Great Enhancement of Carbon Energy Storage through Narrow Pores and Hydrogen-Containing Functional Groups for Aqueous Zn-Ion Hybrid Supercapacitor
Abstract
:1. Introduction
2. Results and Discussion
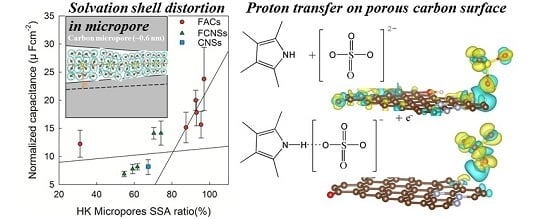
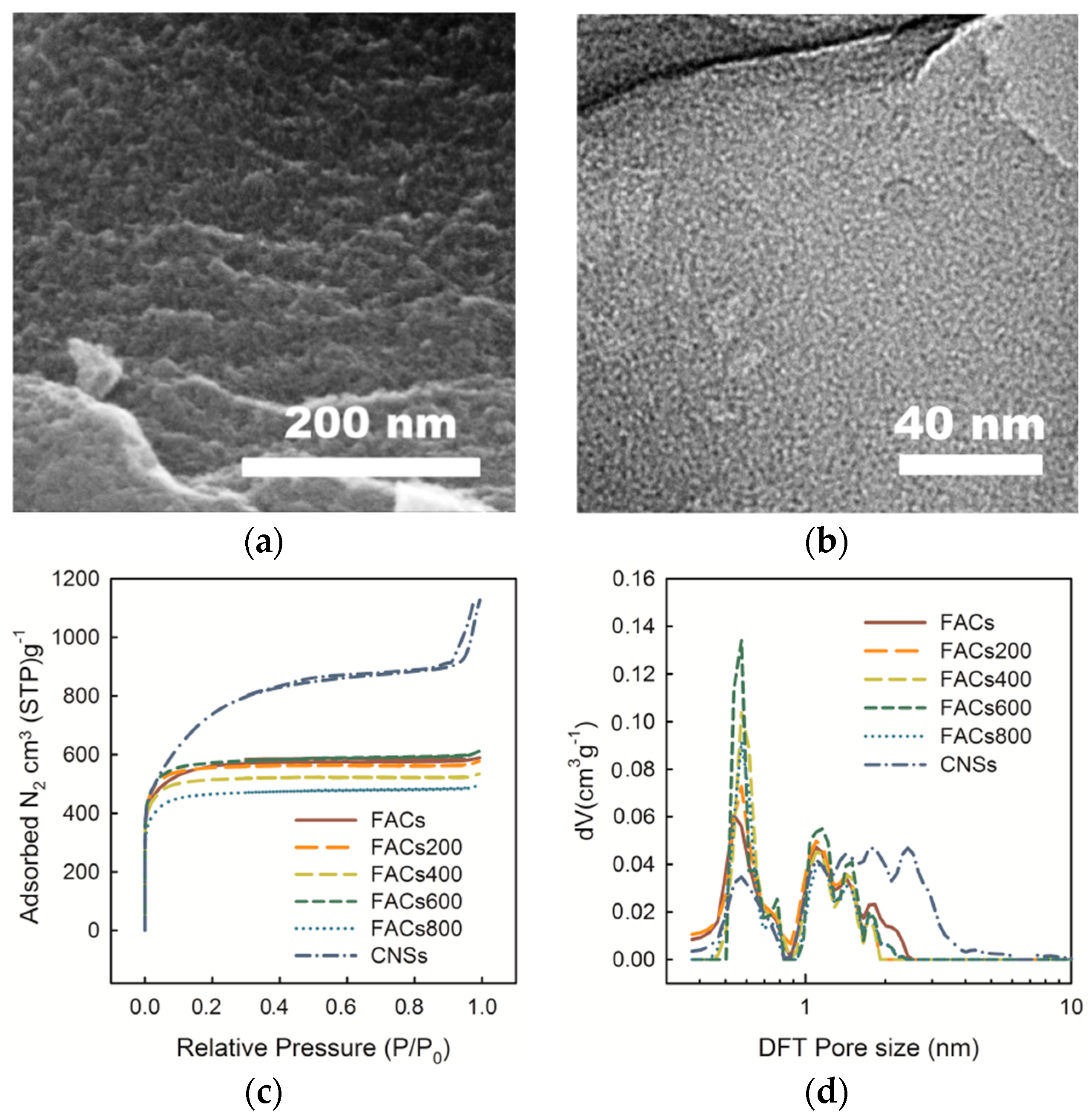
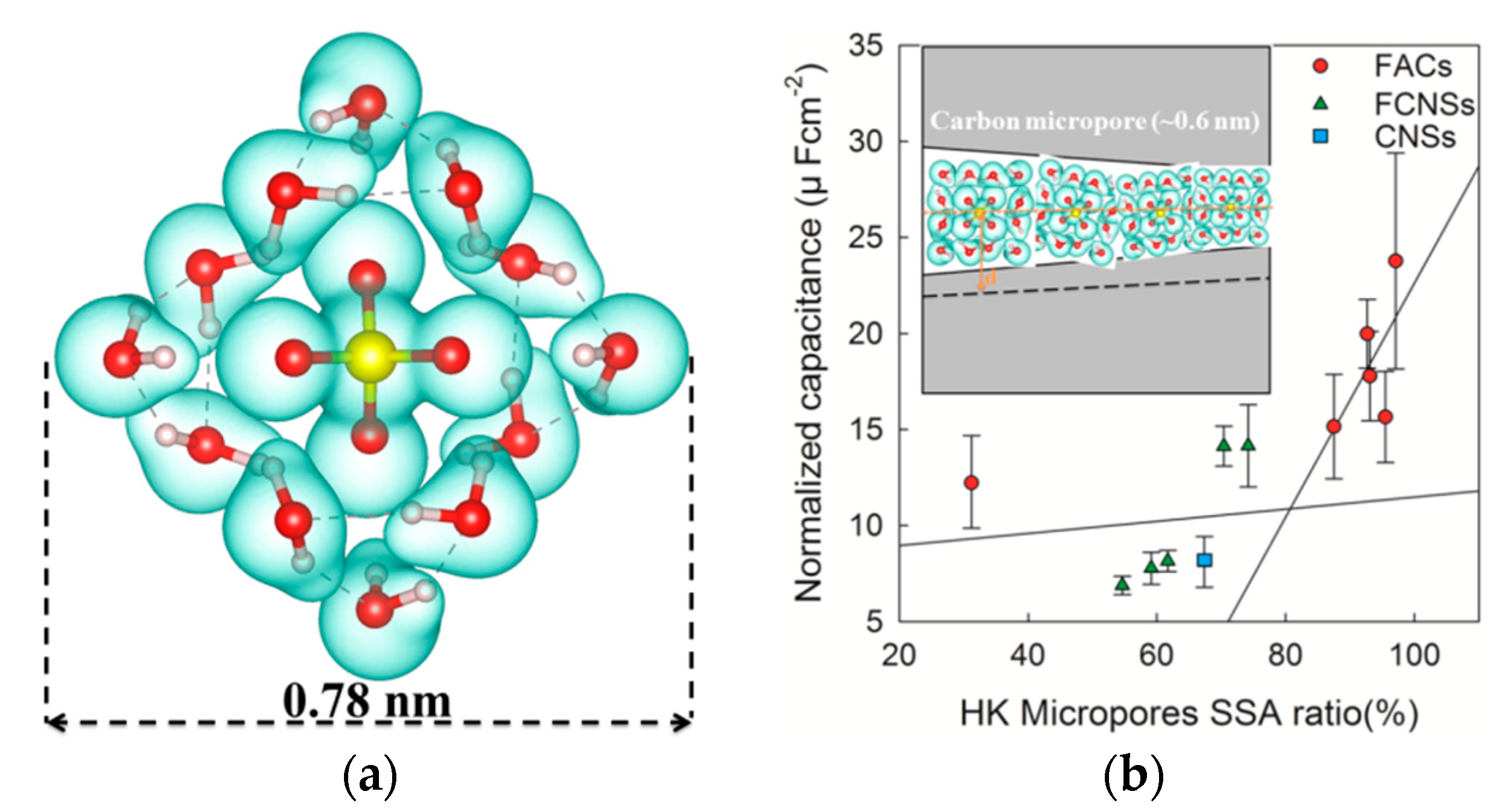
2.1. Effect of the Pore Morphology
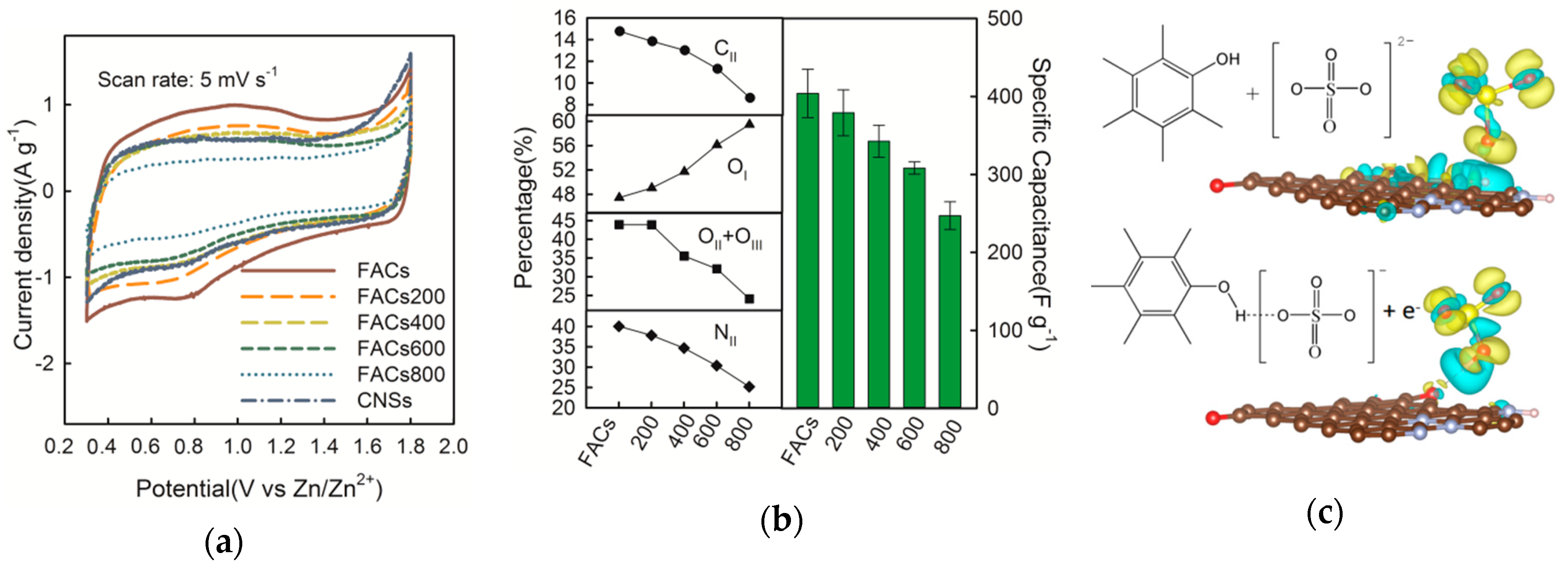
2.2. Effect of the Surface Composition
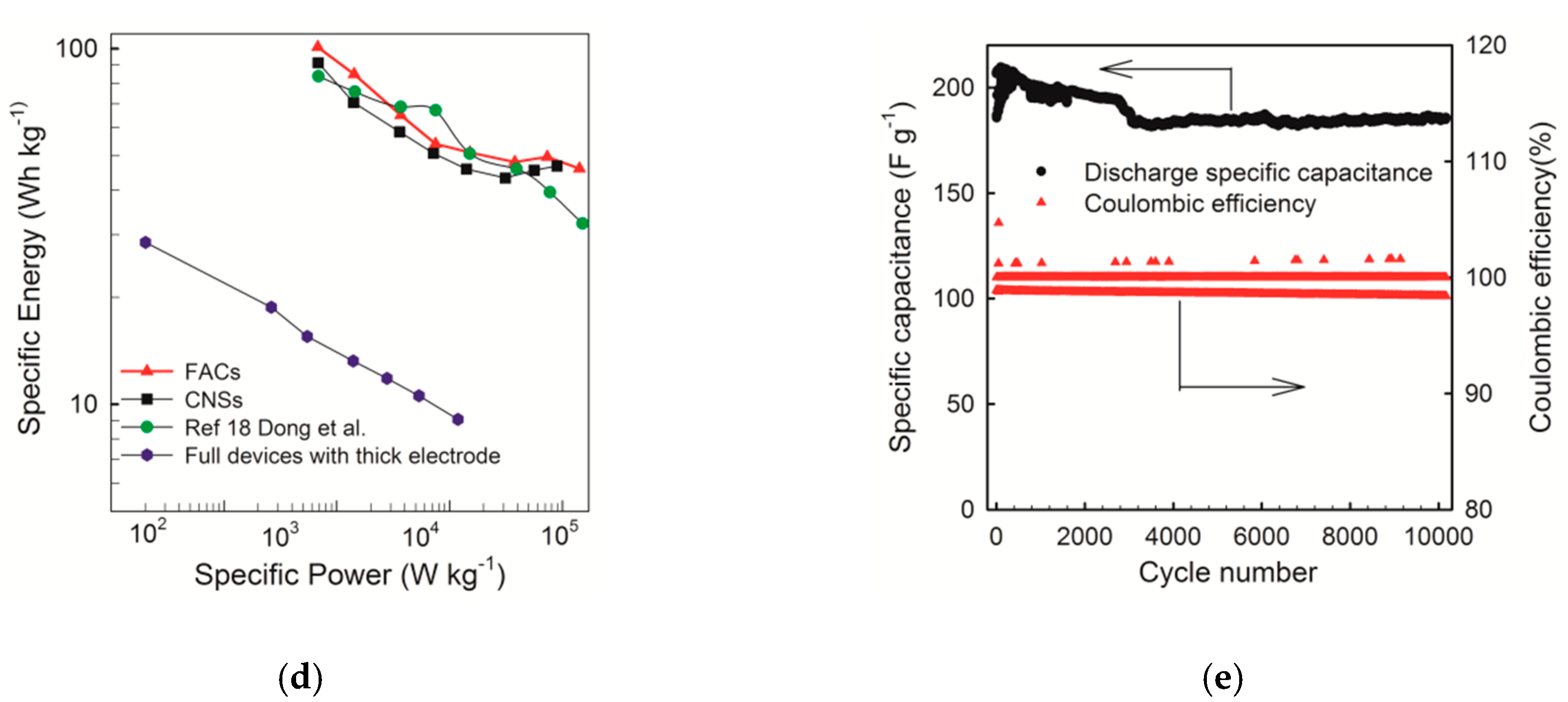
2.3. Electrochemical Performance of FACs
3. Materials and Methods
3.1. Materials Synthesis
3.2. Material Characterization
3.3. Electrochemical Measurements
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, A. R&D considerations for the performance and application of electrochemical capacitors. Electrochim. Acta 2007, 53, 1083–1091. [Google Scholar]
- Wang, X.; Zhou, H.; Sheridan, E.; Walmsley, J.C.; Ren, D.; Chen, D. Geometrically confined favourable ion packing for high gravimetric capacitance in carbon-ionic liquid supercapacitors. Energy Environ. Sci. 2016, 9, 232–239. [Google Scholar] [CrossRef]
- Gao, B.; Zhou, H.; Yang, J. Hierarchically porous carbons derived from polyaniline by “nanotube seeding” for high-performance ionic liquid-based supercapacitors. J. Mater. Chem. A 2017, 5, 524–528. [Google Scholar] [CrossRef]
- Lin, R.; Huang, P.; Ségalini, J.; Largeot, C.; Taberna, P.L.; Chmiola, J.; Gogotsi, Y.; Simon, P. Solvent effect on the ion adsorption from ionic liquid electrolyte into sub-nanometer carbon pores. Electrochim. Acta 2009, 54, 7025–7032. [Google Scholar] [CrossRef]
- Zheng, Y.; Pann, W.; Zhengn, D.; Sun, C. Fabrication of Functionalized Graphene-Based MnO2 Nanoflower through Electrodeposition for High-Performance Supercapacitor Electrodes. J. Electrochem. Soc. 2016, 163, D230–D238. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Sun, X.; Kang, X.; Zhang, Y.; Xu, C.; Zhang, Y. High Intercalation Pseudocapacitance of Free-Standing T-Nb2O5 Nanowires@carbon Cloth Hybrid Supercapacitor Electrodes. J. Electrochem. Soc. 2017, 164, A820–A825. [Google Scholar] [CrossRef]
- Hudak, N.S.; Schlichting, A.D.; Eisenbeiser, K. Structural Supercapacitors with Enhanced Performance Using Carbon Nanotubes and Polyaniline. J. Electrochem. Soc. 2017, 164, A691–A700. [Google Scholar] [CrossRef] [Green Version]
- Makino, S.; Ban, T.; Sugimoto, W. Towards Implantable Bio-Supercapacitors: Pseudocapacitance of Ruthenium Oxide Nanoparticles and Nanosheets in Acids, Buffered Solutions, and Bioelectrolytes. J. Electrochem. Soc. 2015, 162, A5001–A5006. [Google Scholar] [CrossRef] [Green Version]
- Aravindan, V.; Chuiling, W.; Madhavi, S. High power lithium-ion hybrid electrochemical capacitors using spinel LiCrTiO 4 as insertion electrode. J. Mater. Chem. 2012, 22, 16026–16031. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, D.; Tao, L.; Fan, X.; Liu, K.; Liang, G.; Dou, A.; Su, M.; Liu, Y.; Chu, D. Electrochemistry and redox characterization of rock-salt-type lithium metal oxides Li1+z/3Ni1/2-z/2Ti1/2+z/6O2 for Li-ion batteries. J. Alloy. Compd. 2019, 773, 1–10. [Google Scholar] [CrossRef]
- Liu, S.; Tang, S.; Zhang, X.; Wang, A.; Yang, Q.-H.; Luo, J. Porous Al Current Collector for Dendrite-Free Na Metal Anodes. Nano Lett. 2017, 17, 5862–5868. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, C.; Chao, D.; Yan, Q.; Fan, H.J. Nonaqueous Hybrid Lithium-Ion and Sodium-Ion Capacitors. Adv. Mater. 2017, 29, 1702093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ye, K.; Zhu, K.; Cang, R.; Wang, X.; Wang, G.; Cao, D. Assembly of Aqueous Rechargeable Magnesium Ions Battery Capacitor: The Nanowire Mg-OMS-2/Graphene as Cathode and Activated Carbon as Anode. ACS Sustain. Chem. Eng. 2017, 5, 6727–6735. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Tang, Y. A novel zinc-ion hybrid supercapacitor for long-life and low-cost energy storage applications. Energy Storage Mater. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Xie, K.; Yuan, K.; Li, X.; Lu, W.; Shen, C.; Liang, C.; Vajtai, R.; Ajayan, P.; Wei, B. Superior Potassium Ion Storage via Vertical MoS2 “Nano-Rose” with Expanded Interlayers on Graphene. Small 2017, 13, 1701471. [Google Scholar] [CrossRef]
- Dong, L.; Ma, X.; Li, Y.; Zhao, L.; Liu, W.; Cheng, J.; Xu, C.; Li, B.; Yang, Q.-H.; Kang, F. Extremely safe, high-rate and ultralong-life zinc-ion hybrid supercapacitors. Energy Storage Mater. 2018, 13, 96–102. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.-L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef]
- Latham, K.G.; Donne, S.W. Nitrogen Doped Heat-Treated and Activated Hydrothermal Carbon: Examination of Electrochemical Performance Using Step Potential Electrochemical Spectroscopy. J. Electrochem. Soc. 2018, 165, A2840–A2848. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, C.; Wang, Y.; Gao, F.; Cheng, J.; Zhang, J. High-Performance Supercapacitor Based on Nitrogen and Phosphorus Co-Doped Nonporous Polybenzoxazine-Based Carbon Electrodes. J. Electrochem. Soc. 2018, 165, A3313–A3320. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cadek, M.; Béguin, F. Tuning carbon materials for supercapacitors by direct pyrolysis of seaweeds. Adv. Funct. Mater. 2009, 19, 1032–1039. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, C.; Wu, J.-C.; Liu, M.; Zhang, D.; Song, H.; Zhang, X.; Gao, H.; Yang, J.; Chen, D. Boosting the electrochemical performance through proton transfer for the Zn-ion hybrid supercapacitor with both ionic liquid and organic electrolytes. J. Mater. Chem. A 2019, 7, 9708–9715. [Google Scholar] [CrossRef]
- Gao, B.; Zhou, H.; Yang, J. One-step preparation of nitrogen-doped graphene nanosheets for high-performance supercapacitors. Appl. Surf. Sci. 2017, 409, 350–357. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, H.; Lou, F.; Li, Y.; Buan, M.E.M.; Duan, X.; Walmsley, J.C.; Sheridan, E.; Chen, D. Boosted Supercapacitive Energy with High Rate Capability of aCarbon Framework with Hierarchical Pore Structure in an Ionic Liquid. ChemSusChem 2016, 9, 3093–3101. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Peng, Y.; Le, Z.; Wen, M.; Zhang, D.; Chen, Z.; Wu, H.B.; Li, H.; Lu, Y. Mesoporous single-crystal-like TiO2 mesocages threaded with carbon nanotubes for high-performance electrochemical energy storage. Nano Energy 2017, 35, 44–51. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, X.; Cao, X.; Zhang, D.; Xiao, S.; Li, X.; Le, Z.; Li, X.; Li, H. Microwave-assisted synthesis of 1T MoS2/Cu nanowires with enhanced capacity and stability as anode for LIBs. Chem. Eng. J. (Lausanne) 2019, 374, 429–436. [Google Scholar] [CrossRef]
- Wan, Q.; Spanu, L.; Galli, G. Solvation Properties of Microhydrated Sulfate Anion Clusters: Insights from abInitio Calculations. J. Phys. Chem. B 2012, 116, 9460–9466. [Google Scholar] [CrossRef]
- Kislenko, S.A.; Samoylov, I.S.; Amirov, R.H. Molecular dynamics simulation of the electrochemical interface between a graphite surface and the ionic liquid [BMIM][PF6]. Phys. Chem. Chem. Phys. 2009, 11, 5584–5590. [Google Scholar] [CrossRef]
- Tang, Z.; Jiang, J.; Liu, S.; Chen, L.; Liu, R.; Zheng, B.; Fu, R.; Wu, D. Polyaniline-Coated Activated Carbon Aerogel/Sulfur Composite for High-performance Lithium-Sulfur Battery. Nanoscale Res. Lett. 2017, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, T.; Fisher, F.T.; Ruoff, R.S.; Brinson, L.C. Amino-Functionalized Carbon Nanotubes for Binding to Polymers and Biological Systems. Chem. Mater. 2005, 17, 1290–1295. [Google Scholar] [CrossRef]
- Gómez-Serrano, V.; Piriz-Almeida, F.; Durán-Valle, C.J.; Pastor-Villegas, J. Formation of oxygen structures by air activation. A study by FT-IR spectroscopy. Carbon 1999, 37, 1517–1528. [Google Scholar]
- Zhou, J.-H.; Sui, Z.-J.; Zhu, J.; Li, P.; Chen, D.; Dai, Y.-C.; Yuan, W.-K. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR. Carbon 2007, 45, 785–796. [Google Scholar] [CrossRef]
- Le, Z.; Liu, F.; Nie, P.; Li, X.; Liu, X.; Bian, Z.; Chen, G.; Wu, H.B.; Lu, Y. Pseudocapacitive Sodium Storage in Mesoporous Single-Crystal-like TiO2–Graphene Nanocomposite Enables High-Performance Sodium-Ion Capacitors. ACS Nano 2017, 11, 2952–2960. [Google Scholar] [CrossRef]
- Zhan, C.; Naguib, M.; Lukatskaya, M.; Kent, P.R.C.; Gogotsi, Y.; Jiang, D.-E. Understanding the MXene Pseudocapacitance. J. Phys. Chem. Lett. 2018, 9, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Rauda, I.E.; Augustyn, V.; Dunn, B.; Tolbert, S.H. Enhancing Pseudocapacitive Charge Storage in Polymer Templated Mesoporous Materials. Acc. Chem. Res. 2013, 46, 1113–1124. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser Scribing of High-Performance and Flexible Graphene-Based Electrochemical Capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Liu, M.; Li, Y.; Liu, C.; Gao, H.; Cao, Z.; Zhang, D.; Jin, X.; Chen, Q.; Liu, Y.; et al. Carbon Nanosponge Cathode Materials and Graphite-Protected Etched Al Foil Anode for Dual-Ion Hybrid Supercapacitor. J. Electrochem. Soc. 2018, 165, A3100–A3107. [Google Scholar] [CrossRef]
- Lu, X.; Yim, W.-L.; Suryanto, B.H.R.; Zhao, C. Electrocatalytic Oxygen Evolution at Surface-Oxidized Multiwall Carbon Nanotubes. J. Am. Chem. Soc. 2015, 137, 2901–2907. [Google Scholar] [CrossRef]
- Moseley, P.T. Consequences of including carbon in the negative plates of Valve-regulated Lead–Acid batteries exposed to high-rate partial-state-of-charge operation. J. Power Sources 2009, 191, 134–138. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Boettger, J.C.; Smith, J.R.; Birkenheuer, U.; Rösch, N.; Trickey, S.B.; Sabin, J.R.; Apell, S.P. Extracting convergent surface formation energies from slab calculations. J. Phys. Condens. Matter 1998, 10, 893–894. [Google Scholar] [CrossRef]
- Vincenzo, F.; Methfessel, M. Extracting convergent surface energies from slab calculations. J. Phys. Condens. Matter 1996, 8, 6525. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wu, J.-C.; Zhou, H.; Liu, M.; Zhang, D.; Li, S.; Gao, H.; Yang, J. Great Enhancement of Carbon Energy Storage through Narrow Pores and Hydrogen-Containing Functional Groups for Aqueous Zn-Ion Hybrid Supercapacitor. Molecules 2019, 24, 2589. https://doi.org/10.3390/molecules24142589
Liu C, Wu J-C, Zhou H, Liu M, Zhang D, Li S, Gao H, Yang J. Great Enhancement of Carbon Energy Storage through Narrow Pores and Hydrogen-Containing Functional Groups for Aqueous Zn-Ion Hybrid Supercapacitor. Molecules. 2019; 24(14):2589. https://doi.org/10.3390/molecules24142589
Chicago/Turabian StyleLiu, Chao, Jian-Chun Wu, Haitao Zhou, Menghao Liu, Dong Zhang, Shilin Li, Hongquan Gao, and Jianhong Yang. 2019. "Great Enhancement of Carbon Energy Storage through Narrow Pores and Hydrogen-Containing Functional Groups for Aqueous Zn-Ion Hybrid Supercapacitor" Molecules 24, no. 14: 2589. https://doi.org/10.3390/molecules24142589