Competitive Protein Binding Assay of Naproxen by Human Serum Albumin Functionalized Silicon Dioxide Nanoparticles
Abstract
:1. Introduction
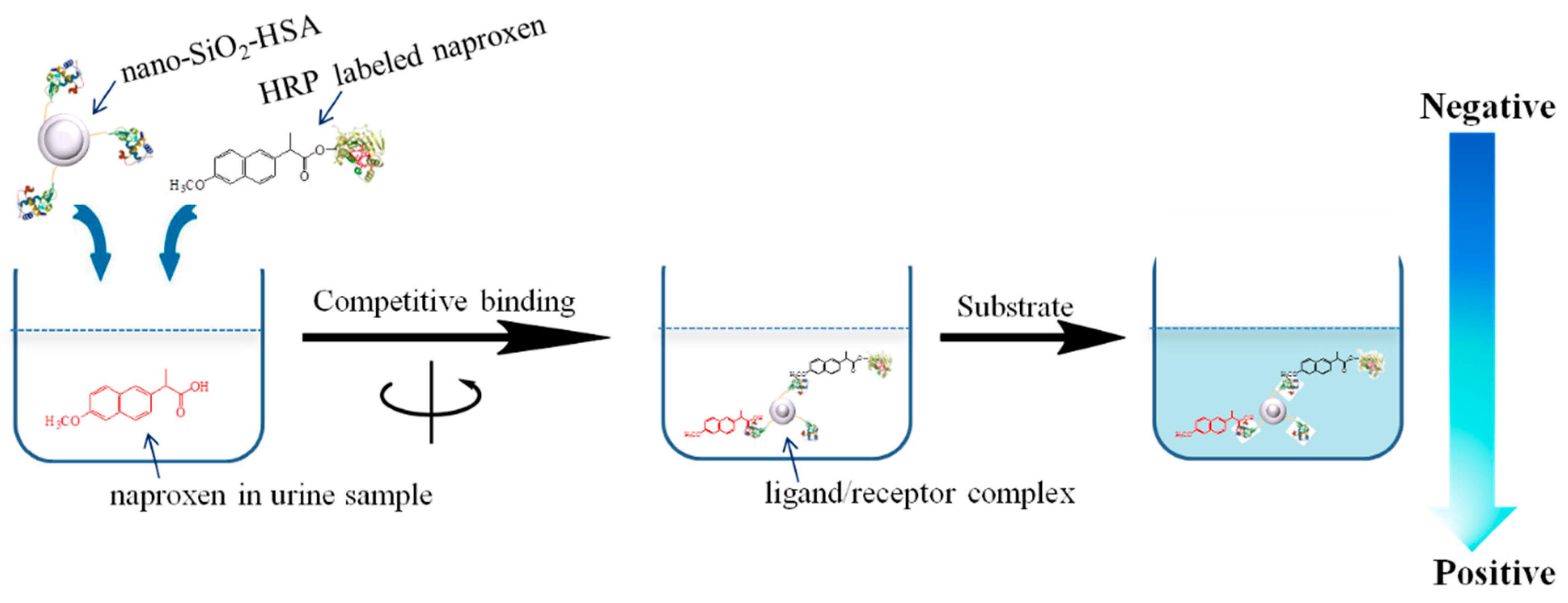
2. Results and Discussion
2.1. Characterization
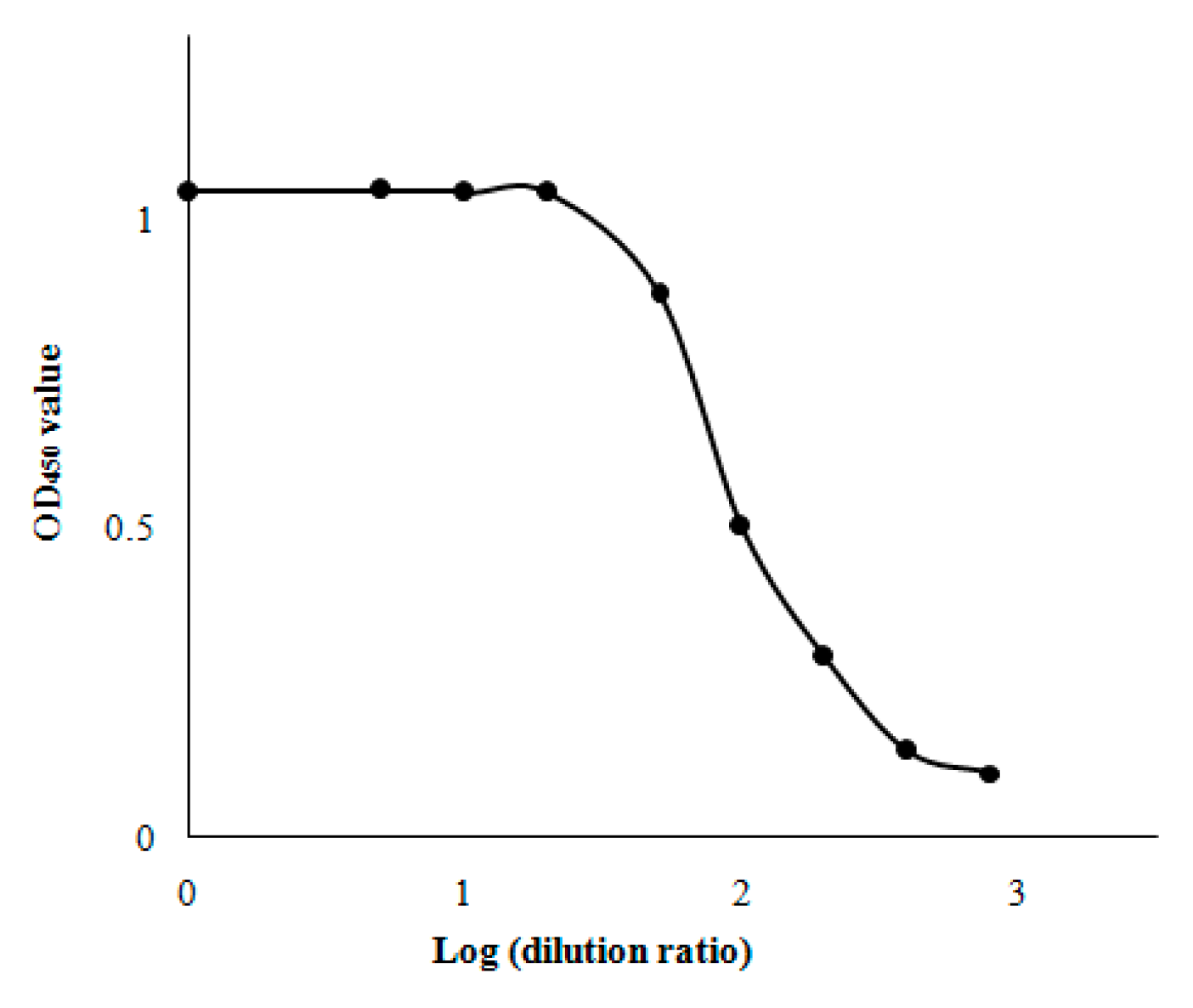
2.2. Optimization of HRP-Naproxen Dilution
2.3. Analytical Figures of Merit
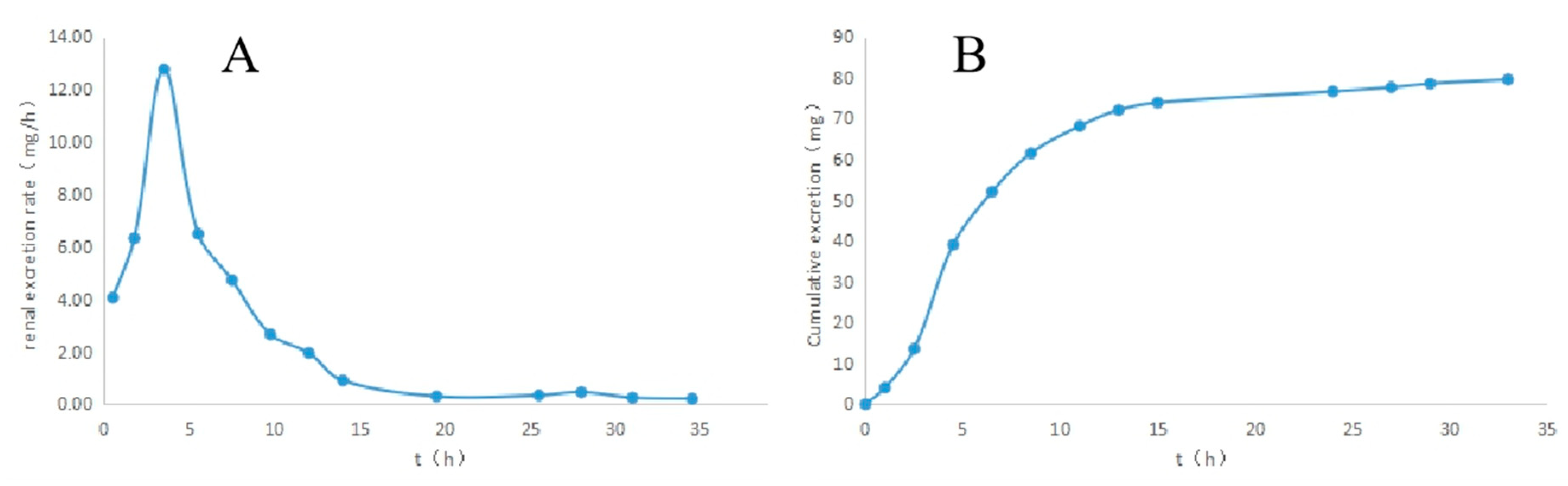
2.4. Determination of Naproxen in Urine by Nano-CPBA Method
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Instrumentation
3.3. Preparation of HSA Immobilized Nano-SiO2 Nanoparticles (SiO2-HSA)
3.4. Preparation of HRP Labeled Naproxen (HRP-Naproxen)
3.5. Optimization of HRP-Naproxen Dilution
3.6. Urine Samples Analysis
3.7. Method Validation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davies, N.M.; Fakhreddin, J.; Skeith, K.J. Steroidal Anti-inflammatory drug-induced enteropathy and severe chronic anemia in a patient with rheumatoid arthritis. Arthritis Rheumatol. 1996, 39, 321–324. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sumatriptan/naproxen sodium: A review in migraine. Drugs 2016, 76, 111–121. [Google Scholar] [CrossRef]
- Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Naproxen up to date: A review of its pharmacological properties and therapeutic efficacy and use in rheumatic diseases and pain states. Drugs 1979, 18, 241–277. [Google Scholar] [CrossRef]
- Henry, D.A. Side-effects of non-steroidal anti-inflammatory drugs. Baillière’s Clin. Rheumatol. 1988, 2, 425–454. [Google Scholar] [CrossRef]
- Süleyman, H.; Demircan, B.; Karagöz, Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharm. Rep. 2007, 59, 247–258. [Google Scholar]
- Mikami, E.; Goto, T.; Ohno, T.; Matsumoto, H.; Nishida, M. Simultaneous analysis of naproxen, nabumetone and its major metabolite 6-methoxy-2-naphthylacetic acid in pharmaceuticals and human urine by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2000, 23, 917–925. [Google Scholar] [CrossRef]
- Brêtas, J.M.; César, I.C.; Brêtas, C.M.; Teixeira, L.D.S.; Bellorio, K.B.; Mundim, I.M.; Pianetti, G.A. Development and validation of an LC-ESI-MS/MS method for the simultaneous quantification of naproxen and sumatriptan in human plasma: Application to a pharmacokinetic study. Anal. Bioanal. Chem. 2016, 408, 3981–3992. [Google Scholar] [CrossRef]
- Yilmaz, B.; Sahin, H.; Erdem, A.F. Determination of naproxen in human plasma by GC-MS. J. Sep. Sci. 2014, 37, 997–1003. [Google Scholar] [CrossRef]
- Pyka, A.; Wiatr, E.; Kwiska, K.; Gurak, D. Validation thin layer chromatography for the determination of naproxen in tablets and comparison with a pharmacopeil method. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 829–847. [Google Scholar] [CrossRef]
- Macia, A.; Borrull, F.; Calull, M.; Benavente, F.; Hernandez, E.; Sanz-Nebot, V.; Barbosa, J.; Aguilar, C. Sensitivity enhancement for the analysis of naproxen in tap water by solid-phase extraction coupled in-line to capillary electrophoresis. J. Sep. Sci. 2008, 31, 872–880. [Google Scholar] [CrossRef]
- Damiani, P.; Bearzotti, M.; Cabezon, M.A. Spectrofluorometric determination of naproxen in tablets. J. Pharm. Biomed. Anal. 2002, 29, 229–238. [Google Scholar] [CrossRef]
- Kaczmarek, M. Chemiluminescence of the reaction system Ce(IV)-non-steroidal anti-inflammatory drugs containing europium(III) ions and its application to the determination of naproxen in pharmaceutical preparations and urine. J. Fluoresc. 2011, 21, 2201–2205. [Google Scholar] [CrossRef] [PubMed]
- Pulgarin, J.A.M.; Molina, A.A.; Sanchez-Ferrer, I. Determination of propranolol and naproxen in urine by using excitation-emission matrix phosphorescence coupled with multivariate calibration algorithms. Curr. Pharm. Anal. 2012, 8, 83–92. [Google Scholar] [CrossRef]
- Lenik, J.; Łyszczek, R. Functionalized β-cyclodextrin based potentiometric sensor for naproxen determination. Mater. Sci. Eng. C 2016, 61, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Sener, E.; Tuncel, M.; Aboul-Enein, H.Y. Rapid determination of naproxen sodium in pharmaceutical formulations by flow injection analysis (FIA) using UV-detection. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 401–408. [Google Scholar] [CrossRef]
- Aresta, A.; Palmisano, F.; Zambonin, C.G. Determination of naproxen in human urine by solid-phase microextraction coupled to liquid chromatography. J. Pharm. Biomed. Anal. 2005, 39, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Chamsaz, M.; Rounaghi, G.H. Ultrasound-assisted magnetic dispersive solid-phase microextraction: A novel approach for the rapid and efficient microextraction of naproxen and ibuprofen employing experimental design with high-performance liquid chromatography. J. Sep. Sci. 2016, 39, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.H.; Manbohi, A.; Heydar, K.T. Electrochemically controlled in-tube solid phase microextraction of naproxen from urine samples using an experimental design. Analyst 2015, 140, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, H.; Ghorbani, M.; Lahoori, N.A.; Shams, A.; Seyedin, O. Preconcentration and determination of naproxen in water samples by functionalized multi-walled carbon nanotubes hollow fiber solid phase microextraction-HPLC. J. Anal. Chem. 2016, 71, 641–647. [Google Scholar] [CrossRef]
- Ayazi, Z.; Rafighi, P. Preparation and application of a carbon nanotube reinforced polyamide-based stir bar for sorptive extraction of naproxen from biological samples prior to its spectrofluorometric determination. Anal. Methods 2015, 7, 3200–3210. [Google Scholar] [CrossRef]
- Madrakian, T.; Ahmadi, M.; Afkhami, A.; Soleimani, M. Selective solid-phase extraction of naproxen drug from human urine samples using molecularly imprinted polymer-coated magnetic multi-walled carbon nanotubes prior to its spectrofluorometric determination. Analyst 2013, 138, 4542–4549. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; Song, C.Y.; Liao, J.; Huang, Z.L.; Li, G.K. Water stable metal-organic framework packed microcolumn for online sorptive extraction and direct analysis of naproxen and its metabolite from urine sample. J. Chromatogr. A 2013, 1294, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.X.; Hu, Y.L.; Li, G.K. Novel metal ion-mediated complex imprinted membrane for selective recognition and direct determination of naproxen in pharmaceuticals by solid surface fluorescence. Talanta 2013, 116, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Rezaeifar, Z.; Es’haghi, Z.; Rounaghi, G.H.; Chamsaz, M. Hyperbranched polyglycerol/graphene oxide nanocomposite reinforced hollow fiber solid/liquid phase microextraction for measurement of ibuprofen and naproxen in hair and waste water samples. J. Chromatogr. B 2016, 1029, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kadhirvel, P.; Azenha, M.; Shinde, S.; Schillinger, E.; Gomes, P.; Sellergren, B.; Silva, A.F. Imidazolium-based functional monomers for the imprinting of the anti-inflammatory drug naproxen: Comparison of acrylic and sol-gel approaches. J. Chromatogr. A 2013, 1314, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.-S.; Xue, Y.; Deng, W.-L.; Liao, X.; Xu, X.-M.; Li, B.-G.; Liu, Y.-M. Ligand fishing with functionalized magnetic nanoparticles coupled with mass spectrometry for herbal medicine analysis. Anal. Bioanal. Chem. 2011, 399, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.-S.; Xue, Y.; Zheng, Y.; Xiong, J.; Liao, X.; Ding, L.-S.; Li, B.-G.; Liu, Y.-M. Ligand fishing from Dioscorea nipponica extract using human serum albumin functionalized magnetic nanoparticles. J. Chromatogr. A 2010, 1217, 4663–4668. [Google Scholar] [CrossRef]
- Scharla, S.H.; Lempert, U.G. Evaluation of an automated competitive protein-binding assay for 25-hydroxyvitamin D. Clin. Lab. 2016, 62, 1781–1786. [Google Scholar] [CrossRef]
- Mondal, B.; Kamatham, N.; Samanta, S.R.; Jagadesan, P.; He, J.; Ramamurthy, V. Synthesis, characterization, guest inclusion, andphotophysical studies of gold nanoparticles stabilized with carboxylicacid groups of organic cavitands. Langmuir 2013, 29, 12703–12709. [Google Scholar] [CrossRef]
- Pérezlópez, B.; Merkoçi, A. Nanoparticles for the development of improved (bio)sensing systems. Anal. Bioanal. Chem. 2011, 399, 1577–1590. [Google Scholar] [CrossRef]
- Yang, T.Y.; Uhlinger, D.J.; Ayers, S.A.; O’Hara, D.M.; Joyce, A.P. Challenges in selectivity, specificity and quantitation range of ligand-binding assays: Case studies using a microfluidics platform. Bioanalysis 2014, 6, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- López-Lorente, A.I.; Simonet, B.M.; Valcárcel, M. Analytical potential of hybrid nanoparticles. Anal. Bioanal. Chem. 2011, 399, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-L.; Li, J.; Li, X.-D.; Ding, L.-S.; Xie, J.; Qing, L.-S. A simple nano-SiO2-based ELISA method for residue detection of 2,4-dichlorophenoxyacetic acid in bean sprouts. Food Anal. Methods 2017, 10, 1500–1506. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Li, J.; Li, X.-D.; Tao, W.-J.; Ding, L.-S.; Luo, P.; Qing, L.-S. An efficient direct competitive Nano-ELISA for residual BSA determination in vaccines. Anal. Bioanal. Chem. 2017, 409, 4607–4614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-L.; Xie, J.; Li, X.-D.; Ding, L.-S.; Liang, J.; Luo, P.; Qing, L.-S. Development of a nano-SiO2 based enzyme-linked ligand binding assay for the determination of ibuprofen in human urine. Talanta 2017, 167, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Runkel, R.; Chaplin, M.; Sevelius, H.; Ortega, E.; Segre, E. Pharmacokinetics of naproxen overdoses. Clin. Pharmacol. Ther. 1976, 20, 269–277. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Naproxen Content in Urine Samples (ng/mL) | Added (ng/mL) | Detected (ng/mL) | Recovery (%) | Mean Recovery (%) | SD | CV (%) |
|---|---|---|---|---|---|---|
| 152 | 180 | 302 | 83.4 | 89.9 | 0.07 | 8.29 |
| 152 | 180 | 311 | 88.4 | |||
| 152 | 180 | 328 | 98.0 | |||
| 152 | 150 | 285 | 88.9 | 95.8 | 0.06 | 6.22 |
| 152 | 150 | 301 | 99.5 | |||
| 152 | 150 | 300 | 99.0 | |||
| 152 | 120 | 275 | 102.9 | 98.0 | 0.04 | 4.49 |
| 152 | 120 | 265 | 94.4 | |||
| 152 | 120 | 268 | 96.6 | |||
| 0 | 120 | 117 | 97.2 | 93.5 | 0.02 | 4.11 |
| 0 | 120 | 107 | 89.5 | |||
| 0 | 120 | 112 | 93.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.-L.; Xie, J.; Liang, J.; Dong, G.-T.; Ding, L.-S.; Luo, P.; Qing, L.-S. Competitive Protein Binding Assay of Naproxen by Human Serum Albumin Functionalized Silicon Dioxide Nanoparticles. Molecules 2019, 24, 2593. https://doi.org/10.3390/molecules24142593
Wang Q-L, Xie J, Liang J, Dong G-T, Ding L-S, Luo P, Qing L-S. Competitive Protein Binding Assay of Naproxen by Human Serum Albumin Functionalized Silicon Dioxide Nanoparticles. Molecules. 2019; 24(14):2593. https://doi.org/10.3390/molecules24142593
Chicago/Turabian StyleWang, Qian-Long, Jing Xie, Jian Liang, Geng-Ting Dong, Li-Sheng Ding, Pei Luo, and Lin-Sen Qing. 2019. "Competitive Protein Binding Assay of Naproxen by Human Serum Albumin Functionalized Silicon Dioxide Nanoparticles" Molecules 24, no. 14: 2593. https://doi.org/10.3390/molecules24142593






