discrimination of new and worn contact lenses | [94] | |
| Titanium dioxide | Wettability | [6] | |
| Environment | Soil | Identification of soil type | [129] |
| Water contamination | Monitoring | [65,130] | |
| Nanomaterials | Fullerene based nanomaterials | Hydration properties | [73,74,97] |
| Polystyrene | Quantification of particles in water solutions | [33] | |
| Microbiology | Bacteria – metabolites | Contribution to NIR signal from cells and metabolites | [131] |
| Bacteria - probiotic | Classification | [87,89] | |
| HIV virus | Detection and quantification | [60] | |
| Bacteria | Selection | [88] | |
| Cells and tissues | Somatic cells in milk | Quantification | [21] |
| Tissue (mice) | Native state of metals | [132] | |
| Tissue (mice) | Ex vivo discrimination | [133] | |
| Plant biology | Soybean | Detection of mosaic virus infection | [70] |
| Soybean | Ability to cope with cold stress in genetically modified cultivars; Detection of mosaic virus infection | [69] | |
| Resurrection plants | Peculiarities of water structure in leaves of anhydrobiotic organism | [72] | |
| Papaya leaves | In vivo detection of begomovirus infection | [71] | |
| Animal medicine | Mastitis in dairy cows | Disease detection | [21,77,78,79,80,81,82] |
| Estrus detection in urine of giant panda | Finding water spectral pattern as biomarker, quantification of hormone | [68,83] | |
| Estrus detection in milk of cows | Ovulation period detection and monitoring | [85] | |
| Estrus detection in urine of Bornean orangutan | Ovulation period detection and monitoring | [84] | |
| Estrus period detection using serum in mares | Detection of oestrus, metestrus, and diestrus in mares, | [86] | |
| Medicine | DNA mutation products | Detection of DNA damage, quantification of damage products | [48] |
| AIDS | HIV virus detection | [60] | |
| Serum | Serum based diagnosis (diabetes, coronary heart disease) | [75] | |
| Prion protein disease | Mechanism of disease | [47] | |
| Skin cream effects | Therapy monitoring | [73,74] | |
| Dialysis efficacy | Monitoring of spent dialysate | [67] | |
| Colorectal cancer | Diagnostics based on serum and urine | [67] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muncan, J.; Tsenkova, R. Aquaphotomics—From Innovative Knowledge to Integrative Platform in Science and Technology. Molecules 2019, 24, 2742. https://doi.org/10.3390/molecules24152742
Muncan J, Tsenkova R. Aquaphotomics—From Innovative Knowledge to Integrative Platform in Science and Technology. Molecules. 2019; 24(15):2742. https://doi.org/10.3390/molecules24152742
Chicago/Turabian StyleMuncan, Jelena, and Roumiana Tsenkova. 2019. "Aquaphotomics—From Innovative Knowledge to Integrative Platform in Science and Technology" Molecules 24, no. 15: 2742. https://doi.org/10.3390/molecules24152742
APA StyleMuncan, J., & Tsenkova, R. (2019). Aquaphotomics—From Innovative Knowledge to Integrative Platform in Science and Technology. Molecules, 24(15), 2742. https://doi.org/10.3390/molecules24152742






