Preparation of a Novel Lignin Nanosphere Adsorbent for Enhancing Adsorption of Lead
Abstract
:1. Introduction
2. Experimental
2.1. Materials
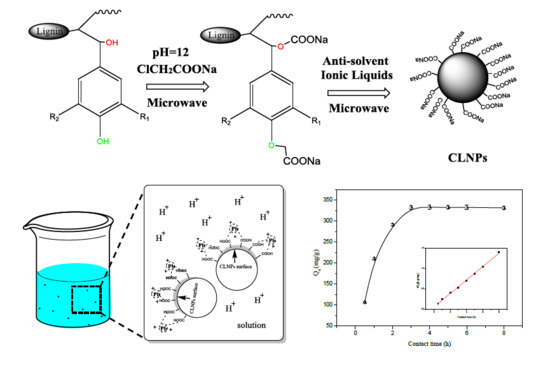
2.2. Preparation of CLNPs
2.3. Characterizations
2.4. Adsorption
3. Results and Discussion
3.1. Characterizations
3.2. Adsorption Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klapiszewski, Ł.; Siwińskastefańska, K.; Kołodyńska, D. Preparation and characterization of novel TiO2/lignin and TiO2-SiO2/lignin hybrids and their use as functional biosorbents for Pb(II). Chem. Eng. J. 2017, 314, 169–181. [Google Scholar] [CrossRef]
- Dang, V.Q.; Kim, J.K.; Sarawade, P.B.; Dang, H.T.; Kim, H.T. Preparation of amino-functionalized silica for copper removal from an aqueous solution. J. Ind. Eng. Chem. 2012, 18, 83–87. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Da̧browski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 0–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, F.; Ju, X.J.; Xie, R.; Sun, Y.M.; Wang, W.; Chu, L.Y. Gating membranes for water treatment: Detection and removal of trace Pb2+ ions based on molecular recognition and polymer phase transition. J. Mater. Chem. A 2013, 1, 9659–9671. [Google Scholar] [CrossRef]
- Li, Z.; Ge, Y. Application of Lignin and Its Derivatives in Adsorption of Heavy Metal Ions in Water: A Review. ACS Sustainable Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Yang, S.; Kim, H.; Narayanan, S.; Mckay, I.S.; Wang, E.N. Dimensionality effects of carbon-based thermal additives for microporous adsorbents. Mater. Des. 2015, 85, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Li, Q.; Li, Z. Lignin microspheres: An effective and recyclable natural polymer-based adsorbent for lead ion removal. Mater. Des. 2016, 95, 141–147. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, Y.; Li, J.; Lei, Y.; Hamel, J.; Giummarella, N.; Henriksson, G.; Zhang, L.; Zhu, H. Bioinspired Ultrastable Lignin Cathode via Graphene Reconfiguration for Energy Storage. ACS Sustainable Chem. Eng. 2017, 5, 3553–3561. [Google Scholar] [CrossRef]
- Zhang, X.; Tu, M.; Paice, M.G. Routes to Potential Bioproducts from Lignocellulosic Biomass Lignin and Hemicelluloses. BioEnergy Res. 2011, 4, 246–257. [Google Scholar] [CrossRef]
- Varanasi, P.; Singh, P.; Auer, M.; Adams, P.D.; Simmons, B.A.; Singh, S. Survey of renewable chemicals produced from lignocellulosic biomass during ionic liquid pretreatment. Biotechnol. Biofuels 2013, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, J.; Li, Q.; Ge, Y. Enhancing antioxidant performance of lignin by enzymatic treatment with laccase. ACS Sustainable Chem. Eng. 2018, 6, 2591–2595. [Google Scholar] [CrossRef]
- Li, Z.; Yan, K.; Ge, Y. Synthesis of porous lignin xanthate resin for Pb2+ removal from aqueous solution. Chem. Eng. J. 2015, 270, 229–234. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, S.Z.; Guo, X.Y.; Huang, H.L. Adsorption of chromium(III) on lignin. Bioresour. Technol. 2008, 99, 7709–7715. [Google Scholar] [CrossRef] [PubMed]
- Dizhbite, T.; Jashina, L.; Dobele, G.; Andersone, A.; Evtuguin, D.; Bikovens, O.; Telysheva, G. Polyoxometalate (POM)-aided modification of lignin from wheat straw biorefinery. Holzforschung 2013, 67, 539–547. [Google Scholar] [CrossRef]
- Peternele, W.S.; Winkler-Hechenleitner, A.A.E.; Gomez Pineda, A. Adsorption of Cd(II) and Pb(II) onto functionalized formic lignin from sugar cane bagasse. Bioresour. Technol. 1999, 68, 95–100. [Google Scholar] [CrossRef]
- Li, Z.; Ge, Y.; Liang, W. Fabrication of a green porous lignin-based sphere for the removal of lead ions from aqueous media. J. Hazard. Mater. 2015, 285, 77–83. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Hou, Y. A simple environment-friendly process for preparing high-concentration alkali lignin nanospheres. Eur. Polym. J. 2019, 112, 15–23. [Google Scholar] [CrossRef]
- Peng, W.X.; Ren, J.L.; Zhong, L.X.; Cao, X.F.; Sun, R.C. Microwave-induced synthesis of carboxymethyl hemicelluloses and their rheological properties. J. Agric. Food Chem. 2011, 59, 570–576. [Google Scholar] [CrossRef]
- Cerrutti, B.M.; Souza, C.S.D.; Castellan, A.; Ruggieroba, R. Carboxymethyl lignin as stabilizing agent in aqueous ceramic suspensions. Ind. Crops Prod. 2012, 36, 108–115. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Hou, Y. Basicity Characterization of Imidazolyl Ionic Liquids and Their Application for Biomass Dissolution. Int. J. Chem. Eng. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- You, T.T.; Mao, J.Z.; Yuan, T.Q.; Wen, J.L.; Xu, F. Structural Elucidation of the Lignins from Stems and Foliage of Arundo donax Linn. J. Agric. Food Chem. 2013, 61, 5361–5370. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cao, Y.; Wang, X.; Castro, M.A.; Fan, Q. Rod-shape porous carbon derived from aniline modified lignin for symmetric supercapacitors. J. Power Sources 2016, 307, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Xiong, F.; Chu, F.; Li, G.; Wang, S.; Qin, T.; Han, Y.; Chen, Y. Preparation and formation mechanism of size-controlled lignin nanospheres by self-assembly. Ind. Crops Prod. 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, X.; Xin, J.; Liu, G.; Chen, J.; Wu, G.; Liu, T.; Zhang, J.; Kong, Z. Thiol–Ene Synthesis of Cysteine-Functionalized Lignin for the Enhanced Adsorption of Cu(II) and Pb(II). Ind. Eng. Chem. Res. 2018, 57, 7872–7880. [Google Scholar] [CrossRef]
- Tseng, R.L.; Wu, F.C.; Juang, R.S. Characteristics and applications of the Lagergren’s first-order equation for adsorption kinetics. J. Taiwan Inst. Chem. Eng. 2010, 41, 661–669. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Sorption of dyes and copper ions onto biosorbents. Process Biochem. 2003, 38, 1047–1061. [Google Scholar] [CrossRef]
- Ulla, W.; Carsten, H.; Gerhard, F.; Rainer, S. Removal of heavy metals from mine waters by natural zeolites. Environ. Sci. Technol. 2005, 39, 4606–4613. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Krukowska, J.; Thomas, P. Comparison of Sorption and Desorption Studies of Heavy Metal Ions From Biochar and Commercial Active Carbon. Chem. Eng. J. 2017, 307, 353–363. [Google Scholar] [CrossRef]
- Liu, B.; Chen, W.; Peng, X.; Cao, Q.; Wang, Q.; Wang, D.; Meng, X.; Yu, G. Biosorption of lead from aqueous solutions by ion-imprinted tetraethylenepentamine modified chitosan beads. Int. J. Biol. Macromol. 2016, 86, 562–569. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Ge, Y. Removal of Lead Ion and Oil Droplet from aqueous solution by Lignin-Grafted Carbon Nanotubes. Chem. Eng. J. 2017, 308, 809–817. [Google Scholar] [CrossRef]
- Armbruster, M.H.; Austin, J.B. The Adsorption of Gases on Plane Surfaces of Mica. J. Am. Chem. Soc. 2002, 60, 467–475. [Google Scholar] [CrossRef]
- Gubbuk, İ.H.; Gup, R.; Kara, H.; Ersoz, M. Adsorption of Cu(II) onto silica gel-immobilized Schiff base derivative. Desalination 2009, 249, 1243–1248. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |












| Lignin | Aliphatic OH (150–145.7 ppm) | Condensed Phenolic OH (145–140.7 ppm) | Guaiacyl and Catechol OH (140–137.6 ppm) | Total Phenolic OH | Carboxyl (136–133.8 ppm) |
|---|---|---|---|---|---|
| (mmol/g) | (mmol/g) | (mmol/g) | (mmol/g) | (mmol/g) | |
| AL | 2.32 | 1.76 | 1.42 | 3.18 | 0.95 |
| CLNPs | 3.02 | 1.60 | 0.43 | 2.03 | 1.80 |
| Adsorbent | T (°C) | Time (min) | pH | K2 | Qe (mg/g) | Reference |
|---|---|---|---|---|---|---|
| Zeolite A | 25 | 30 | 7.5 | - | 213.0 | [28] |
| Commercial active carbon | 25 | 360 | 6.0 | 0.005 | 29.2 | [29] |
| Pb–ITMCB | 40 | 480 | 6.0 | 2.9 × 10−4 | 259.7 | [30] |
| Lignosulfonate sphere | 30 | 150 | 5.0 | 0.02 | 27.1 | [17] |
| Lignin-grafted carbon nanotubes | 25 | 60 | 5.8 | 0.03 | 235.0 | [31] |
| Carboxymethylation formic lignin | 30 | - | 6.0 | - | 107.5 | [16] |
| CLNPs | 30 | 180 | 6.03 | 0.0544 | 333.26 | This study |
| Sample | Pseudo-First-Order Kinetic | Pseudo-Second-Order Kinetic | ||||
|---|---|---|---|---|---|---|
| Qe (mg/g) | K1 (1 min−1) | R2 | Qe (mg/g) | K2 (g/mg min) | R2 | |
| CLNPs | 304.0 | 1.08 | 0.6013 | 350.9 | 0.0544 | 0.9991 |
| Sample | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| B (L/mg) | Qmax (mg/g) | R2 | n | KF (mg/g) | R2 | |
| CLNPs | 0.1734 | 312.5 | 0.973 | 2.86 | 63.09 | 0.448 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Li, Y.; Hou, Y. Preparation of a Novel Lignin Nanosphere Adsorbent for Enhancing Adsorption of Lead. Molecules 2019, 24, 2704. https://doi.org/10.3390/molecules24152704
Liu C, Li Y, Hou Y. Preparation of a Novel Lignin Nanosphere Adsorbent for Enhancing Adsorption of Lead. Molecules. 2019; 24(15):2704. https://doi.org/10.3390/molecules24152704
Chicago/Turabian StyleLiu, Chao, Youming Li, and Yi Hou. 2019. "Preparation of a Novel Lignin Nanosphere Adsorbent for Enhancing Adsorption of Lead" Molecules 24, no. 15: 2704. https://doi.org/10.3390/molecules24152704