Changes in Content of Polyphenols and Ascorbic Acid in Leaves of White Cabbage after Pest Infestation
Abstract
:1. Introduction
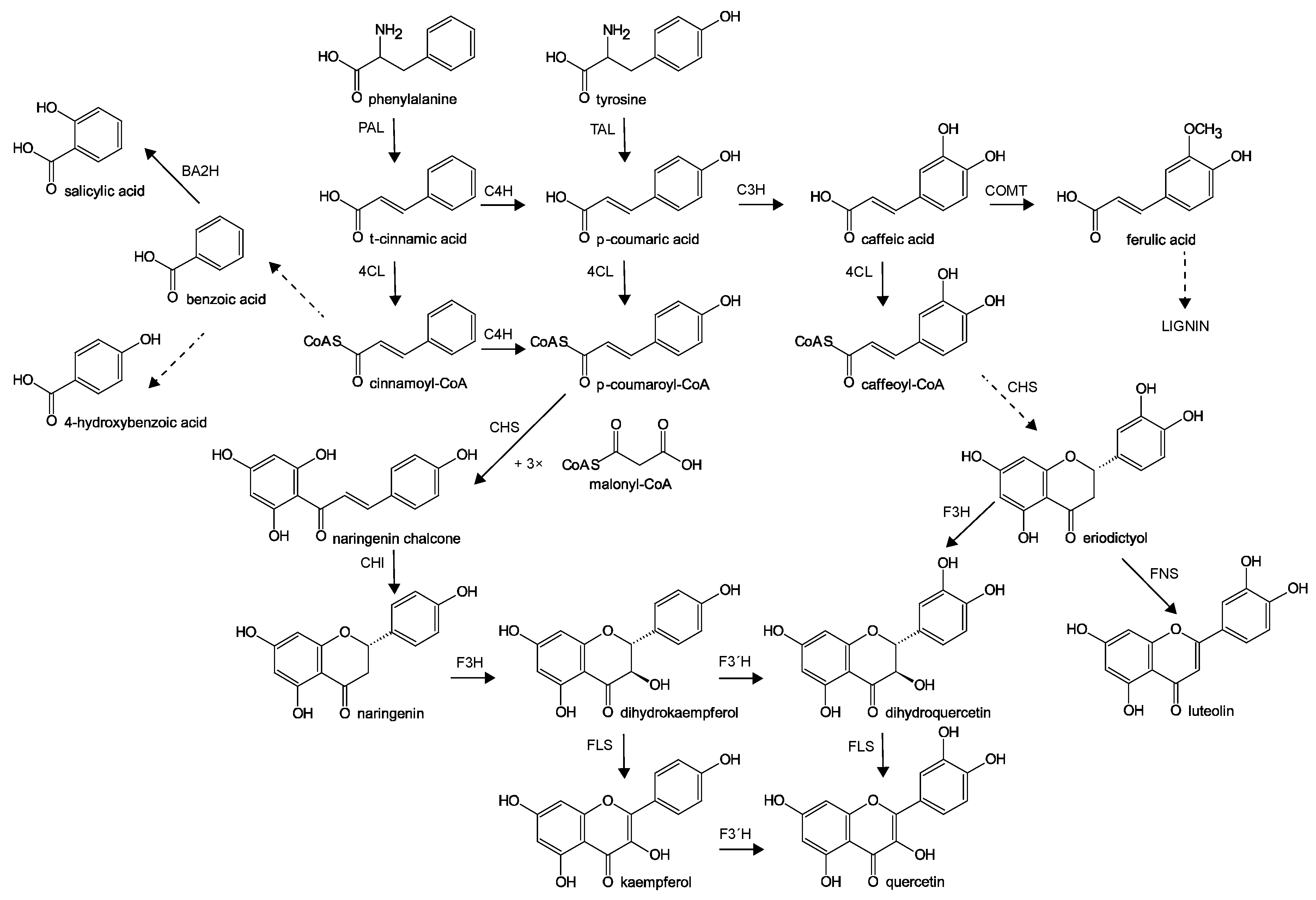
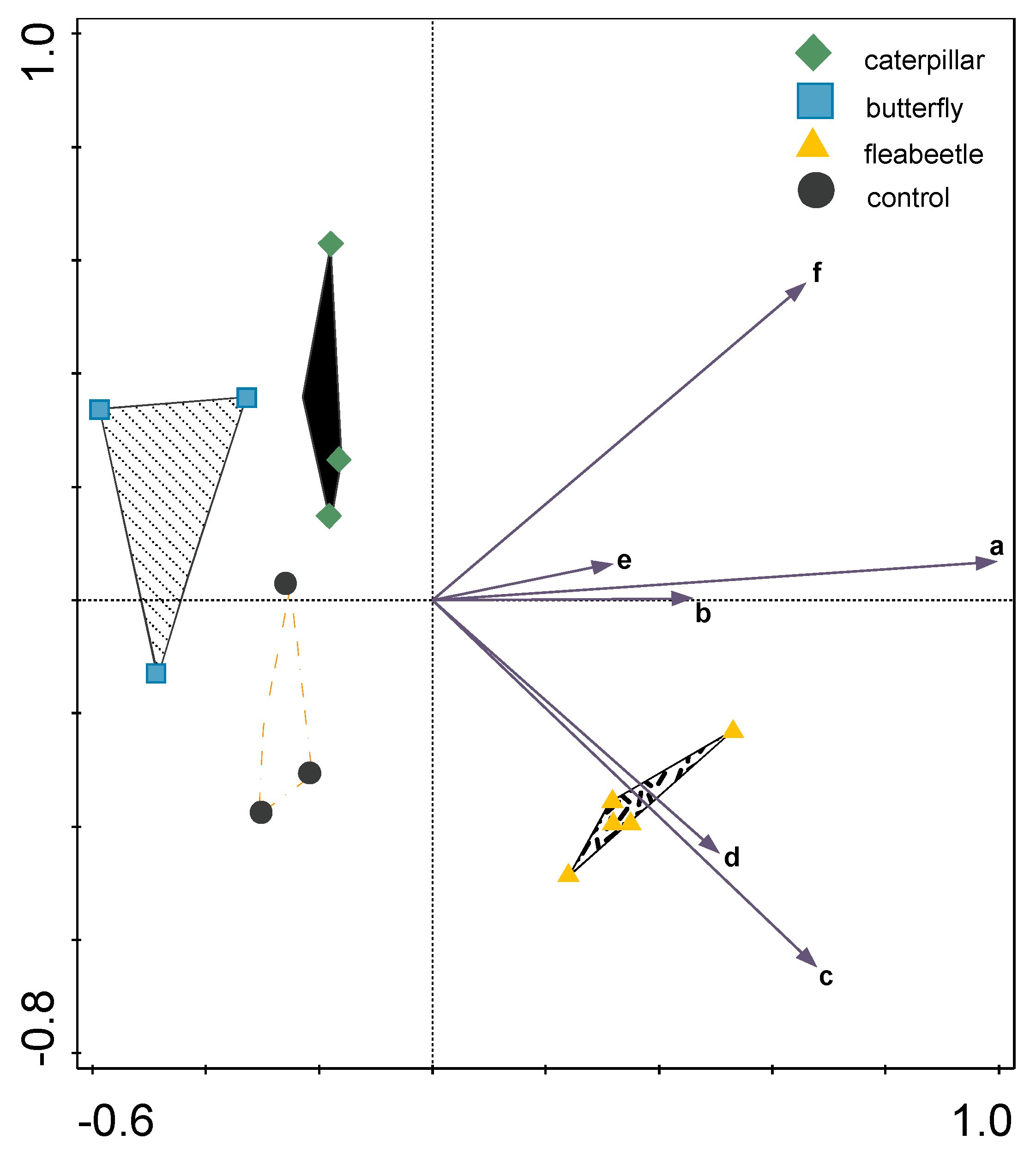
2. Results and Discussion
3. Materials and Methods
3.1. Cultivation
- C—control (untreated plants)
- WBA—boxes with adult butterflies (five butterflies per box) to determine the influence of oviposition and feeding by hatched caterpillars
- WBC—boxes with caterpillars (six caterpillars per box) in 2nd instar
- FB50—boxes with 50 flea beetles per box
- FB100—boxes with 100 flea beetles per box.
3.2. Quantification of Stress-Related Compounds and Ascorbic Acid
3.3. Quantification of Phenols, Flavonoids, and Phenolic Acids
3.4. Quantification of Amino Acids
3.5. Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahuja, I.; Rohloff, J.; Bones, A.M. Defence Mechanisms of Brassicaceae: Implications for Plant-Insect Interactions and Potential for Integrated Pest Management. In Sustainable Agriculture Volume 2; Lichtfouse, E., Hamelin, M., Navarrete, M., Debaeke, P., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 623–670. ISBN 9789048126651. [Google Scholar]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Metspalu, L.; Kruus, E.; Ploomi, A.; Williams, I.H.; Hiiesaar, K.; Jõgar, K.; Veromann, E.; Mänd, M. Flea beetle (Chrysomelidae: Alticinae) species composition and abundance in different cruciferous oilseed crops and the potential for a trap crop system. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2014, 64, 572–582. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S. Plant secondary metabolites (PSMs) of Brassicaceae and their role in plant defense against insect herbivores—A review. J. Appl. Nat. Sci. 2017, 9, 508–519. [Google Scholar] [CrossRef]
- Harborne, J.B.; Grayer, R.J. The Flavonoids Advances in Research Since 1986; Routledge: Abingdon, UK, 2017; ISBN 9780203736692. [Google Scholar]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiselhardt, S.; Yoneya, K.; Blenn, B.; Drechsler, N.; Gershenzon, J.; Kunze, R.; Hilker, M. Egg Laying of Cabbage White Butterfly (Pieris brassicae) on Arabidopsis thaliana Affects Subsequent Performance of the Larvae. PLoS ONE 2013, 8, e59661. [Google Scholar] [CrossRef] [PubMed]
- Kos, M.; Houshyani, B.; Wietsma, R.; Kabouw, P.; Vet, L.E.M.; van Loon, J.J.A.; Dicke, M. Effects of glucosinolates on a generalist and specialist leaf-chewing herbivore and an associated parasitoid. Phytochemistry 2012, 77, 162–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreres, F.; Fernandes, F.; Pereira, D.M.; Pereira, J.A.; Valentão, P.; Andrade, P.B. Phenolics Metabolism in Insects: Pieris brassicae—Brassica oleracea var. costata Ecological Duo. J. Agric. Food Chem. 2009, 57, 9035–9043. [Google Scholar] [CrossRef]
- Ferreres, F.; Valentão, P.; Pereira, J.A.; Bento, A.; Noites, A.; Seabra, R.M.; Andrade, P.B. HPLC-DAD-MS/MS-ESI Screening of Phenolic Compounds in Pieris brassicae L. Reared on Brassica rapa var. rapa L. J. Agric. Food Chem. 2008, 56, 844–853. [Google Scholar] [CrossRef]
- Moloi, M.J.; van der Westhuizen, A.J. The reactive oxygen species are involved in resistance responses of wheat to the Russian wheat aphid. J. Plant Physiol. 2006, 163, 1118–1125. [Google Scholar] [CrossRef]
- Little, D.; Gouhier-Darimont, C.; Bruessow, F.; Reymond, P. Oviposition by Pierid Butterflies Triggers Defense Responses in Arabidopsis. Plant Physiol. 2006, 143, 784–800. [Google Scholar] [CrossRef] [Green Version]
- Kunierczyk, A.; Winge, P.; Jrstad, T.S.; Troczyska, J.; Rossiter, J.T.; Bones, A.M.; Kuśnierczyk, A.; Jørstad, T.S.; Troczyńska, J. Towards global understanding of plant defence against aphids timing and dynamics of early Arabidopsis defence responses to cabbage aphid (Brevicoryne brassicae) attack. Plant Cell Environ. 2008, 31, 1097–1115. [Google Scholar] [CrossRef]
- Kempema, L.A.; Cui, X.; Holzer, F.M.; Walling, L.L. Arabidopsis Transcriptome Changes in Response to Phloem-Feeding Silverleaf Whitefly Nymphs. Similarities and Distinctions in Responses to Aphids. Plant Physiol. 2006, 143, 849–865. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.; Mir, G.M.; Rouf, A.; War, A.R.; Hussain, B. Herbivore and phytohormone induced defensive response in kale against cabbage butterfly, Pieris brassicae Linn. J. Asia-Pac. Entomol. 2018, 21, 367–373. [Google Scholar] [CrossRef]
- Chen, Y.; Ni, X.; Buntin, G.D. Physiological, Nutritional, and Biochemical Bases of Corn Resistance to Foliage-Feeding Fall Armyworm. J. Chem. Ecol. 2009, 35, 297–306. [Google Scholar] [CrossRef]
- He, J.; Chen, F.; Chen, S.; Lv, G.; Deng, Y.; Fang, W.; Liu, Z.; Guan, Z.; He, C. Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. J. Plant Physiol. 2011, 168, 687–693. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Tsai, C.J.; Harding, S.A.; Tschaplinski, T.J.; Lindroth, R.L.; Yuan, Y. Genome-wide analysis of the structural genes regulating defense phenylpropanoid metabolism in Populus. New Phytol. 2006, 172, 47–62. [Google Scholar] [CrossRef]
- Morreel, K.; Goeminne, G.; Storme, V.; Sterck, L.; Ralph, J.; Coppieters, W.; Breyne, P.; Steenackers, M.; Georges, M.; Messens, E.; et al. Genetical metabolomics of flavonoid biosynthesis in Populus: A case study. Plant J. 2006, 47, 224–237. [Google Scholar] [CrossRef]
- Leon, J.; Shulaev, V.; Yalpani, N.; Lawton, M.A.; Raskin, I. Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proc. Natl. Acad. Sci. USA 1995, 92, 10413–10417. [Google Scholar] [CrossRef]
- Khattab, H. The Defense Mechanism of Cabbage Plant Against Phloem-Sucking Aphid (Brevicoryne brassicae L.). Aust. J. Basic Appl. Sci. 2007, 1, 56–62. [Google Scholar]
- Bhonwong, A.; Stout, M.J.; Attajarusit, J.; Tantasawat, P. Defensive role of tomato polyphenol oxidases against cotton bollworm helicoverpa armigera and beet armyworm spodoptera exigua. J. Chem. Ecol. 2009. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, Y.P.; Singh, S.P.; Singh, R. Physical and biochemical aspects of host plant resistance to mustard aphid, Lipaphis erysimi (Kaltenbach) in rapeseed-mustard. Arthropod-Plant Interact. 2017, 11, 551–559. [Google Scholar] [CrossRef]
- Palial, S.; Kumar, S.; Sharma, S. Biochemical changes in the Brassica juncea-fruticulosa introgression lines after Lipaphis erysimi (Kaltenbach) infestation. Phytoparasitica 2018, 46, 499–509. [Google Scholar] [CrossRef]
- Onyilagha, J.C.; Gruber, M.Y.; Hallett, R.H.; Holowachuk, J.; Buckner, A.; Soroka, J.J. Constitutive flavonoids deter flea beetle insect feeding in Camelina sativa L. Biochem. Syst. Ecol. 2012, 42, 128–133. [Google Scholar] [CrossRef]
- Bi, J.L.; Murphy, J.B.; Felton, G.W. Antinutritive and Oxidative Components as Mechanisms of Induced Resistance in Cotton to Helicoverpa zea. J. Chem. Ecol. 1997, 23, 97–117. [Google Scholar] [CrossRef]
- Jiang, Y. Oxidative interactions between the spotted alfalfa aphid (Therioaphis trifolii maculata) (Homoptera: Aphididae) and the host plant Medicago sativa. Bull. Entomol. Res. 1996, 86, 533–540. [Google Scholar] [CrossRef]
- Broekgaarden, C.; Poelman, E.H.; Steenhuis, G.; Voorrips, R.E.; Dicke, M.; Vosman, B. Genotypic variation in genome-wide transcription profiles induced by insect feeding: Brassica oleracea—Pieris rapae interactions. BMC Genom. 2007, 8, 239. [Google Scholar] [CrossRef]
- Goggin, F.L.; Avila, C.A.; Lorence, A. Vitamin C content in plants is modified by insects and influences susceptibility to herbivory. BioEssays 2010, 32, 777–790. [Google Scholar] [CrossRef]
- Hilker, M.; Fatouros, N.E. Plant Responses to Insect Egg Deposition. Annu. Rev. Entomol. 2015, 60, 493–515. [Google Scholar] [CrossRef]
- Walker, K.S.; Bray, J.L.; Lehman, M.E.; Lentz-Ronning, A.J. Effects of host plant phenolic acids and nutrient status on oviposition and feeding of the cabbage white butterfly, Pieris rapae. Bios 2014, 85, 95–101. [Google Scholar] [CrossRef]
- Usha Rani, P.; Pratyusha, S. Role of castor plant phenolics on performance of its two herbivores and their impact on egg parasitoid behaviour. BioControl 2014, 59, 513–524. [Google Scholar] [CrossRef]
- Bruessow, F.; Gouhier-Darimont, C.; Buchala, A.; Metraux, J.P.; Reymond, P. Insect eggs suppress plant defence against chewing herbivores. Plant J. 2010, 62, 876–885. [Google Scholar] [CrossRef] [Green Version]
- Van Poecke, R.M.P.; Posthumus, M.A.; Dicke, M. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: Chemical, behavioral, and gene-expression analysis. J. Chem. Ecol. 2001, 27, 1911–1928. [Google Scholar] [CrossRef]
- Dučaiová, Z.; Sajko, M.; Mihaličová, S.; Repčák, M. Dynamics of accumulation of coumarin-related compounds in leaves of Matricaria chamomilla after methyl jasmonate elicitation. Plant Growth Regul. 2016, 79, 81–94. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Simek, J.; Kovalikova, Z.; Dohnal, V.; Tuma, J. Accumulation of cadmium in potential hyperaccumulators Chlorophytum comosum and Callisia fragrans and role of organic acids under stress conditions. Environ. Sci. Pollut. Res. 2018, 25, 28129–28139. [Google Scholar] [CrossRef]
- Sajko, M.; Kovalíková-Dučaiová, Z.; Paľove-Balang, P.; Repčák, M. Physiological Responses of Matricaria chamomilla to Potassium Nitrate Supply and Foliar Application of Ethephon. J. Plant Growth Regul. 2018, 37, 360–369. [Google Scholar] [CrossRef]
- Smilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781139627061. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors of Department of Biology. |
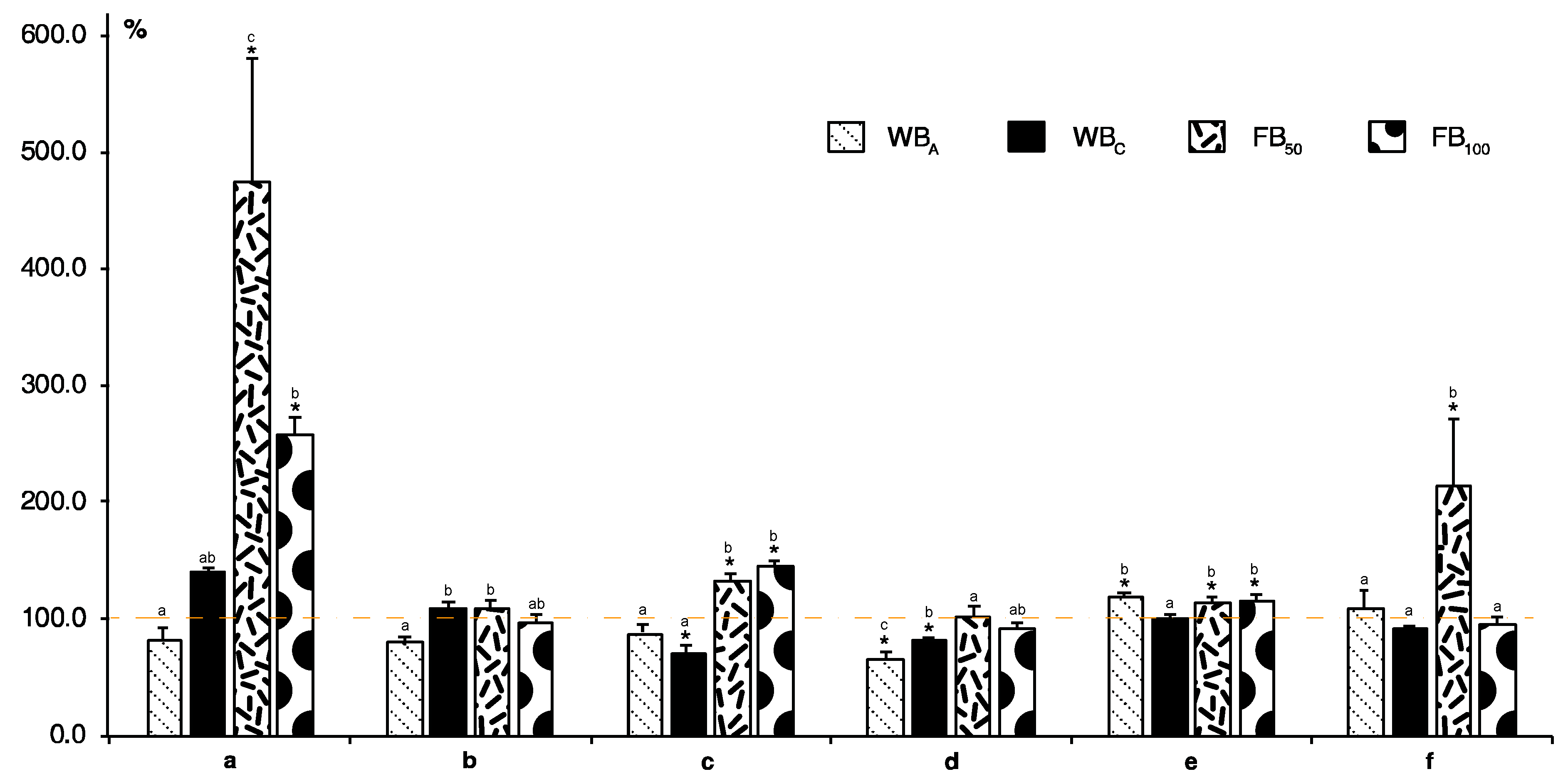


| Control | WBA | WBC | FB50 | FB100 |
|---|---|---|---|---|
| 0.034 ± 0.003 a | 0.052 ± 0.004 a | 0.054 ± 0.013 a | 0.116 ± 0.006 b | 0.155 ± 0.033 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalikova, Z.; Kubes, J.; Skalicky, M.; Kuchtickova, N.; Maskova, L.; Tuma, J.; Vachova, P.; Hejnak, V. Changes in Content of Polyphenols and Ascorbic Acid in Leaves of White Cabbage after Pest Infestation. Molecules 2019, 24, 2622. https://doi.org/10.3390/molecules24142622
Kovalikova Z, Kubes J, Skalicky M, Kuchtickova N, Maskova L, Tuma J, Vachova P, Hejnak V. Changes in Content of Polyphenols and Ascorbic Acid in Leaves of White Cabbage after Pest Infestation. Molecules. 2019; 24(14):2622. https://doi.org/10.3390/molecules24142622
Chicago/Turabian StyleKovalikova, Zuzana, Jan Kubes, Milan Skalicky, Nikola Kuchtickova, Lucie Maskova, Jiri Tuma, Pavla Vachova, and Vaclav Hejnak. 2019. "Changes in Content of Polyphenols and Ascorbic Acid in Leaves of White Cabbage after Pest Infestation" Molecules 24, no. 14: 2622. https://doi.org/10.3390/molecules24142622





