Fe-Containing MOFs as Seeds for the Preparation of Highly Active Fe/Al-SBA-15 Catalysts in the N-Alkylation of Aniline
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalytic Characterization
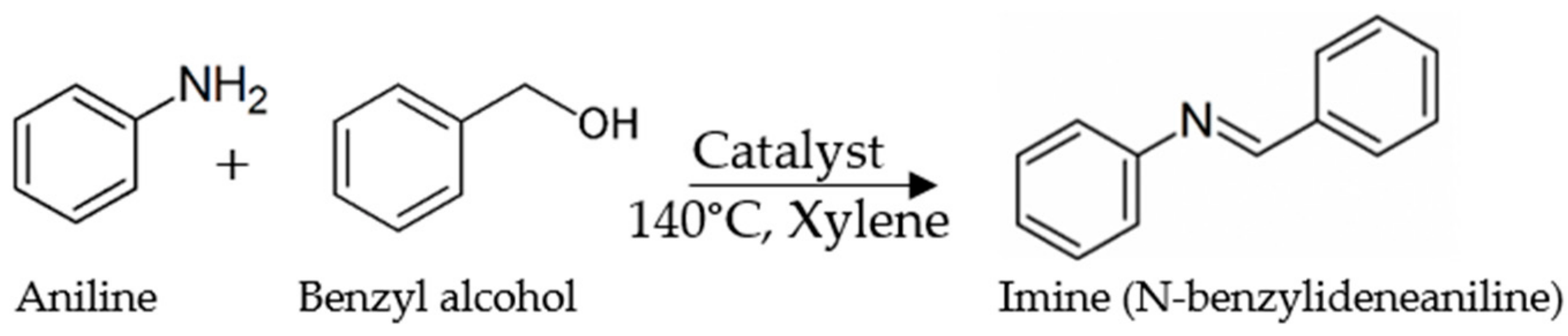
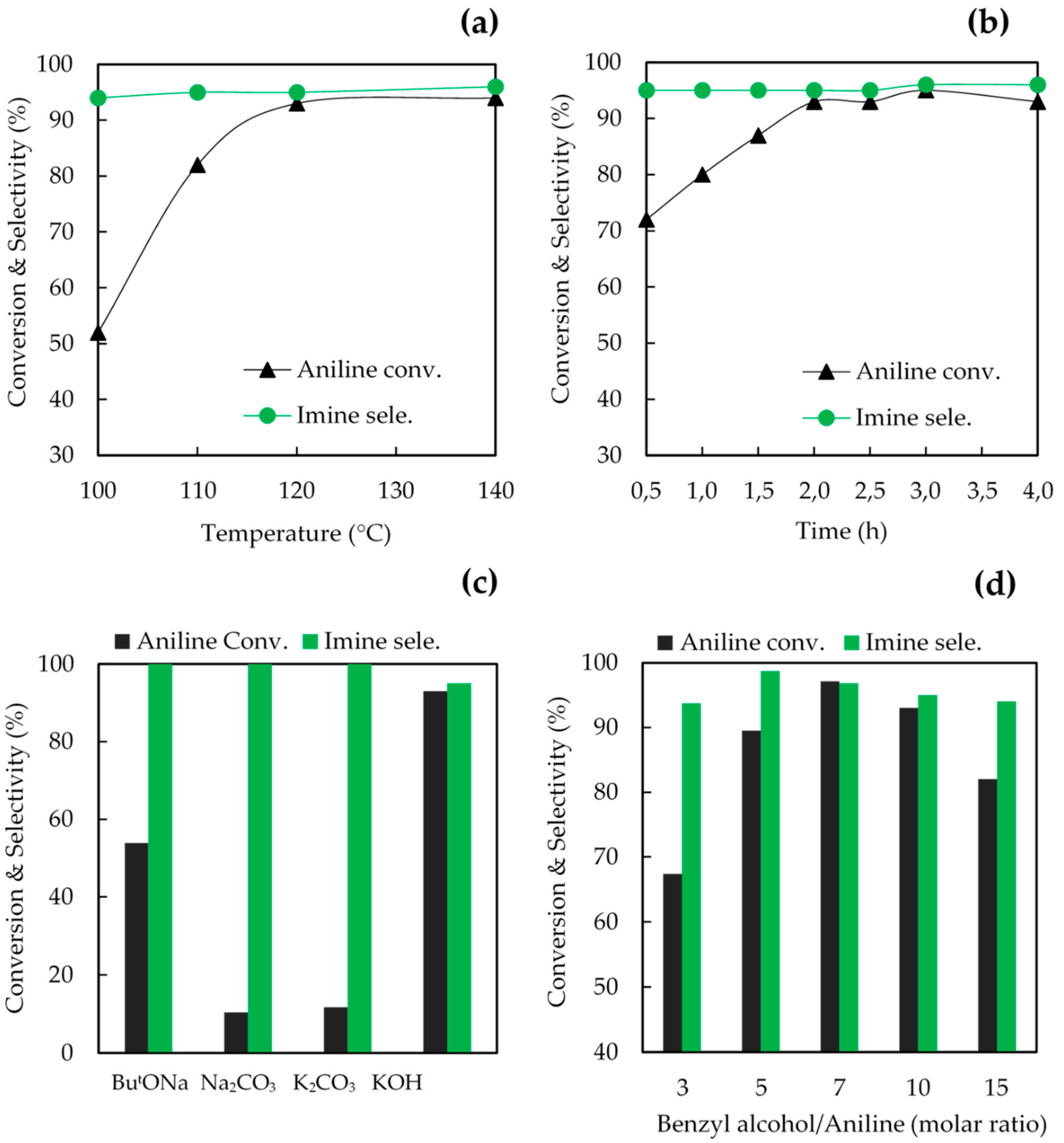
2.2. Catalytic Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.1.1. Synthesis of Al-SBA-15
3.1.2. Synthesis of Catalysts
3.2. Catalyst Characterization
3.3. Reaction Testing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohanty, A.; Roy, S. Re-entry of tin in N-alkylation: First example of a homogeneous heterobimetallic Pd-Sn catalyst for base and additive free alkylation of amine and surrogates with alcohol. Tetrahedron Lett. 2016, 57, 2749–2753. [Google Scholar]
- Luque, R.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Catalytic performance of Al-MCM-41 materials in the N-alkylation of aniline. J. Mol. Catal. A Chem. 2007, 269, 190–196. [Google Scholar] [CrossRef]
- Liu, H.; Chuah, G.K.; Jaenicke, S. N-alkylation of amines with alcohols over alumina-entrapped Ag catalysts using the “borrowing hydrogen” methodology. J. Catal. 2012, 292, 130–137. [Google Scholar]
- Mamidala, R.; Mukundam, V.; Dhanunjayarao, K.; Venkatasubbaiah, K. Cyclometalated palladium pre-catalyst for N-alkylation of amines using alcohols and regioselective alkylation of sulfanilamide using aryl alcohols. Tetrahedron 2017, 73, 2225–2233. [Google Scholar]
- Sun, Y.W.; Lu, X.H.; Wei, X.L.; Zhou, D.; Xia, Q.H. Solvent-free synthesis of imines via N-alkylation of aromatic amines with alcohols over Co2+-exchanged zeolites. Catal. Commun. 2014, 43, 213–217. [Google Scholar]
- Yang, H.; Mao, R.; Luo, C.; Lu, C.; Cheng, G. An efficient homogeneous gold(I) catalyst for N-alkylation of amines with alcohols by hydrogen autotransfer. Tetrahedron 2014, 70, 8829–8835. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Q.; Zhu, X.; Chen, J. Green and scalable aldehyde-catalyzed transition metal-free dehydrative N-alkylation of amides and amines with alcohols. Adv. Synth. Catal. 2013, 355, 73–80. [Google Scholar] [CrossRef]
- Ramachandran, R.; Prakash, G.; Viswanathamurthi, P.; Malecki, J.G. Ruthenium(II) complexes containing phosphino hydrazone/thiosemicarbazone ligand: An efficient catalyst for regioselective N-alkylation of amine via borrowing hydrogen methodology. Inorg. Chim. Acta 2018, 477, 122–129. [Google Scholar]
- Ulu, Ö.D.; Gürbüz, N.; Özdemir, İ. Alkylation of cyclic amines with alcohols catalyzed by Ru(II) complexes bearing N -Heterocyclic carbenes. Tetrahedron 2018, 7, 645–651. [Google Scholar] [CrossRef]
- Yu, X.J.; He, H.Y.; Yang, L.; Fu, H.Y.; Zheng, X.L.; Chen, H.; Li, R.X. Hemilabile N -heterocyclic carbene (NHC)-nitrogen-phosphine mediated Ru (II)-catalyzed N -alkylation of aromatic amine with alcohol efficiently. Catal. Commun. 2017, 95, 54–57. [Google Scholar] [CrossRef]
- Martinez, R.; Ramon, D.J.; Yus, M. Selective N-monoalkylation of aromatic amines with benzylic alcohols by a hydrogen autotransfer process catalyzed by unmodified magnetite. Org. Biomol. Chem. 2009, 7, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.I.; Kanno, S.; Kon, K.; Hakim Siddiki, S.M.A.; Tanaka, H.; Sakata, Y. N-alkylation of ammonia and amines with alcohols catalyzed by Ni-loaded CaSiO3. Catal. Today 2014, 232, 134–138. [Google Scholar] [CrossRef]
- Sun, J.; Jin, X.; Zhang, F.; Hu, W.; Liu, J.; Li, R. Ni–Cu/γ-Al2O3 catalyzed N-alkylation of amines with alcohols. Catal. Commun. 2012, 24, 30–33. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, R. Heterogeneous MOF catalysts for the synthesis of trans-4, 5-diaminocyclopent-2-enones from furfural and secondary amines. Catal. Commun. 2019, 120, 11–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Zhou, H.C. Synthesis of MOFs for heterogeneous catalysis via linker design. Polyhedron 2018, 154, 189–201. [Google Scholar] [CrossRef]
- Xuan, K.; Pu, Y.; Li, F.; Luo, J.; Zhao, N.; Xiao, F. Metal-organic frameworks MOF-808-X as highly efficient catalysts for direct synthesis of dimethyl carbonate from CO2 and methanol. Chin. J. Catal. 2019, 40, 553–566. [Google Scholar] [CrossRef]
- Chen, L.; Luque, R.; Li, Y.W. Encapsulation of metal nanostructures into metal-organic frameworks. Dalton Trans. 2018, 47, 3663–3668. [Google Scholar] [CrossRef]
- Saberi, F.; Rodríguez-Padrón, D.; Doustkhah, E.; Ostovar, S.; Franco, A.; Shaterian, H.R.; Luque, R. Mechanochemically modified aluminosilicates for efficient oxidation of vanillyl alcohol. Catal. Commun. 2019, 118, 65–69. [Google Scholar] [CrossRef]
- Márquez-Medina, M.D.; Rodríguez-Padrón, D.; Balu, A.M.; Romero, A.A.; Muñoz-Batista, M.J.; Luque, R. Mechanochemically synthesized supported magnetic Fe-nanoparticles as catalysts for efficient vanillin production. Catalysts 2019, 9, 290. [Google Scholar] [CrossRef]
- Yepez, A.; Prinsen, P.; Pineda, A.; Balu, A.M.; Garcia, A.; Lam, F.L.Y.; Luque, R. A comprehensive study on the continuous flow synthesis of supported iron oxide nanoparticles on porous silicates and their catalytic applications. React. Chem. Eng. 2018, 3, 757–768. [Google Scholar] [CrossRef]
- Xu, C.P.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Comun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; De, S.; Balu, M.A.; Romero, A.A.; Luque, R. Selective oxidation of isoeugenol to vanillin over mechanochemically synthesized aluminosilicate supported transition metal catalysts. Chem. Sel. 2017, 2, 9546–9551. [Google Scholar] [CrossRef]
- Márquez-Medina, M.D.; Mhadmhan, S.; Balu, A.M.; Romero, A.A.; Luque, R. Post-synthetic mechanochemical incorporation of Al-species into the framework of porous materials: Toward more sustainable redox chemistries. ACS Sustain. Chem. Eng. 2019, 7, 9537–9543. [Google Scholar]
- Liyu, C.; Huirong, C.; Rafael, L.; Yingwei, L. Metal-organic framework encapsulated Pd nanoparticles: Towards advanced heterogeneous catalysts. Chem. Sci. 2014, 5, 3708–3714. [Google Scholar]
- Gonzalez-Arellano, C.; Arancon, R.A.D.; Luque, R. Al-SBA-15 catalysed cross-esterification and acetalisation of biomass-derived platform chemicals. Green Chem. 2014, 16, 4985–4993. [Google Scholar] [CrossRef]
- Cheng, M.; Zhao, H.; Yang, J.; Zhao, J.; Yan, L.; Song, H.; Chou, L. The catalytic dehydrogenation of isobutane and the stability enhancement over Fe incorporated SBA-15. Microporous Mesoporous Mater. 2018, 266, 117–125. [Google Scholar] [CrossRef]
- Zhu, L.; Qu, H.; Zhang, L.; Zhou, Q. Direct synthesis, characterization and catalytic performance of Al–Fe-SBA-15 materials in selective catalytic reduction of NO with NH3. Catal. Commun. 2016, 73, 118–122. [Google Scholar] [CrossRef]
- Sun, B.; Li, L.; Fei, Z.; Gu, S.; Lu, P.; Ji, W. Prehydrolysis approach to direct synthesis of Fe, Al, Cr-incorporated SBA-15 with good hydrothermal stability and enhanced acidity. Microporous Mesoporous Mater. 2014, 186, 14–20. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z. Coupling MOF-based photocatalysis with Pd catalysis over Pd@MIL-100(Fe) for efficient N-alkylation of amines with alcohols under visible light. J. Catal. 2016, 342, 151–157. [Google Scholar] [CrossRef]
- Benzaquén, T.B.; Ochoa Rodriguez, P.A.; Cánepa, A.L.; Casuscelli, S.G.; Elías, V.R.; Eimer, G.A. Heterogeneous Fenton reaction for the treatment of ACE in residual waters of pharmacological origin using Fe-SBA-15 nanocomposites. Mol. Catal. 2018. [Google Scholar] [CrossRef]
- Liang, R.; Shen, L.; Jing, F.; Qin, N.; Wu, L. Preparation of MIL-53(Fe)-reduced graphene oxide nanocomposites by a simple self-assembly strategy for increasing interfacial contact: Efficient visible-light photocatalysts. ACS Appl. Mater. Interfaces 2015, 7, 9507–9515. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.; Ma, Y.; Wan, J.; Wang, Y.; Wang, J.; Brusseau, M.L. Activation performance and mechanism of a novel heterogeneous persulfate catalyst: Metal Organic Framework MIL-53(Fe) with Fe(II)/Fe(III) mixed-valence coordinative unsaturated iron center. Catal. Sci. Technol. 2017, 7, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.; Balu, A.M.; Barrón, V.; Pineda, A.; Coleto, Á.G.; Romero, A.Á.; Luque, R. Solventless mechanochemical synthesis of magnetic functionalized catalytically active mesoporous SBA-15 nanocomposites. J. Mater. Chem. A 2014, 2, 387–393. [Google Scholar] [CrossRef]
- Pineda, A.; Balu, M.A.; Campelo, M.J.; Romero, A.A.; Carmona, D.; Balas, F.; Santamaria, J.; Luque, R. A Dry milling approach for the synthesis of highly active nanoparticles supported on porous materials. ChemSusChem 2011, 4, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
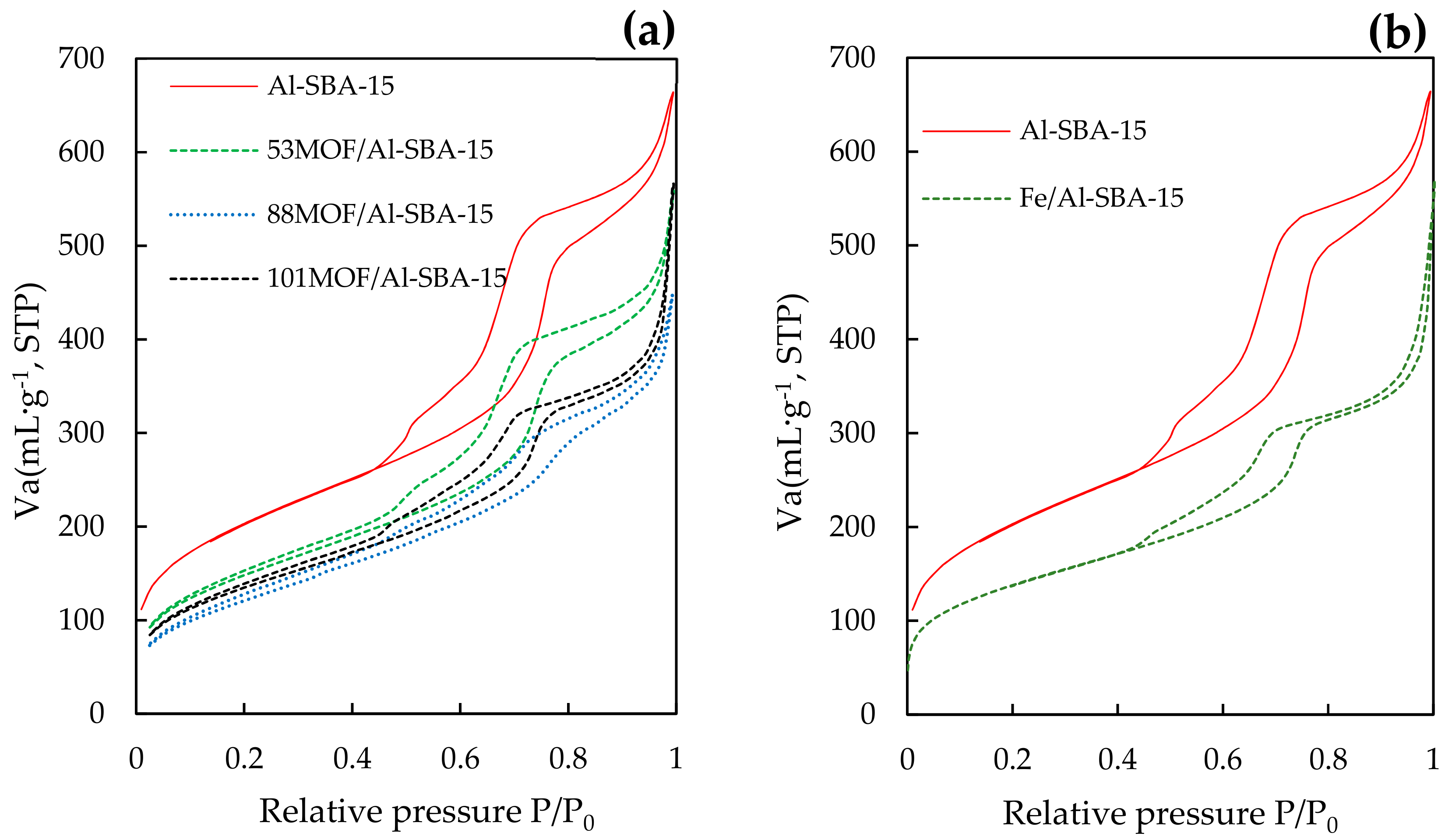
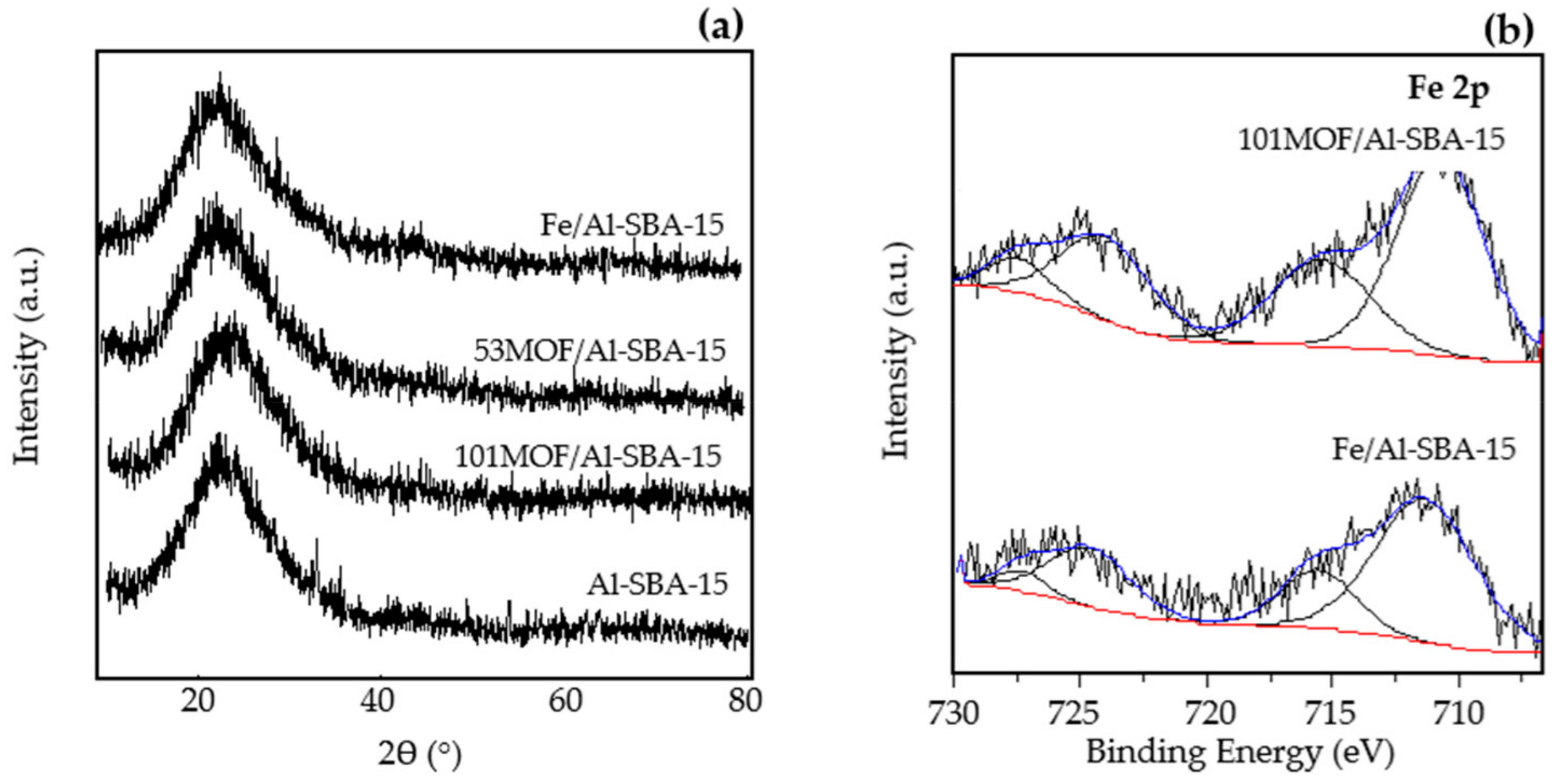
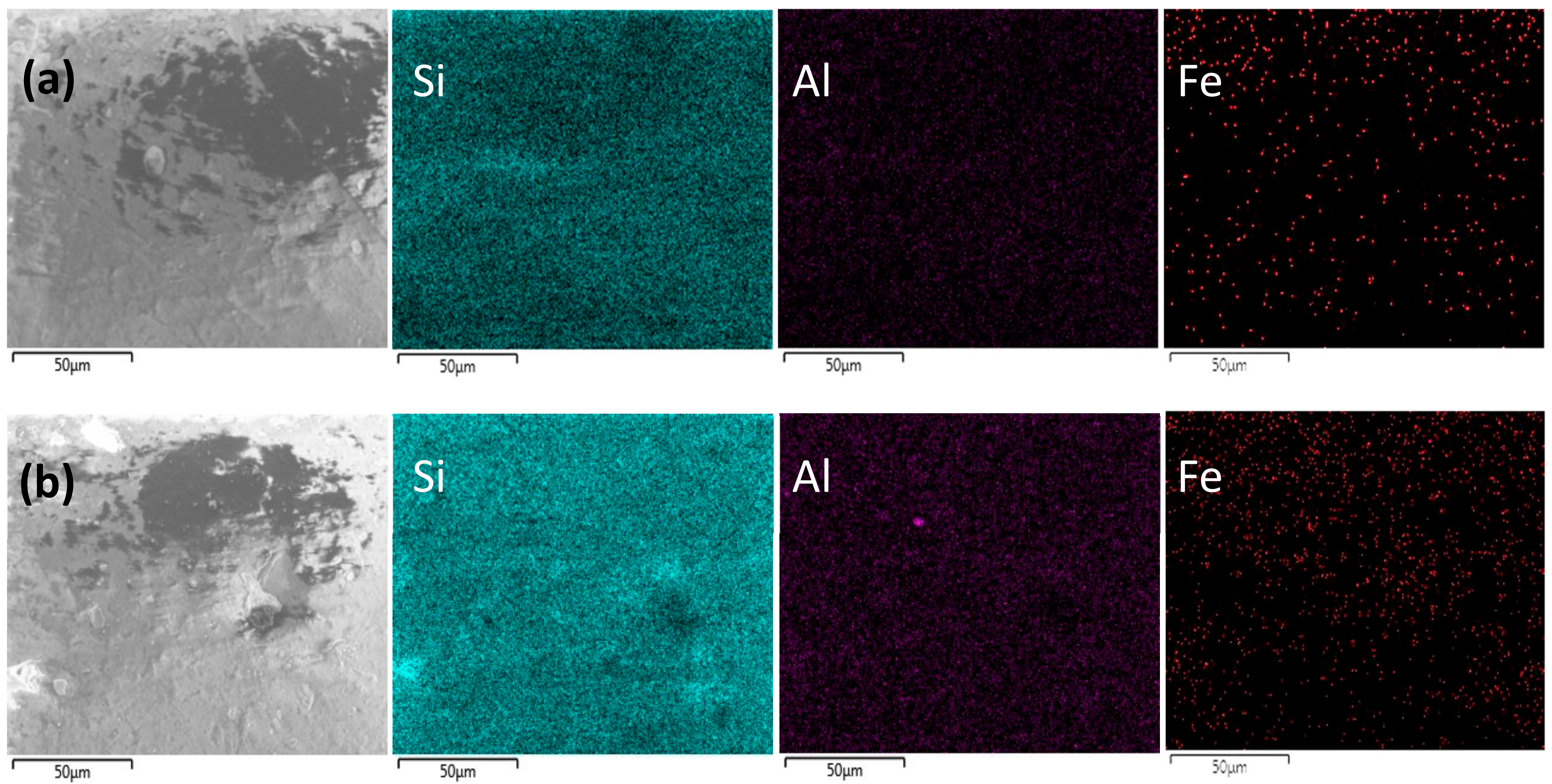

| Samples | SBET a (m2·g−1) | Dp b (nm) | Vp c (mL·g−1) | Fe Content d (wt.%) | Acidity e (µmol·g−1) | |
|---|---|---|---|---|---|---|
| PY | DMPY | |||||
| Al-SBA-15 | 736 | 8.5 | 0.8 | - | 82 | 61 |
| 53MOF/Al-SBA-15 | 538 | 3.2 | 0.8 | 0.22/0.21 * | 220 | 124 |
| 88MOF/Al-SBA-15 | 475 | 3.0 | 0.7 | 0.18 | 219 | 72 |
| 101MOF/Al-SBA-15 | 490 | 3.7 | 0.8 | 0.30 | 94 | 51 |
| Fe/Al-SBA-15 | 500 | 6.2 | 0.5 | 1.00 | 198 | 30 |
| Samples | Conversion (%) | Imine Select (%) | Imine Yield (%) |
|---|---|---|---|
| Blank (no catalyst) | 54 | 76 | 41 |
| Al-SBA-15 | 61 | 71 | 43 |
| FeCl3 | 75 | 88 | 66 |
| 53MOF | 77 | 82 | 63 |
| 88MOF | 73 | 87 | 64 |
| 101MOF | 78 | 80 | 62 |
| 53MOF/Al-SBA-15 | 94 | 96 | 90 |
| 88MOF/Al-SBA-15 | 90 | 94 | 85 |
| 101MOF/Al-SBA-15 | 85 | 93 | 79 |
| Fe/Al-SBA-15 | 77 | 98 | 76 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhadmhan, S.; Marquez-Medina, M.D.; Romero, A.A.; Reubroycharoen, P.; Luque, R. Fe-Containing MOFs as Seeds for the Preparation of Highly Active Fe/Al-SBA-15 Catalysts in the N-Alkylation of Aniline. Molecules 2019, 24, 2695. https://doi.org/10.3390/molecules24152695
Mhadmhan S, Marquez-Medina MD, Romero AA, Reubroycharoen P, Luque R. Fe-Containing MOFs as Seeds for the Preparation of Highly Active Fe/Al-SBA-15 Catalysts in the N-Alkylation of Aniline. Molecules. 2019; 24(15):2695. https://doi.org/10.3390/molecules24152695
Chicago/Turabian StyleMhadmhan, Sareena, Maria Dolores Marquez-Medina, Antonio A. Romero, Prasert Reubroycharoen, and Rafael Luque. 2019. "Fe-Containing MOFs as Seeds for the Preparation of Highly Active Fe/Al-SBA-15 Catalysts in the N-Alkylation of Aniline" Molecules 24, no. 15: 2695. https://doi.org/10.3390/molecules24152695
APA StyleMhadmhan, S., Marquez-Medina, M. D., Romero, A. A., Reubroycharoen, P., & Luque, R. (2019). Fe-Containing MOFs as Seeds for the Preparation of Highly Active Fe/Al-SBA-15 Catalysts in the N-Alkylation of Aniline. Molecules, 24(15), 2695. https://doi.org/10.3390/molecules24152695






