Divergent Effects of Resveratrol on Rat Cardiac Fibroblasts and Cardiomyocytes
Abstract
:1. Introduction
2. Results
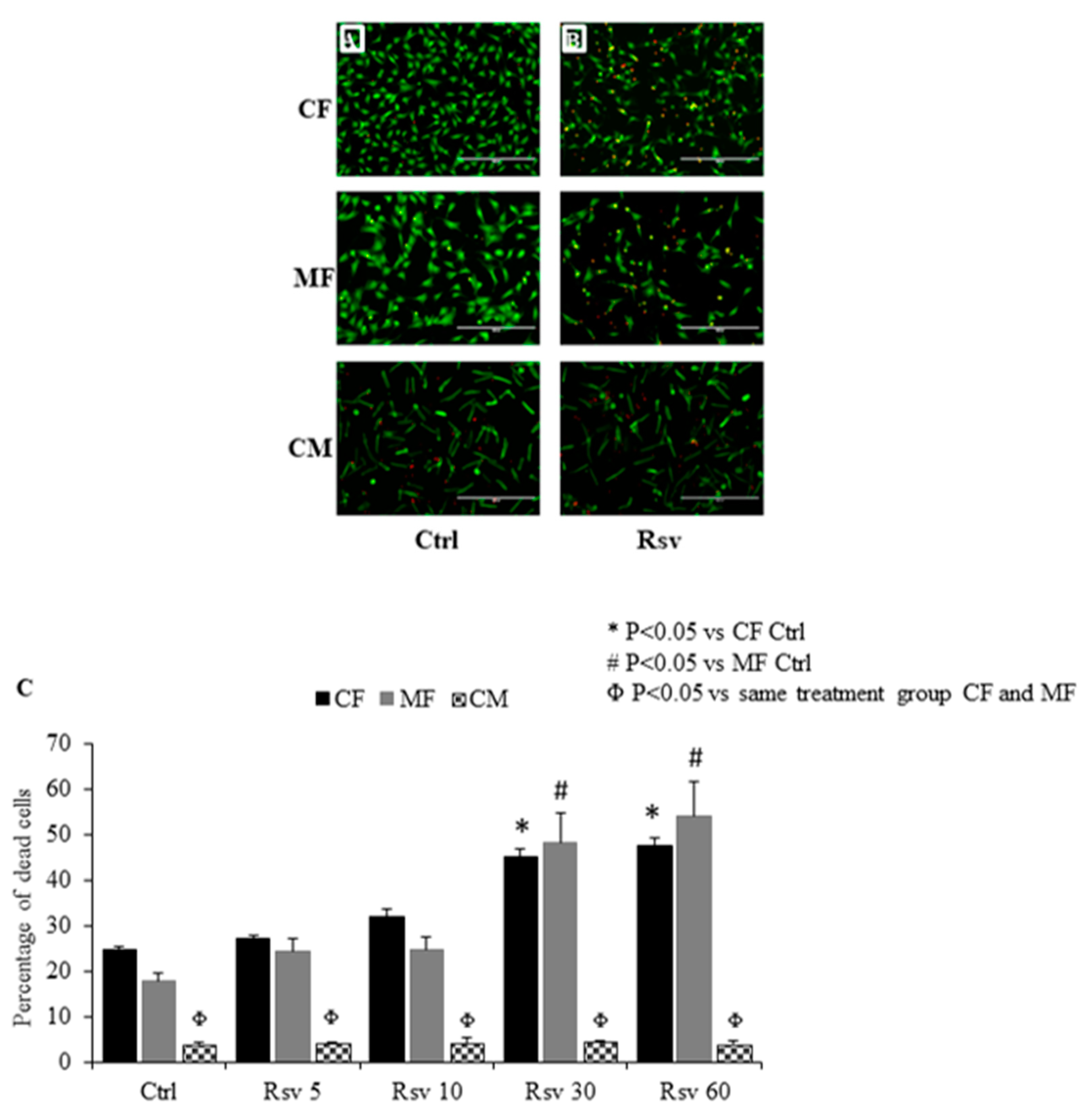
2.1. Comparing the Dose Dependent Effect of Resveratrol on Fibroblast, Myofibroblast and Cardiomyocyte Cell Death
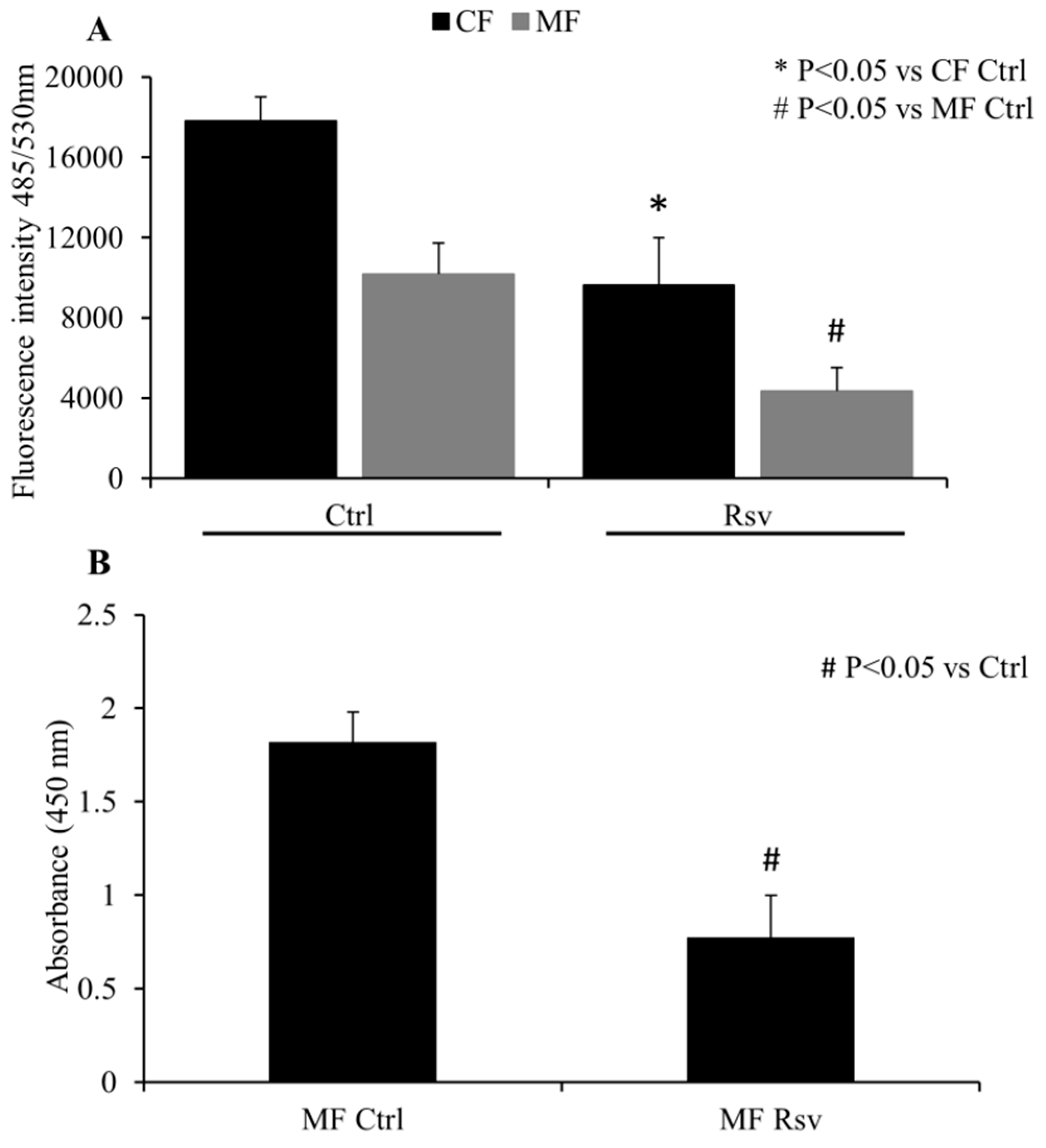
2.2. Quantification of CF and MF Proliferation
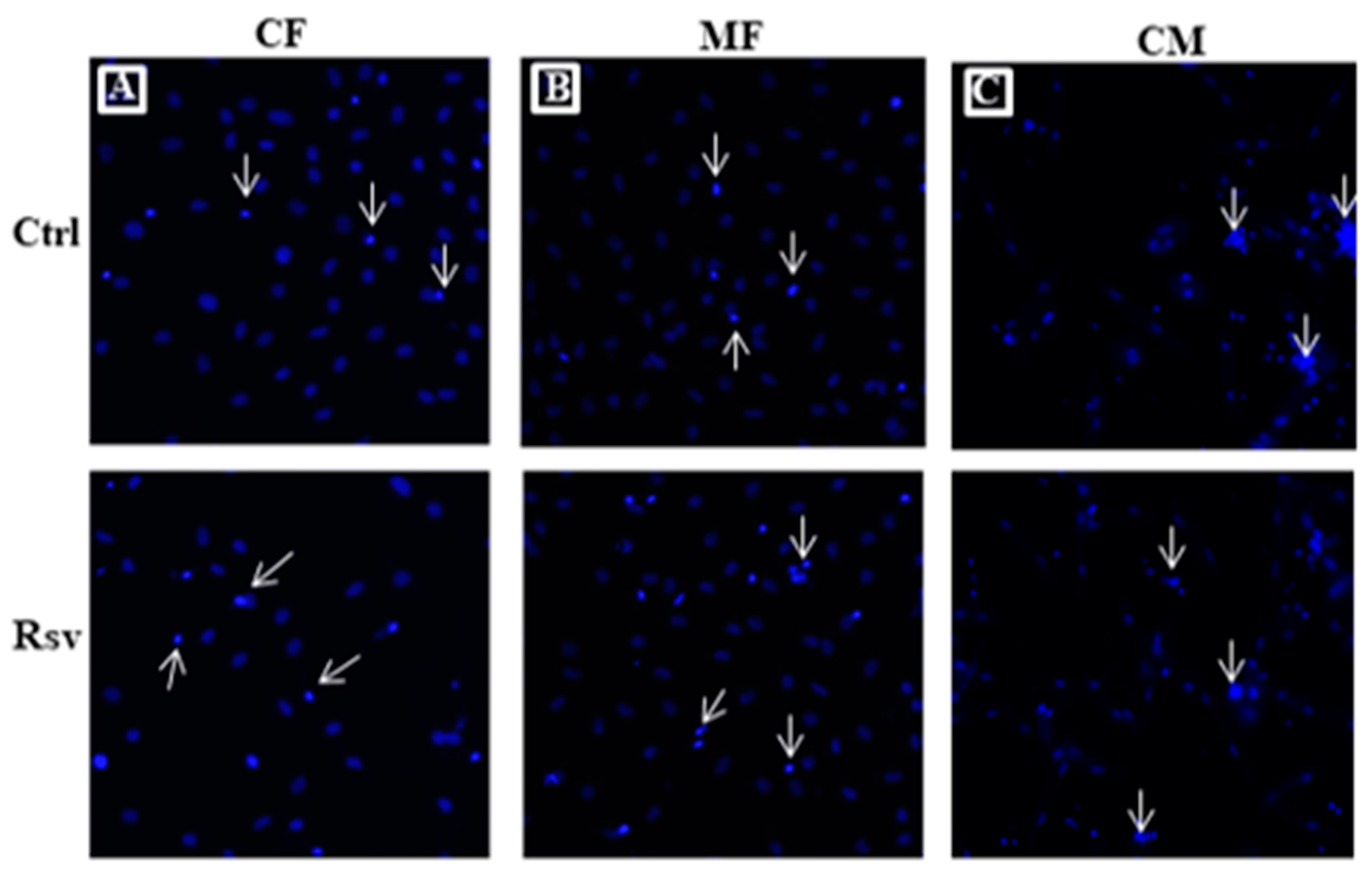
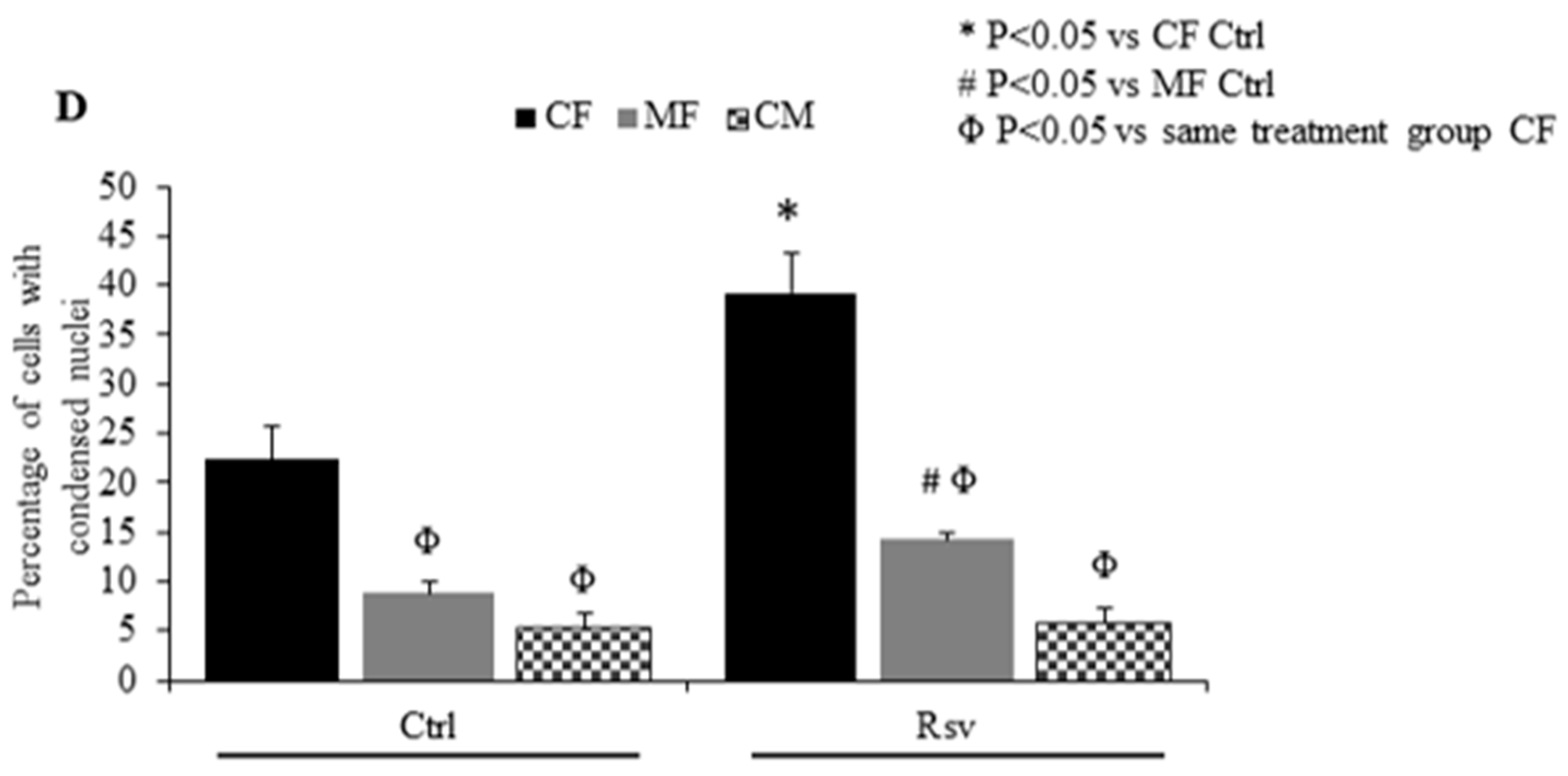
2.3. Effect of Rsv on Fibroblasts and Cardiomyocyte Apoptosis
2.4. Effect of Estradiol, Tamoxifen and Fulvestrant on Cardiac Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Adult Cardiomyocyte and Fibroblast Isolation and Culture
4.2. Dose Dependent Effect of Resveratrol on Cardiac Fibroblasts, Myofibroblasts and Cardiomyocytes
4.3. Quantification of CF and MF Proliferation
4.4. Measurement of Apoptotic Cells
4.5. Fibroblast Treatment with Estrogen, Tamoxifen and Fulvestrant
4.6. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr. Physiol. 2015, 6, 187–214. [Google Scholar] [CrossRef] [PubMed]
- Foundation, H.A.S. The Burden of Heart Failure. In 2016 Report on the health of Canadians; 2016; Available online: https://www.heartandstroke.ca/-/media/pdf-files/canada/2017-heart-month/heartandstroke-reportonhealth-2016.ashx?la=en&hash=91708486C91708481BC91708014E91708424AB91708484E91708719B91708447AEEB91708488C91708485EB91708493E (accessed on 15 July 2019).
- Dostal, D.; Glaser, S.; Baudino, T.A. Cardiac fibroblast physiology and pathology. Compr. Physiol. 2015, 5, 887–909. [Google Scholar] [CrossRef] [PubMed]
- Ivey, M.J.; Tallquist, M.D. Defining the Cardiac Fibroblast. Circ. J. Off. J. Jpn. Circ. Soc. 2016, 80, 2269–2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severs, N.J. The cardiac muscle cell. Bioessays: News Rev. Mol. Cell. Dev. Biol. 2000, 22, 188–199. [Google Scholar] [CrossRef]
- Marin-Garcia, J. Cell death in the pathogenesis and progression of heart failure. Heart Fail. Rev. 2016, 21, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Briasoulis, A.; Androulakis, E.; Christophides, T.; Tousoulis, D. The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail. Rev. 2016, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A. Nutraceuticals and Dietary Supplements to Improve Quality of Life and Outcomes in Heart Failure Patients. Curr. Pharm. Des. 2017, 23, 1265–1272. [Google Scholar] [CrossRef]
- Johnston, T.P.; Korolenko, T.A.; Pirro, M.; Sahebkar, A. Preventing cardiovascular heart disease: Promising nutraceutical and non-nutraceutical treatments for cholesterol management. Pharmacol. Res. 2017, 120, 219–225. [Google Scholar] [CrossRef]
- Dyck, G.J.B.; Raj, P.; Zieroth, S.; Dyck, J.R.B.; Ezekowitz, J.A. The Effects of Resveratrol in Patients with Cardiovascular Disease and Heart Failure: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 904. [Google Scholar] [CrossRef]
- Raj, P.; Zieroth, S.; Netticadan, T. An overview of the efficacy of resveratrol in the management of ischemic heart disease. Ann. New York Acad. Sci. 2015, 1348, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Lieben Louis, X.; Raj, P.; Chan, L.; Zieroth, S.; Netticadan, T.; Wigle, J.T. Are the cardioprotective effects of the phytoestrogen resveratrol sex dependent? Can. J. Physiol. Pharmacol. 2018, 10. [Google Scholar] [CrossRef]
- Baarine, M.; Thandapilly, S.J.; Louis, X.L.; Mazue, F.; Yu, L.; Delmas, D.; Netticadan, T.; Lizard, G.; Latruffe, N. Pro-apoptotic versus anti-apoptotic properties of dietary resveratrol on tumoral and normal cardiac cells. Genes Nutr. 2011, 6, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Murphy, A.J.; Dart, A.M. A Clinical Perspective of Anti-Fibrotic Therapies for Cardiovascular Disease. Front. Pharmacol. 2017, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Serpi, R.; Tolonen, A.M.; Tenhunen, O.; Pievilainen, O.; Kubin, A.M.; Vaskivuo, T.; Soini, Y.; Kerkela, R.; Leskinen, H.; Ruskoaho, H. Divergent effects of losartan and metoprolol on cardiac remodeling, c-kit+ cells, proliferation and apoptosis in the left ventricle after myocardial infarction. Clin. Transl. Sci. 2009, 2, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Embry, C.; Marsiske, M.; Lu, X.; Doss, H.; Leeuwenburgh, C.; Manini, T.M. Safety and metabolic outcomes of resveratrol supplementation in older adults: Results of a twelve-week, placebo-controlled pilot study. Exp. Gerontol. 2014, 57, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Fodor, K.; Tit, D.M.; Pasca, B.; Bustea, C.; Uivarosan, D.; Endres, L.; Iovan, C.; Abdel-Daim, M.M.; Bungau, S. Long-Term Resveratrol Supplementation as a Secondary Prophylaxis for Stroke. Oxidative Med. Cell. Longev. 2018, 2018, 4147320. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nature reviews. Cardiology 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Novelle, M.G.; Wahl, D.; Dieguez, C.; Bernier, M.; de Cabo, R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res. Rev. 2015, 21, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Thandapilly, S.J.; Louis, X.L.; Yang, T.; Stringer, D.M.; Yu, L.; Zhang, S.; Wigle, J.; Kardami, E.; Zahradka, P.; Taylor, C.; et al. Resveratrol prevents norepinephrine induced hypertrophy in adult rat cardiomyocytes, by activating NO-AMPK pathway. Eur. J. Pharmacol. 2011, 668, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Yu, L.; Thandapilly, S.J.; Louis, X.L.; Netticadan, T. Resveratrol protects adult cardiomyocytes against oxidative stress mediated cell injury. Arch. Biochem. Biophys. 2012, 527, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Thandapilly, S.J.; Louis, X.L.; Behbahani, J.; Movahed, A.; Yu, L.; Fandrich, R.; Zhang, S.; Kardami, E.; Anderson, H.D.; Netticadan, T. Reduced hemodynamic load aids low-dose resveratrol in reversing cardiovascular defects in hypertensive rats. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2013, 36, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, P.; Juric, D.; Louis, X.L.; Thandapilly, S.J.; Yu, L.; Taylor, C.; Netticadan, T. Resveratrol arrests and regresses the development of pressure overload- but not volume overload-induced cardiac hypertrophy in rats. J. Nutr. 2010, 140, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Juric, D.; Wojciechowski, P.; Das, D.K.; Netticadan, T. Prevention of concentric hypertrophy and diastolic impairment in aortic-banded rats treated with resveratrol. Am. J. Physiology. Heart Circ. Physiol. 2007, 292, H2138–H2143. [Google Scholar] [CrossRef] [Green Version]
- Raj, P.; Aloud, B.M.; Louis, X.L.; Yu, L.; Zieroth, S.; Netticadan, T. Resveratrol is equipotent to perindopril in attenuating post-infarct cardiac remodeling and contractile dysfunction in rats. J. Nutr. Biochem. 2016, 28, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, J.; Liu, J.; Li, N.; Wang, S.; Liu, H.; Zeng, M.; Zhang, Y.; Bu, P. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-beta/Smad3 pathway. Am. J. Physiology. Heart Circ. Physiol. 2015, 308, H424–H434. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Resveratrol modulation of signal transduction in apoptosis and cell survival: A mini-review. Cancer Detect. Prev. 2006, 30, 217–223. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, X.; Xie, W.; Li, Y.; Ma, M.; Yuan, T.; Luo, B. Resveratrol exerts an anti-apoptotic effect on human bronchial epithelial cells undergoing cigarette smoke exposure. Mol. Med. Rep. 2015, 11, 1752–1758. [Google Scholar] [CrossRef]
- Di Liberto, V.; Makela, J.; Korhonen, L.; Olivieri, M.; Tselykh, T.; Malkia, A.; Do Thi, H.; Belluardo, N.; Lindholm, D.; Mudo, G. Involvement of estrogen receptors in the resveratrol-mediated increase in dopamine transporter in human dopaminergic neurons and in striatum of female mice. Neuropharmacology 2012, 62, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Jackson, E.K.; Gillespie, D.G.; Zacharia, L.C.; Imthurn, B.; Rosselli, M. Resveratrol, a red wine constituent, blocks the antimitogenic effects of estradiol on human female coronary artery smooth muscle cells. J. Clin. Endocrinol Metab 2010, 95, E9–E17. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Jing, H.; Gan, L.; Li, H.; Luo, B. Resveratrol attenuated estrogen-deficient-induced cardiac dysfunction: Role of AMPK, SIRT1, and mitochondrial function. Am. J. Transl. Res. 2016, 8, 2641–2649. [Google Scholar] [PubMed]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Et Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Lu, X.; Wang, L.; Yang, W. Resveratrol attenuates high fat diet-induced mouse cardiomyopathy through upregulation of estrogen related receptor-alpha. Eur. J. Pharmacol. 2019, 843, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Yurdagul, A., Jr.; Kleinedler, J.J.; McInnis, M.C.; Khandelwal, A.R.; Spence, A.L.; Orr, A.W.; Dugas, T.R. Resveratrol promotes endothelial cell wound healing under laminar shear stress through an estrogen receptor-alpha-dependent pathway. Am. J. Physiology. Heart Circ. Physiol. 2014, 306, H797–H806. [Google Scholar] [CrossRef]
- Khandelwal, A.R.; Hebert, V.Y.; Dugas, T.R. Essential role of ER-alpha-dependent NO production in resveratrol-mediated inhibition of restenosis. Am. J. Physiology. Heart Circ. Physiol. 2010, 299, H1451–H1458. [Google Scholar] [CrossRef]
- Olson, E.R.; Naugle, J.E.; Zhang, X.; Bomser, J.A.; Meszaros, J.G. Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. Am. J. Physiology. Heart Circ. Physiol. 2005, 288, H1131–H1138. [Google Scholar] [CrossRef] [Green Version]
- Jahan, F.; Landry, N.M.; Rattan, S.G.; Dixon, I.M.C.; Wigle, J.T. The Functional Role of Zinc Finger E Box-Binding Homeobox 2 (Zeb2) in Promoting Cardiac Fibroblast Activation. Int. J. Mol. Sci. 2018, 19, 3207. [Google Scholar] [CrossRef]
- Santiago, J.J.; Dangerfield, A.L.; Rattan, S.G.; Bathe, K.L.; Cunnington, R.H.; Raizman, J.E.; Bedosky, K.M.; Freed, D.H.; Kardami, E.; Dixon, I.M. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: Expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2010, 239, 1573–1584. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound Resv is available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lieben Louis, X.; Meikle, Z.; Chan, L.; DeGagne, G.; Cummer, R.; Meikle, S.; Krishnan, S.; Yu, L.; Netticadan, T.; Wigle, J.T. Divergent Effects of Resveratrol on Rat Cardiac Fibroblasts and Cardiomyocytes. Molecules 2019, 24, 2604. https://doi.org/10.3390/molecules24142604
Lieben Louis X, Meikle Z, Chan L, DeGagne G, Cummer R, Meikle S, Krishnan S, Yu L, Netticadan T, Wigle JT. Divergent Effects of Resveratrol on Rat Cardiac Fibroblasts and Cardiomyocytes. Molecules. 2019; 24(14):2604. https://doi.org/10.3390/molecules24142604
Chicago/Turabian StyleLieben Louis, Xavier, Zach Meikle, Laura Chan, Garret DeGagne, Rebecca Cummer, Shannon Meikle, Sampath Krishnan, Liping Yu, Thomas Netticadan, and Jeffrey T. Wigle. 2019. "Divergent Effects of Resveratrol on Rat Cardiac Fibroblasts and Cardiomyocytes" Molecules 24, no. 14: 2604. https://doi.org/10.3390/molecules24142604





