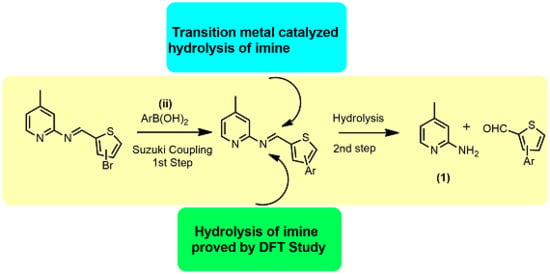
Role of Pyridine Nitrogen in Palladium-Catalyzed Imine Hydrolysis: A Case Study of (E)-1-(3-bromothiophen-2-yl)-N-(4-methylpyridin-2-yl)methanimine
Abstract
:1. Introduction
2. Results and Discussion
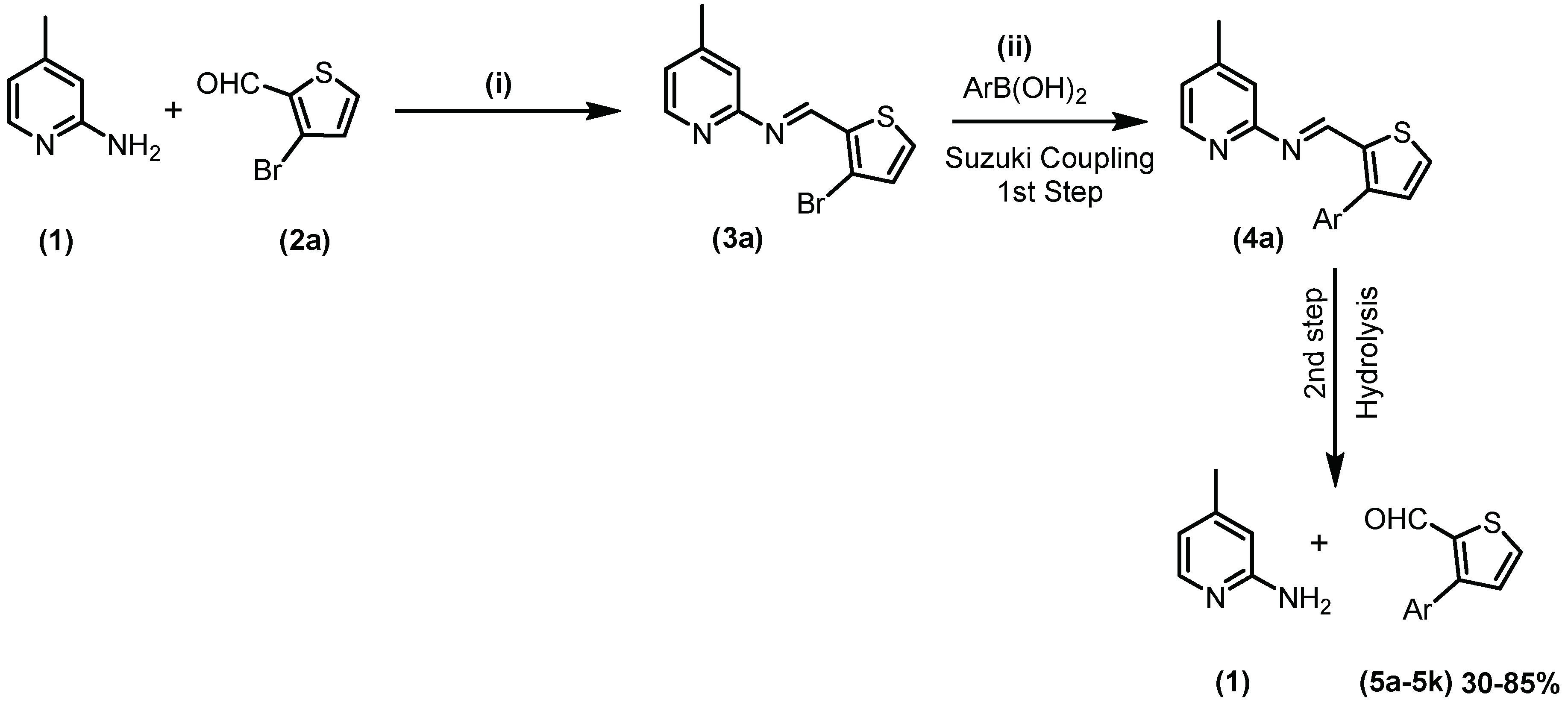
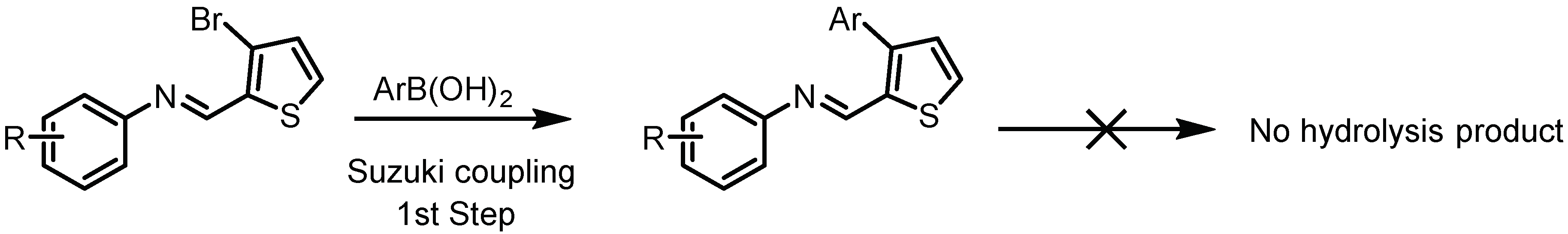
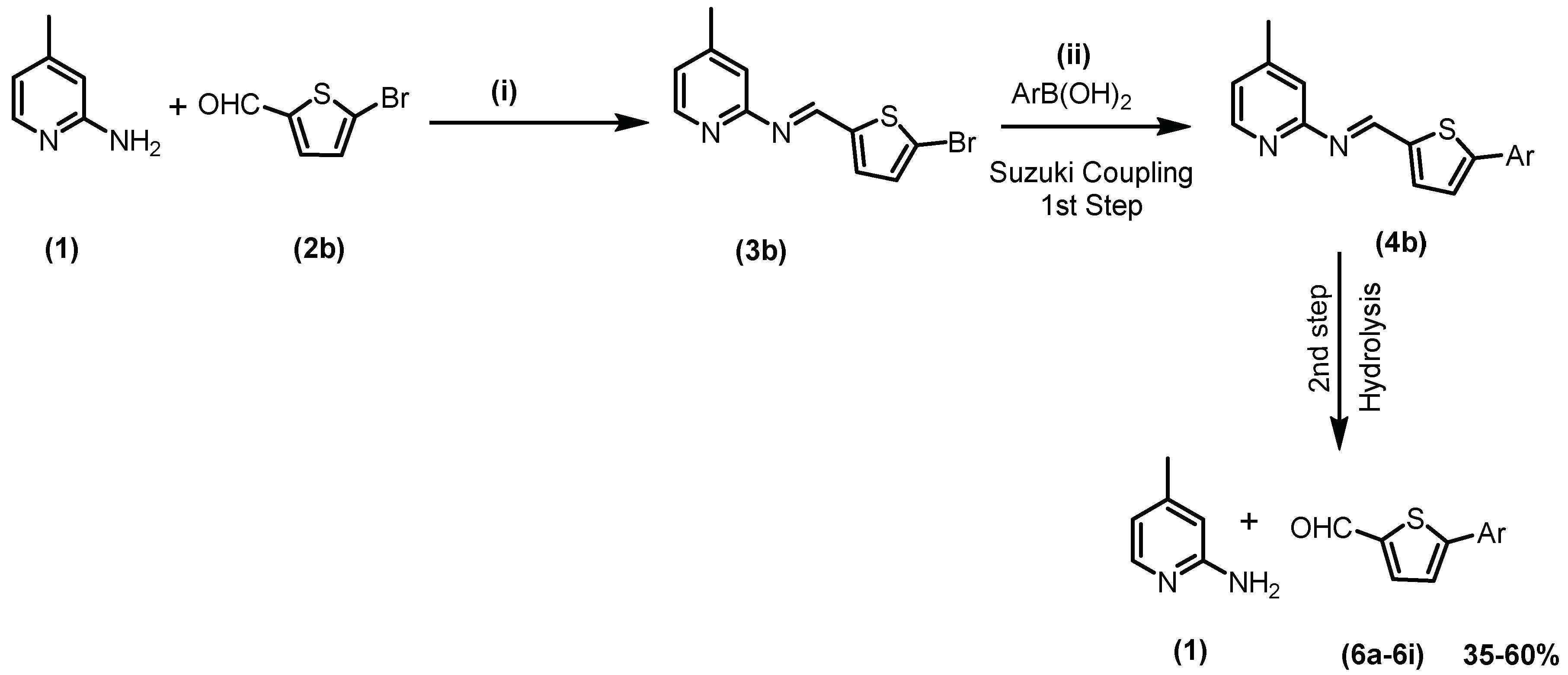
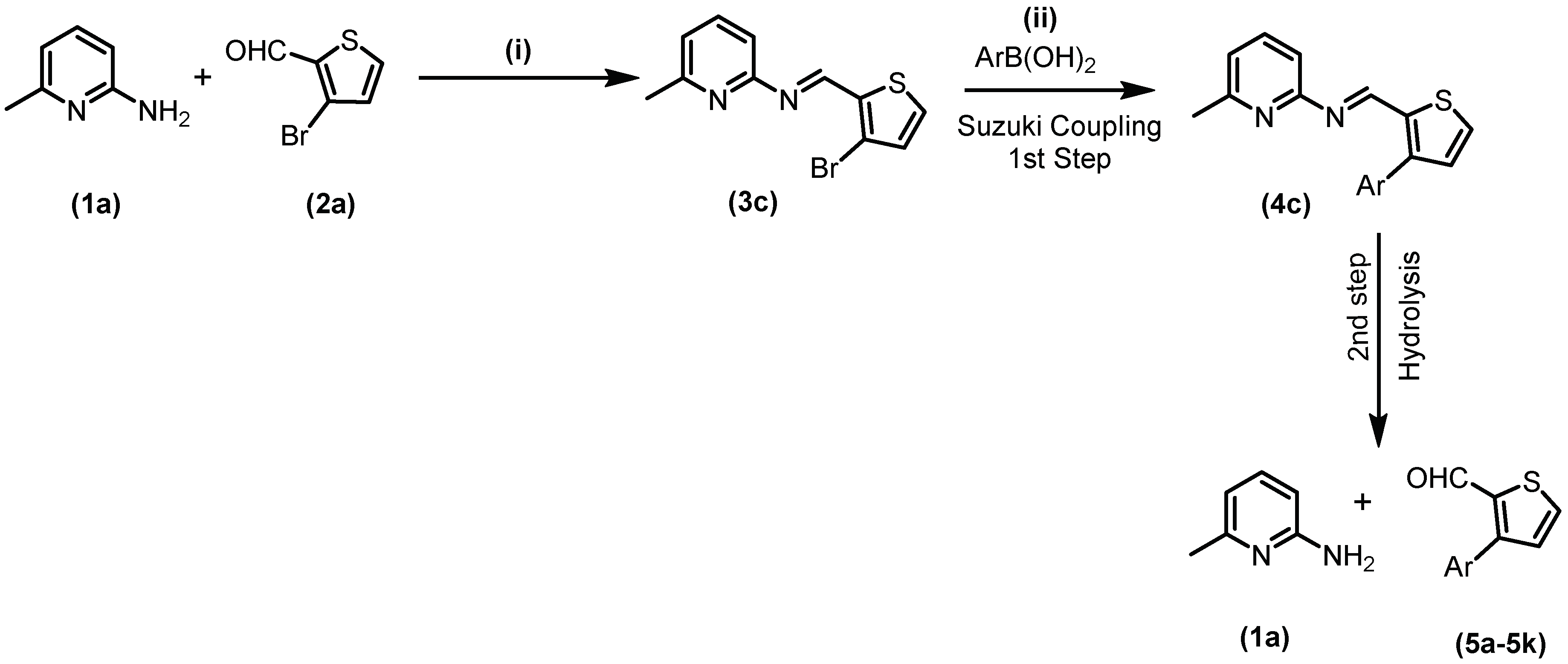
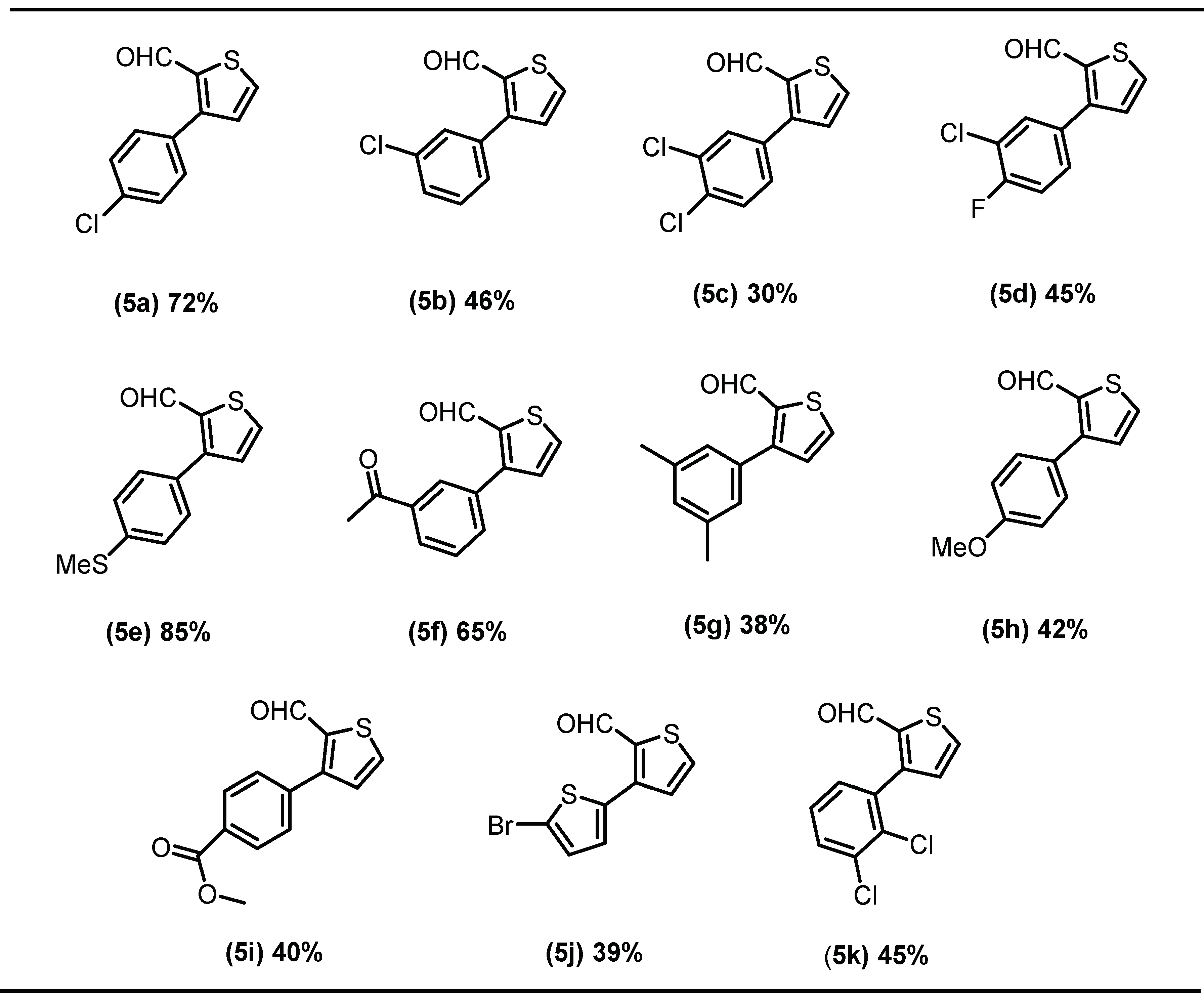
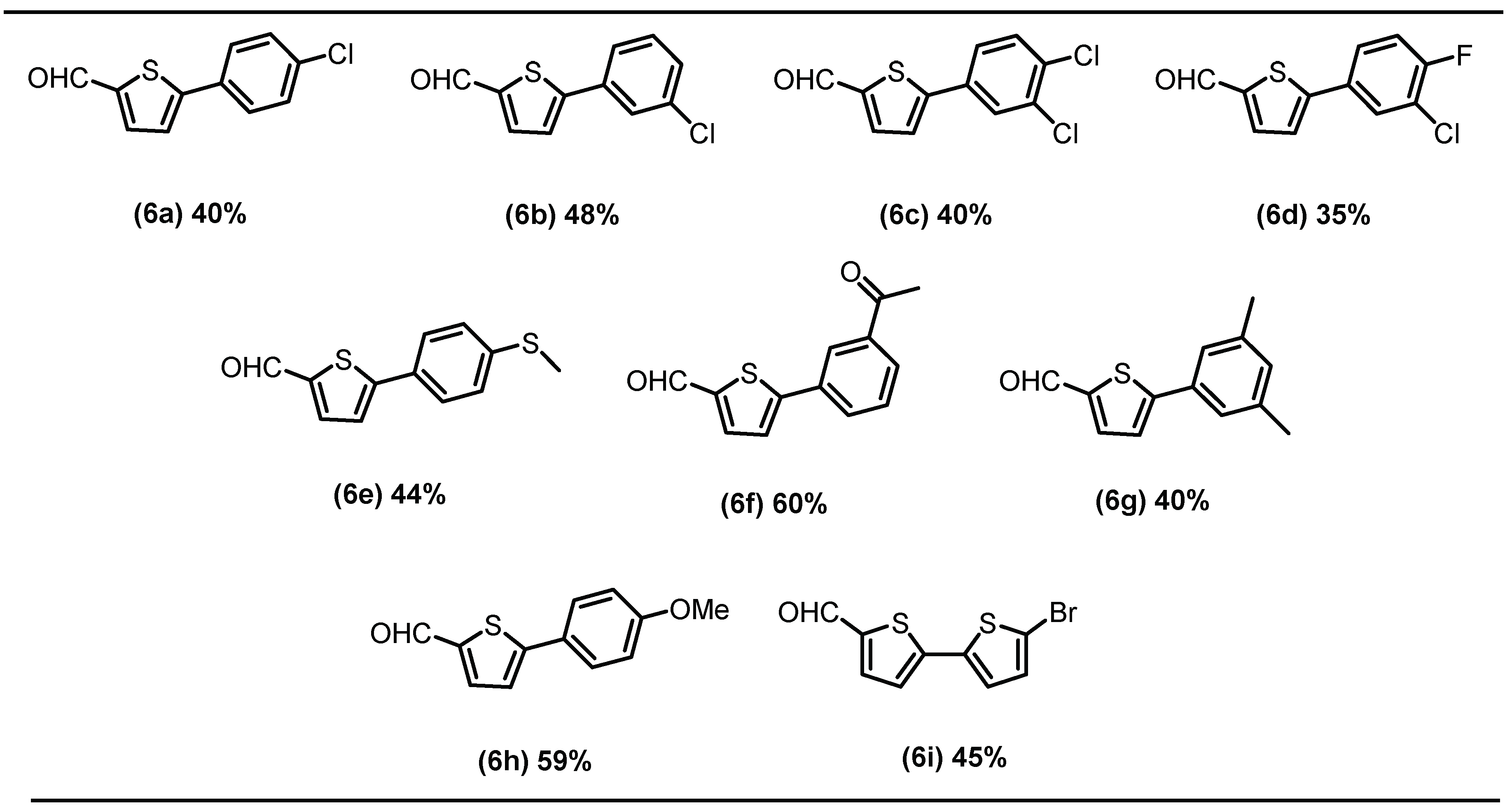
2.1. Chemistry
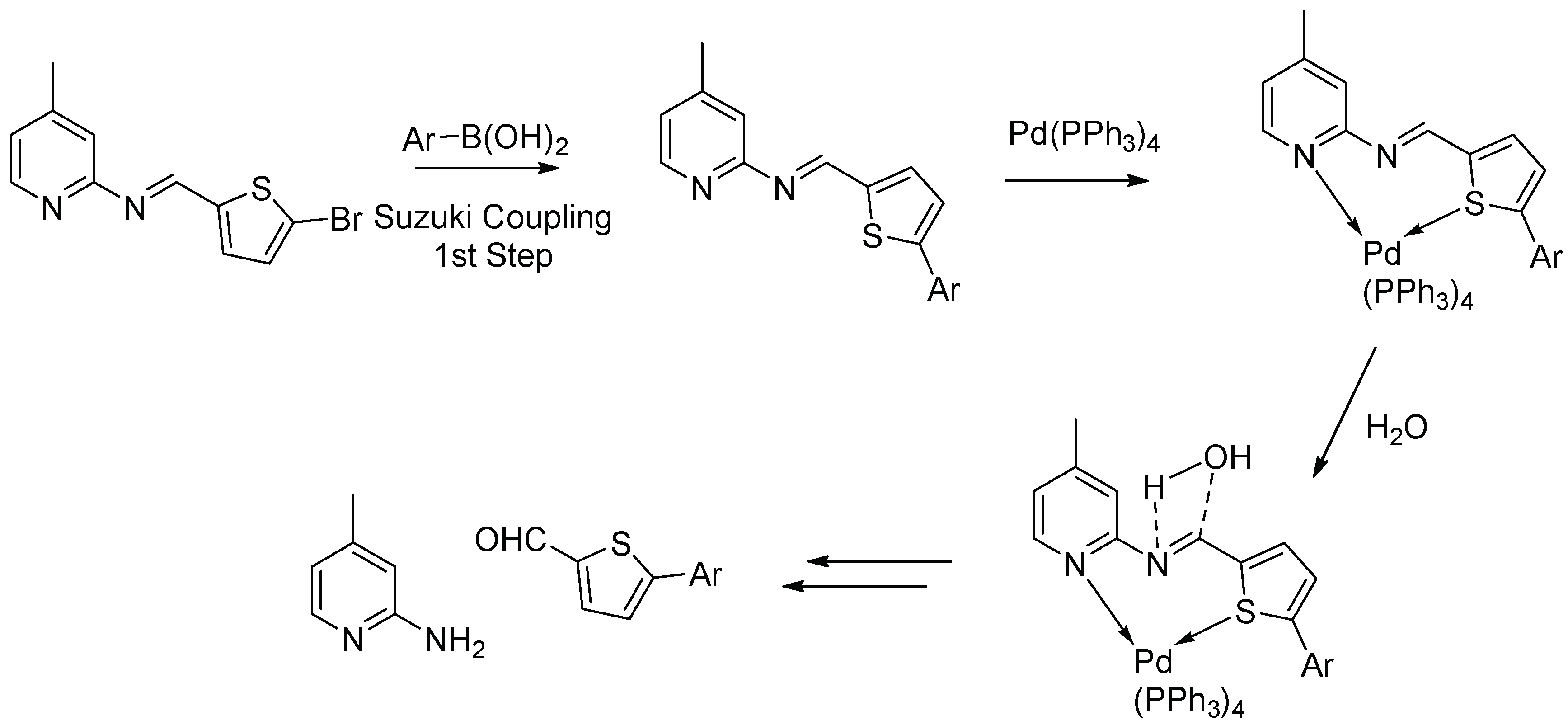
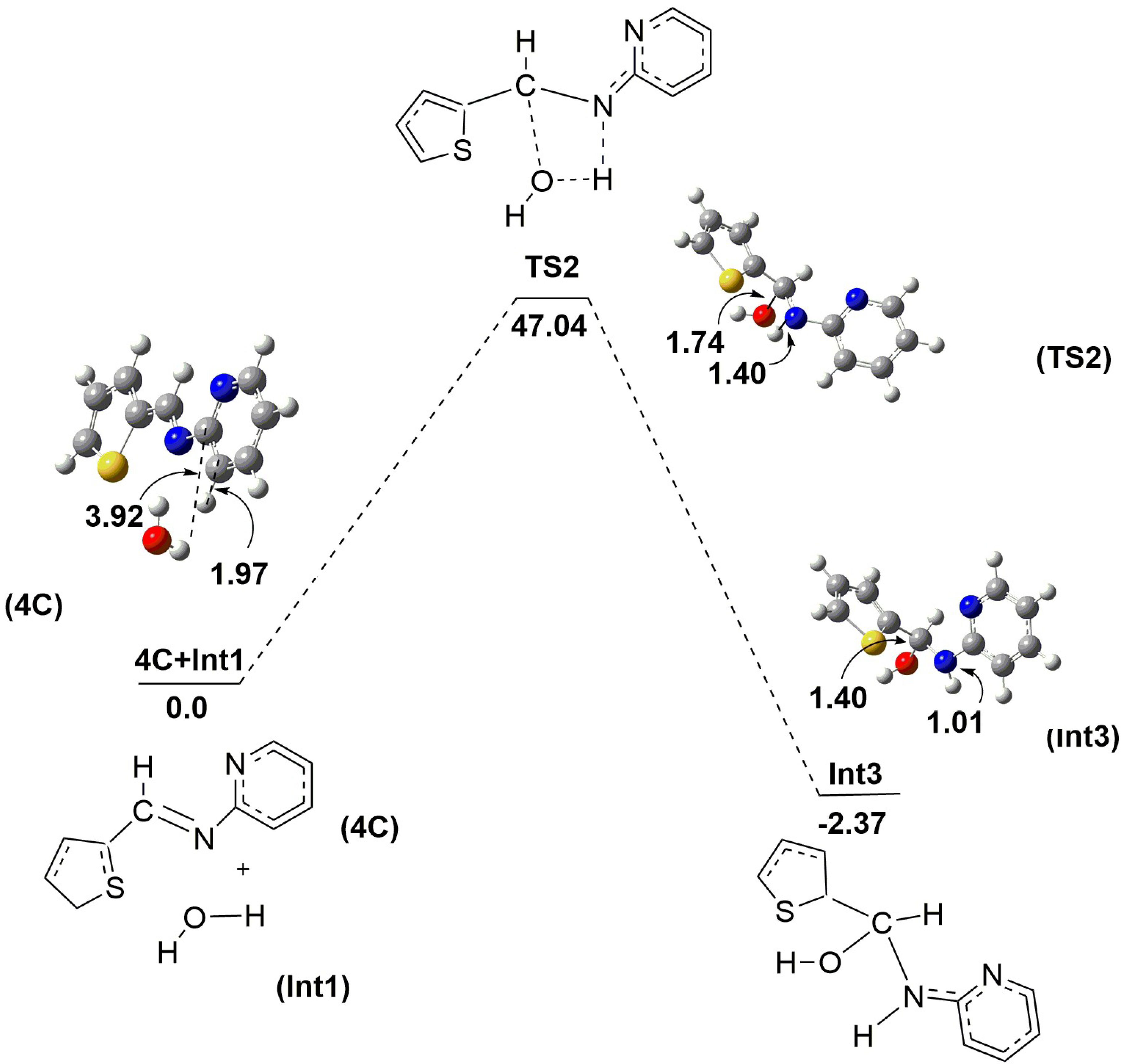
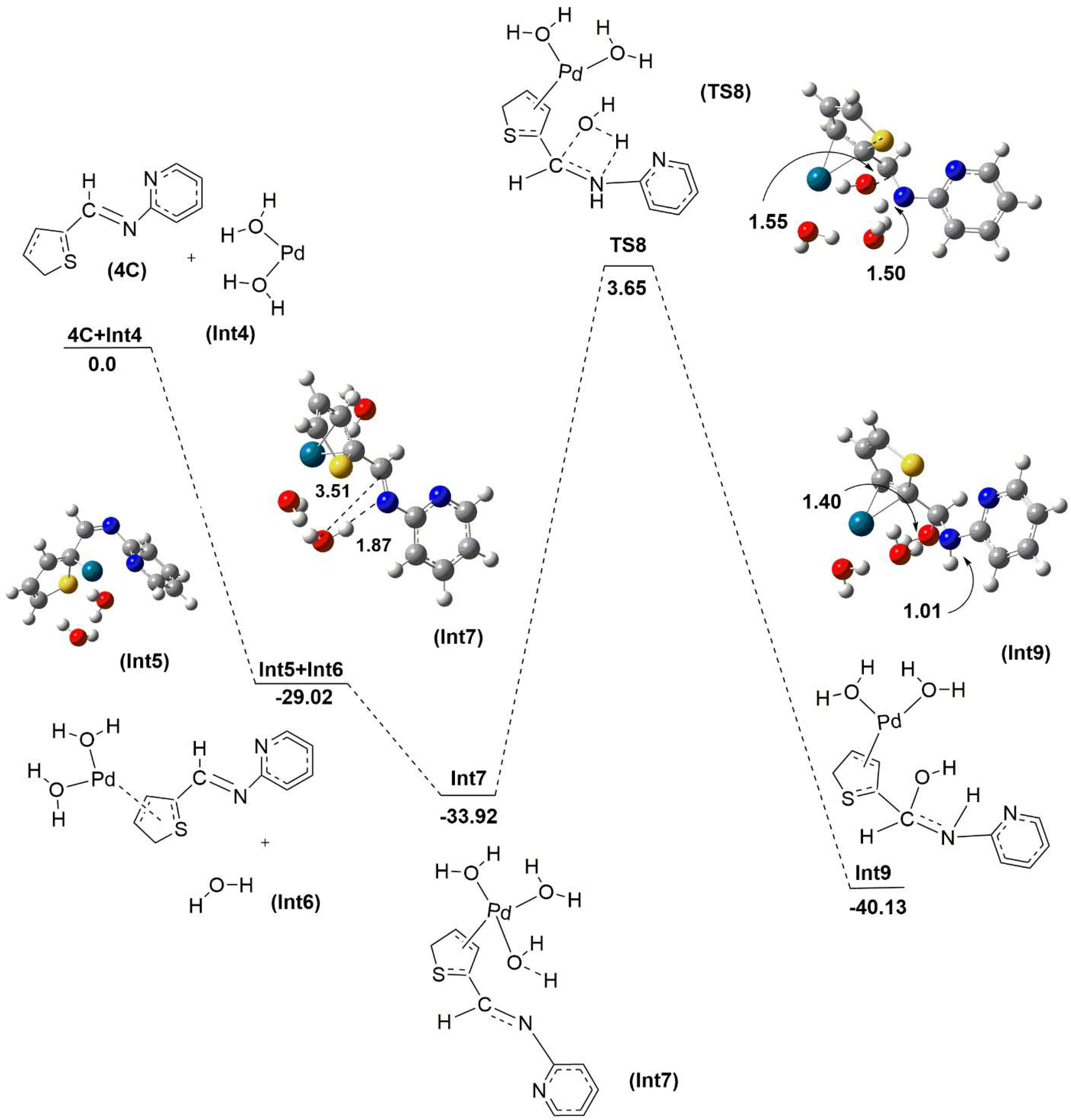
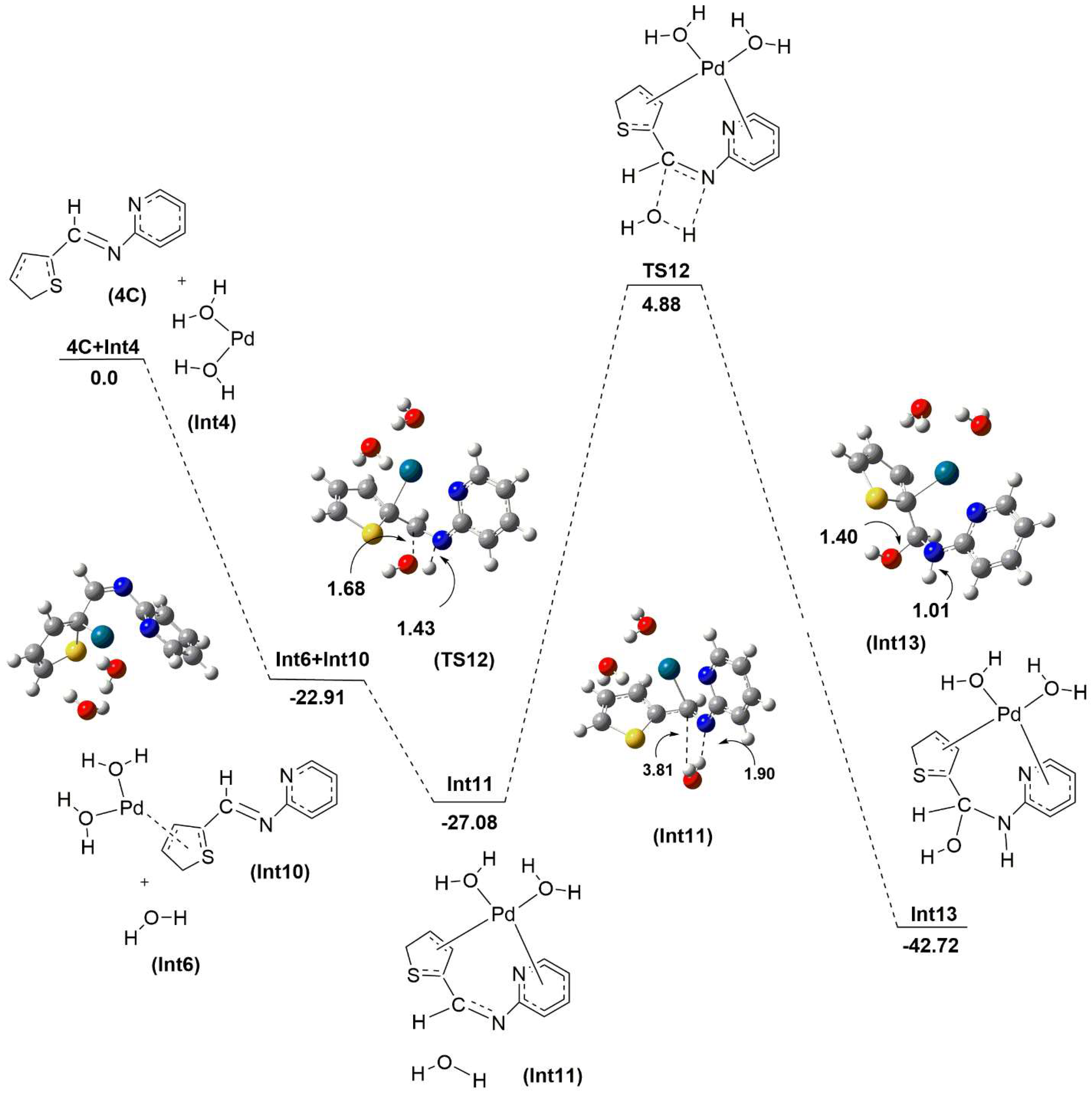
2.2. Mechanistic Studies via DFT Calculations for the Hydrolysis of Imine
3. Materials and Methods
3.1. General Information
3.2. General Synthetic Procedure of Schiff Bases
3.3. General Procedure for Suzuki Coupling of Schiff Base with Aryl/het-Aryl Boronic Acids
3.4. Characterization Data
3.5. Computational Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sondhi, S.M.; Singh, N.; Kumar, A.; Lozach, O.; Meijer, L. Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff’s bases. Bioorg. Med. Chem. 2006, 14, 3758–3765. [Google Scholar] [CrossRef] [PubMed]
- Mounika, K.; Pragathi, A.; Gyanakumari, C. Synthesis characterization and biological activity of a Schiff base derived from 3-ethoxy salicylaldehyde and 2-amino benzoic acid and its transition metal complexes. J. Sci. Res. 2010, 2, 513. [Google Scholar] [CrossRef]
- Wei, D.; Li, N.; Lu, G.; Yao, K. Synthesis, catalytic and biological activity of novel dinuclear copper complex with Schiff base. Sci. Chin. Ser. B Chem. 2006, 49, 225–229. [Google Scholar] [CrossRef]
- Miri, R.; Razzaghi-asl, N.; Mohammadi, M.K. QM study and conformational analysis of an isatin Schiff base as a potential cytotoxic agent. J. Mol. Model. 2013, 19, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, A.; Pandeya, S. Synthesis & anticonvulsant activity (Chemo Shock) of Schiff and Mannich bases of Isatin derivatives with 2-Amino pyridine (mechanism of action). Int. J. PharmTech Res. 2012, 4, 590–598. [Google Scholar]
- Avaji, P.G.; Kumar, C.V.; Patil, S.A.; Shivananda, K.; Nagaraju, C. Synthesis, spectral characterization, in-vitro microbiological evaluation and cytotoxic activities of novel macrocyclic bis hydrazone. Eur. J. Med. Chem. 2009, 44, 3552–3559. [Google Scholar] [CrossRef]
- Tisato, F.; Refosco, F.; Bandoli, G. Structural survey of technetium complexes. Coord. Chem. Rev. 1994, 135, 325–397. [Google Scholar] [CrossRef]
- Zhang, N.; Fan, Y.-H.; Zhang, Z.; Zuo, J.; Zhang, P.-F.; Wang, Q.; Liu, S.-B.; Bi, C.-F. Syntheses, crystal structures and anticancer activities of three novel transition metal complexes with Schiff base derived from 2-acetylpyridine and l-tryptophan. Inorg. Chem. Comm. 2012, 22, 68–72. [Google Scholar] [CrossRef]
- Hajrezaie, M.; Paydar, M.; Zorofchian Moghadamtousi, S.; Hassandarvish, P.; Gwaram, N.S.; Zahedifard, M.; Rouhollahi, E.; Karimian, H.; Looi, C.Y.; Ali, H.M. A Schiff Base-derived copper (II) complex is a potent inducer of apoptosis in colon cancer cells by activating the intrinsic pathway. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Gharamaleki, J.A.; Akbari, F.; Karbalaei, A.; Ghiassi, K.B.; Olmstead, M.M. Synthesis, Characterization and Crystal Structure of a New Schiff Base Ligand from a Bis (Thiazoline) Template and Hydrolytic Cleavage of the Imine Bond Induced by a Co (II) Cation. Open J. Inorg. Chem. 2016, 6, 76. [Google Scholar] [CrossRef]
- Teoh, S.-G.; Yeap, G.-Y.; Loh, C.-C.; Foong, L.-W.; Teo, S.-B.; Fun, H.-K. Inner coordination sphere tin (IV) complexes with some O,N,N-terdentate {N-(2-hydroxybenzaldehyde)-1-amino-2-phenyleneimine and N-(2-hydroxy-1-naphthaldehyde)-1-amino-2-phenyleneimine} and O,N,N,O-quadridentate {N,N′-bis(2-hydroxybenzaldehyde)-1,2-phenylenediimine and N,N′-bis (2-hydroxy-1-naphthaldehyde)-1,2-phenylenediimine} Schiff bases. Polyhedron 1997, 16, 2213–2221. [Google Scholar]
- Bu, X.R.; Jackson, C.R.; Van Derveer, D.; You, X.Z.; Meng, Q.J.; Wang, R.X. New copper (II) complexes incorporating unsymmetrical tetradentate ligands with cis-N2O2 chromophores: synthesis, molecular structure, substituent effect and thermal stability. Polyhedron 1997, 16, 2991–3001. [Google Scholar] [CrossRef]
- Gourbatsis, S.; Perlepes, S.P.; Butler, I.S.; Hadjiliadis, N. Zinc (II) complexes derived from the di-Schiff-base ligand N,N′-bis[1-(pyridin-2-yl)ethylidene]ethane-1,2-diamine (LA) and its hydrolytic-cleavage product N-[1-pyridin-2-yl)ethylidene]ethane-1,2-diamine (L): preparation, characterization and crystal structure of the 5-coordinate species [ZnLCl2]. Polyhedron 1999, 18, 2369–2375. [Google Scholar]
- Cano, J.; Benito, A.; Martínez-Máñez, R.; Soto, J.; Payá, J.; Lloret, F.; Julve, M.; Marcos, M.D.; Sinn, E. Ferrocene containing chelating ligands 3. Synthesis, spectroscopic characterization, electrochemical behaviour and interaction with metal ions of new ligands obtained by condensation of ferrocenecarboxaldehyde with 2-amino-benzoic acid derivatives. Crystal structures of 2-ferrocenylmethylamino-5-methyl-benzoic acid and 2-bis (ferrocenylmethyl) ammonium-5-methyl-benzoic acid perchlorate. Inorg. Chim. Acta 1995, 231, 45–56. [Google Scholar]
- Mukherjee, P.; Drew, M.G.; Ghosh, A. Anion-Directed Template Synthesis and Hydrolysis of Mono-Condensed Schiff Base of 1,3-Pentanediamine and o-Hydroxyacetophenone in NiII and CuII Complexes. Eur. J. Inorg. Chem. 2008, 2008, 3372–3381. [Google Scholar] [CrossRef]
- Dong, Y.-B.; Zhao, X.; Huang, R.-Q.; Smith, M.D.; zur Loye, H.-C. New Ag (I)-containing coordination polymers generated from multidentate Schiff-base ligands. Inorg. Chem. 2004, 43, 5603–5612. [Google Scholar] [CrossRef]
- Sarkar, B.; Ray, M.S.; Drew, M.G.; Figuerola, A.; Diaz, C.; Ghosh, A. Trinuclear Cu (II) complexes containing peripheral ketonic oxygen bridges and a μ 3-OH core: Steric influence on their structures and existence. Polyhedron 2006, 25, 3084–3094. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Drew, M.G.; Ghosh, A. Anion directed templated synthesis of mono-and di-Schiff base complexes of Ni (II). Polyhedron 2007, 26, 3513–3522. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Chakraborty, P.; Drew, M.G.; Ghosh, A. Nickel (II) complexes of terdentate or symmetrical tetradentate Schiff bases: Evidence of the influence of the counter anions in the hydrolysis of the imine bond in Schiff base complexes. Inorg. Chim. Acta 2009, 362, 502–508. [Google Scholar] [CrossRef]
- Naiya, S.; Sarkar, B.; Song, Y.; Ianelli, S.; Drew, M.G.; Ghosh, A. Carbonyl compound dependent hydrolysis of mono-condensed Schiff bases: A trinuclear Schiff base complex and a mononuclear mixed-ligand ternary complex of copper (II). Inorg. Chim. Acta 2010, 363, 2488–2495. [Google Scholar] [CrossRef]
- Rizwan, K.; Rasool, N.; Rehman, R.; Mahmood, T.; Ayub, K.; Rasheed, T.; Ahmad, G.; Malik, A.; Khan, S.A.; Akhtar, M.N.; et al. Facile synthesis of N-(4-bromophenyl)-1-(3-bromothiophen-2-yl)methanimine derivatives via Suzuki cross-coupling reaction: their characterization and DFT studies. Chem. Cent. J. 2018, 12, 84. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Arnó, M. Understanding the mechanism of the N-heterocyclic carbene-catalyzed ring-expansion of 4-formyl-β-lactams to succinimide derivatives. Tetrahedron 2009, 65, 3432–3440. [Google Scholar] [CrossRef]
- Williams, D.B.G.; Lawton, M. Drying of organic solvents: quantitative evaluation of the efficiency of several desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef]
- Vaidehi, B.; Tejaswi, K.S.; Prabhakar, N.; Devi, L. Synthesis, characterization and biological evaluation of 4-Nitro schiff bases. Int. J. Pharma Bio Sci. 2013, 4, 829–837. [Google Scholar]
- Dang, T.T.; Rasool, N.; Dang, T.T.; Reinke, H.; Langer, P. Synthesis of tetraarylthiophenes by regioselective Suzuki cross-coupling reactions of tetrabromothiophene. Tetrahedron Lett. 2007, 48, 845–847. [Google Scholar] [CrossRef]
- Rizwan, K.; Zubair, M.; Rasool, N.; Ali, S.; Zahoor, A.F.; Rana, U.A.; Khan, S.U.-D.; Shahid, M.; Zia-Ul-Haq, M.; Jaafar, H.Z. Regioselective synthesis of 2-(bromomethyl)-5-aryl-thiophene derivatives via palladium (0) catalyzed suzuki cross-coupling reactions: as antithrombotic and haemolytically active molecules. Chem. Cent. J. 2014, 8, 74. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D. 01; Gaussian Inc.: Wallingford, UK, 2010. [Google Scholar]
- Roy, D.; Todd, K.; John, M. Gauss View; Version 5; Semichem, Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
Sample Availability: Not available. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, G.; Rasool, N.; Rizwan, K.; Altaf, A.A.; Rashid, U.; Hussein, M.Z.; Mahmood, T.; Ayub, K. Role of Pyridine Nitrogen in Palladium-Catalyzed Imine Hydrolysis: A Case Study of (E)-1-(3-bromothiophen-2-yl)-N-(4-methylpyridin-2-yl)methanimine. Molecules 2019, 24, 2609. https://doi.org/10.3390/molecules24142609
Ahmad G, Rasool N, Rizwan K, Altaf AA, Rashid U, Hussein MZ, Mahmood T, Ayub K. Role of Pyridine Nitrogen in Palladium-Catalyzed Imine Hydrolysis: A Case Study of (E)-1-(3-bromothiophen-2-yl)-N-(4-methylpyridin-2-yl)methanimine. Molecules. 2019; 24(14):2609. https://doi.org/10.3390/molecules24142609
Chicago/Turabian StyleAhmad, Gulraiz, Nasir Rasool, Komal Rizwan, Ataf Ali Altaf, Umer Rashid, Mohd Zobir Hussein, Tariq Mahmood, and Khurshid Ayub. 2019. "Role of Pyridine Nitrogen in Palladium-Catalyzed Imine Hydrolysis: A Case Study of (E)-1-(3-bromothiophen-2-yl)-N-(4-methylpyridin-2-yl)methanimine" Molecules 24, no. 14: 2609. https://doi.org/10.3390/molecules24142609