Prediction of Transformation Products of Monensin by Electrochemistry Compared to Microsomal Assay and Hydrolysis
Abstract
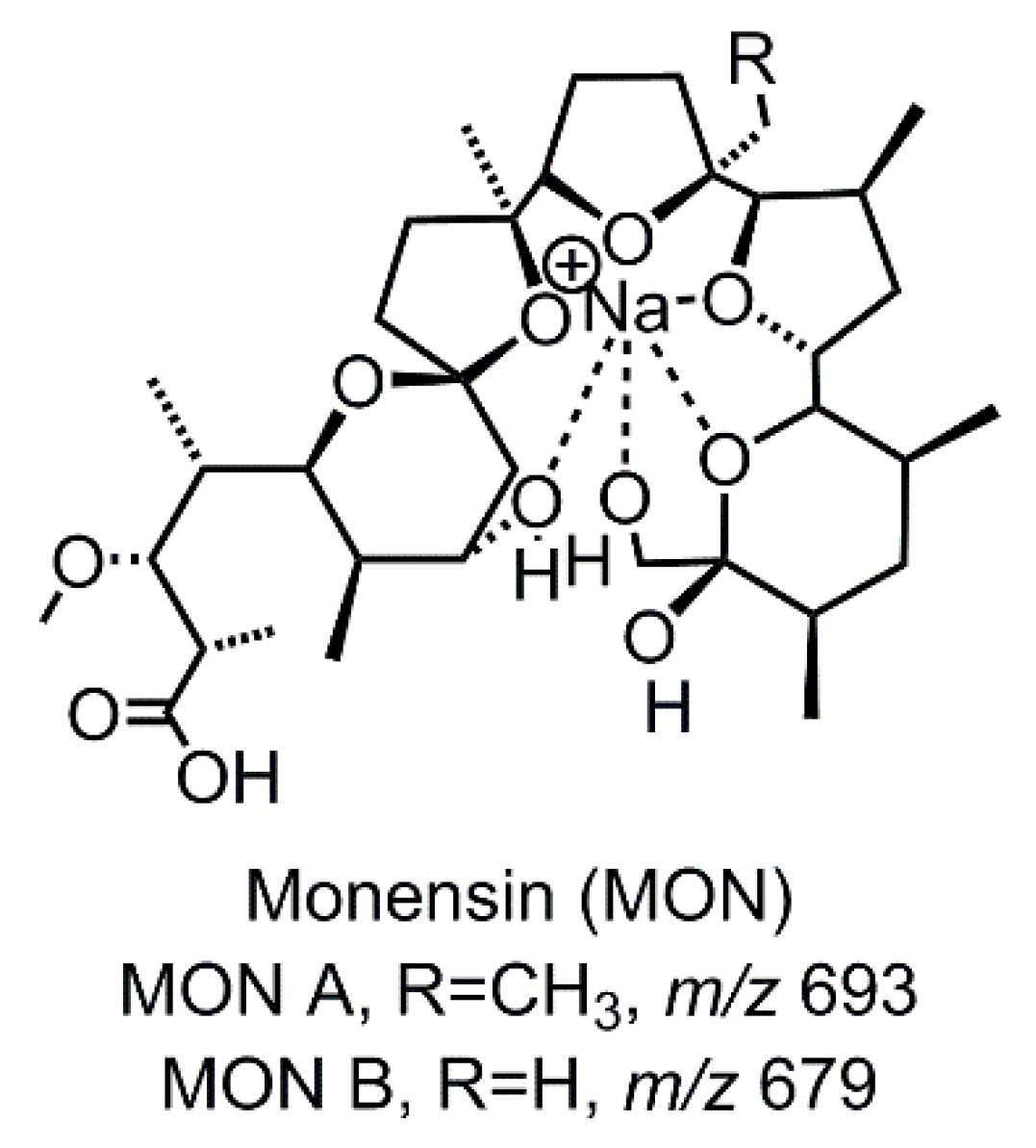
:1. Introduction
2. Results and Discussion
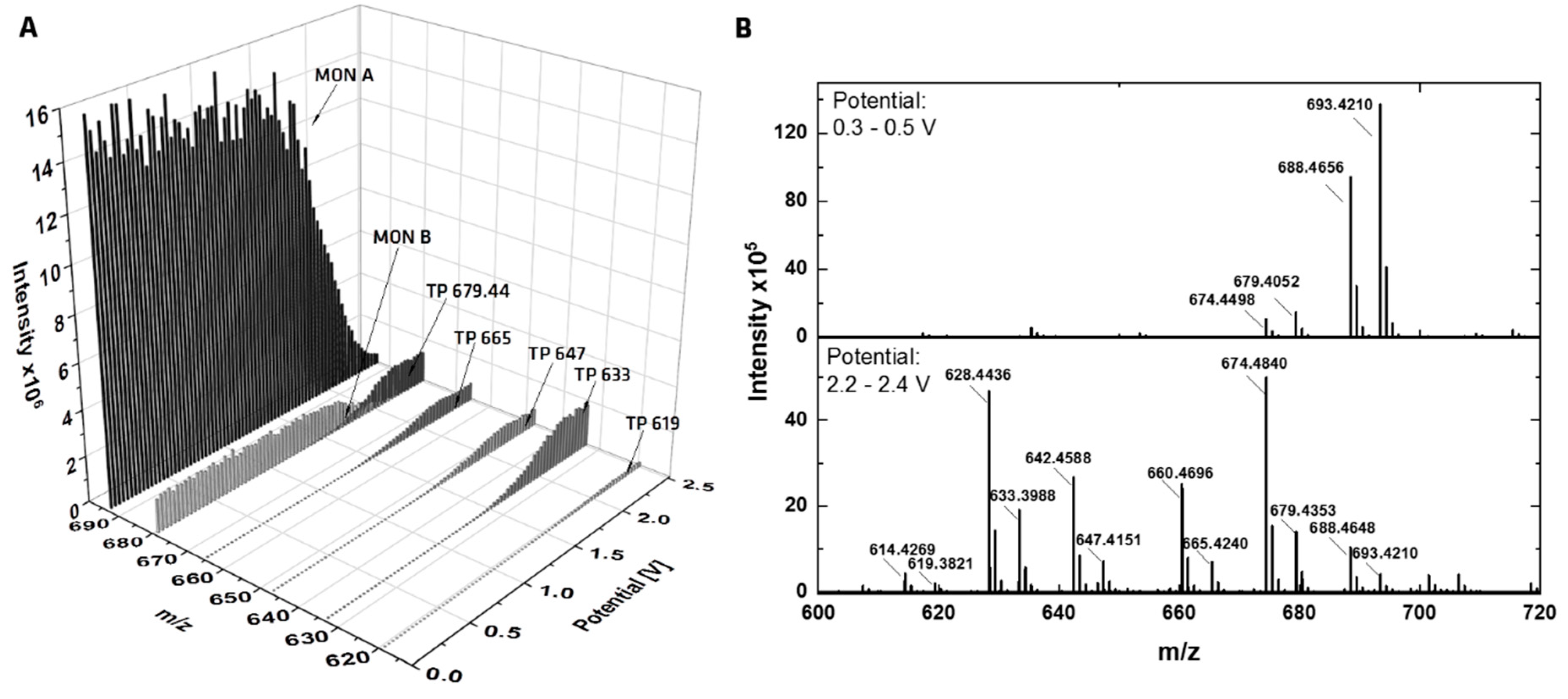
2.1. Electrochemical Investigation
2.2. Microsomal Tests
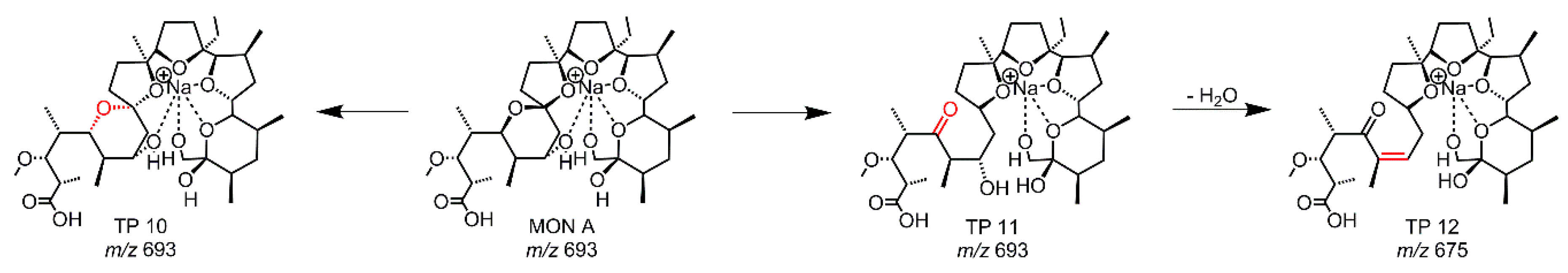
2.3. Hydrolysis of MON
3. Materials and Methods
3.1. Chemicals
3.2. Microsomal Sources and Incubation
3.3. EC/ESI-HRMS
3.4. Hydrolysis
3.5. LC/HRMS
3.6. EC/ESI-MS—LC/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bletsou, A.A.; Jeon, J.; Hollender, J.; Archontaki, E.; Thomaidis, N.S. Targeted and non-targeted liquid chromatography-mass spectrometric workflows for identification of transformation products of emerging pollutants in the aquatic environment. TrAC Trends Anal. Chem. 2015, 66, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Boxall, A.B.A.; Fogg, L.A.; Blackwell, P.A.; Blackwell, P.; Kay, P.; Pemberton, E.J.; Croxford, A. Veterinary Medicines in the Environment. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2004; pp. 1–91. [Google Scholar]
- Farré, M.l.; Pérez, S.; Kantiani, L.; Barceló, D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal. Chem. 2008, 27, 991–1007. [Google Scholar] [CrossRef]
- Kantiani, L.; Llorca, M.; Sanchís, J.; Farré, M.; Barceló, D. Emerging food contaminants: A review. Analy. Bioanaly. Chem. 2010, 398, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Bartikova, H.; Podlipna, R.; Skalova, L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere 2016, 144, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Speight, J.G. Chemical Transformations in the Environment. In Environmental Organic Chemistry for Engineers; Butterworth-Heinemann: Oxford, UK, 2017; pp. 305–353. [Google Scholar]
- Fatta-Kassinos, D.; Vasquez, M.I.; Kummerer, K. Transformation products of pharmaceuticals in surface waters and wastewater formed during photolysis and advanced oxidation processes-degradation, elucidation of byproducts and assessment of their biological potency. Chemosphere 2011, 85, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Pico, Y.; Barcelo, D. Transformation products of emerging contaminants in the environment and high-resolution mass spectrometry: A new horizon. Anal. Bioanal. Chem. 2015, 407, 6257–6273. [Google Scholar] [CrossRef] [PubMed]
- Agüera, A.; Martínez Bueno, M.J.; Fernández-Alba, A.R. New trends in the analytical determination of emerging contaminants and their transformation products in environmental waters. Environ. Sci. Pollut. Res. 2013, 20, 3496–3515. [Google Scholar] [CrossRef]
- Kotthoff, L.; Keller, J.; Lörchner, D.; Mekonnen, T.F.; Koch, M. Transformation products of organic contaminants and residues—overview of current simulation methods. Molecules 2019, 24, 753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Luo, G.; Ding, X.; Lu, C. Preclinical experimental models of drug metabolism and disposition in drug discovery and development. Acta Pharm. Sin. B 2012, 2, 549–561. [Google Scholar] [CrossRef] [Green Version]
- Brandon, E.F.A.; Raap, C.D.; Meijerman, I.; Beijnen, J.H.; Schellens, J.H.M. An update on in vitro test methods in human hepatic drug biotransformation research: Pros and cons. Toxicol. Appl. Pharmacol. 2003, 189, 233–246. [Google Scholar] [CrossRef]
- Jahn, S.; Karst, U. Electrochemistry coupled to (liquid chromatography/) mass spectrometry--current state and future perspectives. J. Chromatogr. A 2012, 1259, 16–49. [Google Scholar] [CrossRef] [PubMed]
- Portychova, L.; Schug, K.A. Instrumentation and applications of electrochemistry coupled to mass spectrometry for studying xenobiotic metabolism: A review. Anal. Chim. Acta 2017, 993, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bruins, A.P. An overview of electrochemistry combined with mass spectrometry. TrAC Trends Anal. Chem. 2015, 70, 14–19. [Google Scholar] [CrossRef]
- OECD. Test No. 111: Hydrolysis as a Function of pH. Available online: https://www.oecd-ilibrary.org/environment/test-no-111-hydrolysis-as-a-function-of-ph_9789264069701-en (accessed on 11 October 2018).
- Rocha, B.A.; Assis, M.D.; Peti, A.P.; Moraes, L.A.; Moreira, F.L.; Lopes, N.P.; Pospisil, S.; Gates, P.J.; de Oliveira, A.R. In vitro metabolism of monensin A: Microbial and human liver microsomes models. Xenobiotica 2014, 44, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Nebbia, C.; Ceppa, L.; Dacasto, M.; Nachtmann, C.; Carletti, M. Oxidative monensin metabolism and cytochrome P450 3A content and functions in liver microsomes from horses, pigs, broiler chicks, cattle and rats. J. Vet. Pharmacol. Therap. 2001, 24, 399–403. [Google Scholar] [CrossRef]
- Davison, K.L. Monensin absorption and metabolism in calves and chickens. J. Agri. Food Chem. 1984, 32, 1273–1277. [Google Scholar] [CrossRef]
- Donoho, A.; Manthey, J.; Occolowitz, J.; Zornes, L. Metabolism of monensin in the steer and rat. J. Agric. Food Chem. 1978, 26, 1090–1095. [Google Scholar] [CrossRef]
- Kiehl, D.E.; Julian, R.K.; Kennington, A.S. Electrospray ionization mass spectrometry with in-source collision-induced dissociation of monensin factors and related metabolites. Rapid Commun. Mass Spectrom. 1998, 12, 903–910. [Google Scholar] [CrossRef]
- Munaretto, J.S.; Yonkos, L.; Aga, D.S. Transformation of ionophore antimicrobials in poultry litter during pilot-scale composting. Environ. Pollut. 2016, 212, 392–400. [Google Scholar] [CrossRef]
- Sun, P.; Cabrera, M.L.; Huang, C.H.; Pavlostathis, S.G. Biodegradation of veterinary ionophore antibiotics in broiler litter and soil microcosms. Environ. Sci. Technol. 2014, 48, 2724–2731. [Google Scholar] [CrossRef]
- Arikan, O.A.; Mulbry, W.; Rice, C.; Lansing, S. The fate and effect of monensin during anaerobic digestion of dairy manure under mesophilic conditions. PLoS ONE 2018, 13, e0192080. [Google Scholar] [CrossRef] [PubMed]
- Sassman, S.A.; Lee, L.S. Sorption and degradation in soils of veterinary ionophore antibiotics: Monensin and lasalocid. Environ. Toxicol. Chem. 2007, 26, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Yao, H.; Minakata, D.; Crittenden, J.C.; Pavlostathis, S.G.; Huang, C.H. Acid-catalyzed transformation of ionophore veterinary antibiotics: Reaction mechanism and product implications. Environ. Sci. Technol. 2013, 47, 6781–6789. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Pavlostathis, S.G.; Huang, C.H. Photodegradation of veterinary ionophore antibiotics under UV and solar irradiation. Environ. Sci. Technol. 2014, 48, 13188–13196. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Junior, J.N.; Rocha, B.A.; Assis, M.D.; Peti, A.P.F.; Moraes, L.A.B.; Iamamoto, Y.; Gates, P.J.; de Oliveira, A.R.M.; Lopes, N.P. Biomimetic oxidation studies of monensin A catalyzed by metalloporphyrins: Identification of hydroxyl derivative product by electrospray tandem mass spectrometry. Rev. Bras. Farmacogn. 2013, 23, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Rocha, B.A.; de Oliveira, A.R.; Pazin, M.; Dorta, D.J.; Rodrigues, A.P.; Berretta, A.A.; Peti, A.P.; de Moraes, L.A.; Lopes, N.P.; Pospisil, S.; et al. Jacobsen catalyst as a cytochrome P450 biomimetic model for the metabolism of monensin A. Biomed. Res. Int. 2014, 2014, 152102. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Huang, C.H.; Pavlostathis, S.G. Inhibition and biotransformation potential of veterinary ionophore antibiotics under different redox conditions. Environ. Sci. Technol. 2014, 48, 13146–13154. [Google Scholar] [CrossRef]
- Lopes, N.P.; Stark, C.B.; Hong, H.; Gates, P.J.; Staunton, J. Fragmentation studies on monensin A and B by accurate-mass electrospray tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2002, 16, 414–420. [Google Scholar] [CrossRef]
- Lopes, N.P.; Stark, C.B.W.; Gates, P.J.; Staunton, J. Fragmentation studies on monensin A by sequential electrospray mass spectrometry. Analyst 2002, 127, 503–506. [Google Scholar] [CrossRef]
- Simon, H.; Hoffmann, G.; Hubner, F.; Humpf, H.U.; Karst, U. Electrochemical simulation of metabolic reactions of the secondary fungal metabolites alternariol and alternariol methyl ether. Anal. Bioanal. Chem. 2016, 408, 2471–2483. [Google Scholar] [CrossRef]
- Keller, J.; Haase, H.; Koch, M. Electrochemical simulation of biotransformation reactions of citrinin and dihydroergocristine compared to UV irradiation and Fenton-like reaction. Anal. Bioanal. Chem. 2017, 409, 4037–4045. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| TP Simulation Method | Compound | Retention Time [s] | calc. m/z | Molecular Formula | Suggested Transformation | TP Intensity |
|---|---|---|---|---|---|---|
| Std | MON A | 162 | 693.4184 | C36H62O11Na | - | s |
| Std | MON B | 120 | 679.4028 | C35H60O11Na | - | w |
| EC-GC | TP 1 | 92 | 619.3817 | C33H56O9Na | -CO2 -2x CH2 | w |
| EC-GC | TP 2 | 102 | 665.4235 | C35H62O10Na | -CO2 -2H +H2O | w |
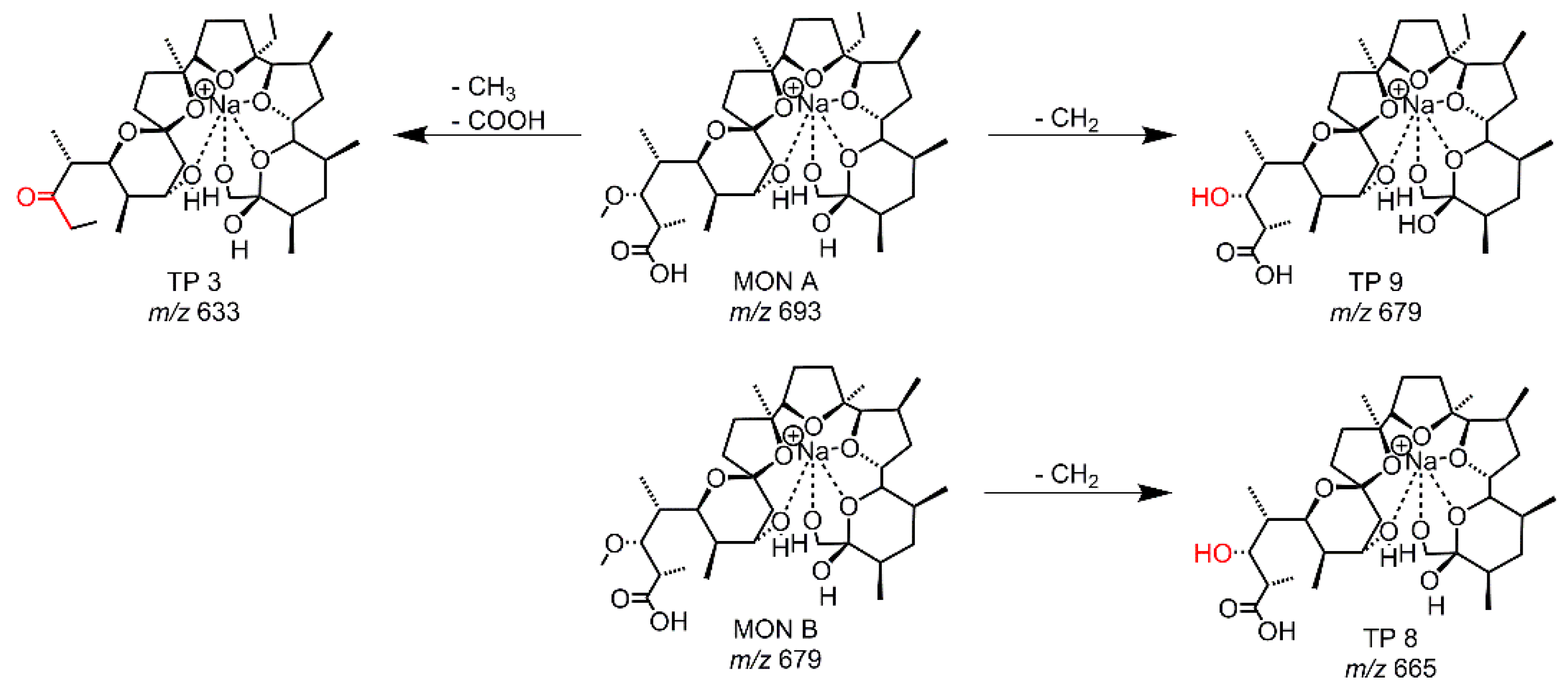
| EC-GC, RLM | TP 3 | 109 | 633.3973 | C34H58O9Na | -CO2 -CH2 | s |
| EC-GC | TP 4 | 132 | 647.4130 | C35H60O9Na | -CO2 -2H | ms |
| EC-GC | TP 5 | 133 | 679.4392 | C36H64O10Na | -CO2 -2H +OCH3 +H | s |
| EC-GC | TP 6 | 144 | 679.4392 | C36H64O10Na | -CO2 -2H +OCH3 +H | ms |
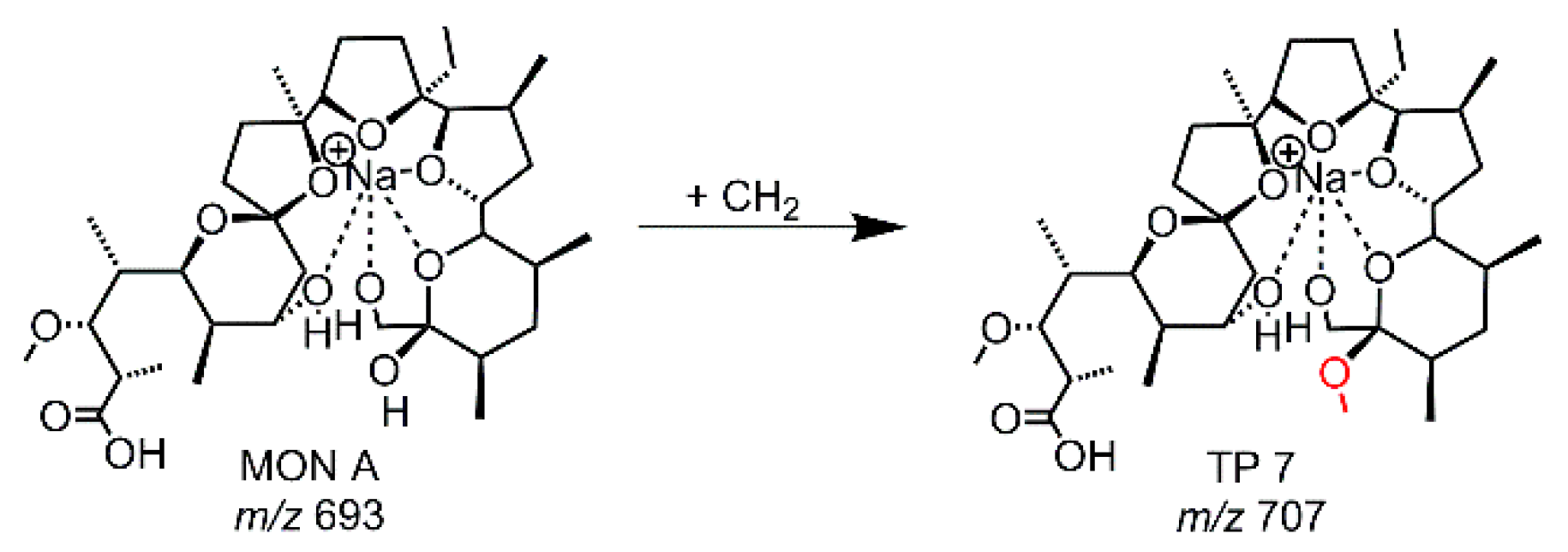
| EC-MD | TP 7 | 107 | 707.4341 | C37H64O11Na | +CH2 | vw |
| RLM | TP 8 | 79 | 665.3877 | C34H58O11Na | -2 CH2 | vw |
| RLM | TP 9 | 95 | 679.4028 | C35H60O11Na | -CH2 | w |
| Hyd | TP 10 | 75 | 693.4184 | C36H62O11Na | diastereomer | s |
| Hyd | TP 11 | 99 | 693.4184 | C36H62O11Na | ring cleavage | ms |
| Hyd | TP 12 | 86 | 675.4079 | C36H60O10Na | -H2O | s |
| Day | ||||||||
|---|---|---|---|---|---|---|---|---|
| pH 3 | 1 | 2 | 3 | 4 | 5 | 8 | 15 | 30 |
| MON | x | x | x | x | x | x | x | x |
| TP10 | x | x | x | x | x | x | x | x |
| TP11 | x | x | x | x | x | x | x | x |
| TP12 | x | x | x | x | x | x | x | |
| Day | ||||||||
| pH 4 | 1 | 2 | 3 | 4 | 5 | 8 | 15 | 30 |
| MON | x | x | x | x | x | x | x | x |
| TP10 | x | x | x | x | x | x | x | |
| TP11 | x | x | x | x | x | x | ||
| TP12 | x | x | x | x | x | |||
| Day | ||||||||
| pH 5 | 1 | 2 | 3 | 4 | 5 | 8 | 15 | 30 |
| MON | x | x | x | x | x | x | x | x |
| TP10 | x | x | x | x | x | |||
| TP11 | x | x | ||||||
| TP12 | ||||||||
| Experiments Parameters | Mass Range Parameters | ||
|---|---|---|---|
| gas temperature | 350 °C | collision energy | 10 V |
| ion source gas 1 (nitrogen) | 20 L/min | declustering potential | 80 V |
| ion source gas 2 (nitrogen | 15 L/min | mass range | 200–1000 Da |
| curtain gas (nitrogen) | 25 L/min | ||
| ion spray voltage floating | +5500 V | ||
| V Potassium Biphthalate Buffer–0.1 M [mL] | V Hydrochloric Acid–0.1 M [mL] | V Sodium Hydroxide–0.1 M [mL] | pH Measured | |
|---|---|---|---|---|
| pH 3 | 5 | 2.032 | - | 3.06 |
| pH 4 | 5 | - | 0.04 | 4.02 |
| pH 5 | 5 | - | 2.385 | 5.04 |
| Experiments Parameters | Mass Range Parameters | ||
|---|---|---|---|
| gas temperature | 400 °C | MS 1 | |
| ion source gas 1 (nitrogen) | 50 L/min | collision energy | 10 V |
| ion source gas 2 (nitrogen | 55 L/min | declustering potential | 80 V |
| curtain gas (nitrogen) | 45 L/min | mass range | 100–800 Da |
| ion spray voltage floating | +5500 V | MS2 | |
| collision energy | 85 V | ||
| collision energy spread | 20 V | ||
| declustering potential | 80 V | ||
| mass range | 50–800 Da | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotthoff, L.; Lisec, J.; Schwerdtle, T.; Koch, M. Prediction of Transformation Products of Monensin by Electrochemistry Compared to Microsomal Assay and Hydrolysis. Molecules 2019, 24, 2732. https://doi.org/10.3390/molecules24152732
Kotthoff L, Lisec J, Schwerdtle T, Koch M. Prediction of Transformation Products of Monensin by Electrochemistry Compared to Microsomal Assay and Hydrolysis. Molecules. 2019; 24(15):2732. https://doi.org/10.3390/molecules24152732
Chicago/Turabian StyleKotthoff, Lisa, Jan Lisec, Tanja Schwerdtle, and Matthias Koch. 2019. "Prediction of Transformation Products of Monensin by Electrochemistry Compared to Microsomal Assay and Hydrolysis" Molecules 24, no. 15: 2732. https://doi.org/10.3390/molecules24152732





